Abstract
The aim of our study was to compare the diagnostic performance of 16--slice multidetector computed tomography with that of superparamagnetic iron oxide (SPIO)-enhanced magnetic resonance (MR) imaging in the detection of small hepatic metastases and in the differentiation of hepatic metastases from cysts. Twenty-three patients with 55 liver metastases and 14 liver cysts underwent SPIO-enhanced MR imaging and multiphasic CT using 16-MDCT. Two observers independently analyzed each image, in random order. Sensitivity and diagnostic accuracy for lesion detection and differentiation as metastases or cysts for MDCT and SPIO-enhanced MR imaging were calculated using receiver operating characteristic analysis. For all observers, the Az values of SPIO-enhanced MR imaging for lesion detection and differentiation of liver metastases from cysts (mean 0.955, 0.999) were higher than those of MDCT (mean 0.925, 0.982), but not statistically significantly so (P>0.05). Sensitivity of SPIO-enhanced MR imaging with regard to the detection of liver metastases (mean 94.5%) was significantly higher than that of MDCT (mean 80.0%) (P<0.05). SPIO-enhanced MR imaging and 16-MDCT showed similar diagnostic accuracies for detection and differentiation of liver metastases from cysts, but sensitivity of SPIO-enhanced imaging in the detection of liver metastases was superior to that of 16-MDCT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The early detection and precise characterization of liver metastases has become a crucial issue in treatment planning and is also considered to be important for improved therapeutic outcomes. Computed tomography (CT) has been widely used as a primary imaging modality for diagnosis and preoperative staging work-ups in patients suffering from primary cancers of the abdomen and chest, as the result of its easy availability and simultaneous evaluation of primary cancer, as well as metastases. However, the reported sensitivity of single-detector row helical CT for the detection of hepatic metastases, as compared with other recently established liver imaging modalities [1–6], has been somewhat unsatisfactory. Additionally, the accurate characterization of small hepatic metastases with helical CT alone, even when thinner collimation is used, might prove difficult, particularly with regard to the differentiation of metastases from small hepatic cysts, which are thought to be the most frequently encountered cause of low attenuation on CT [7–9]. This may constitute the critical shortcoming of helical CT in the accurate selection of surgical candidates. From this perspective, the demand for more accurate imaging modalities for the detection and characterization of hepatic metastases, such as magnetic resonance (MR) imaging, with the administration of liver-specific MR contrast media, including superparamagnetic iron oxide (SPIO) [2, 10–12], mangafodipir trisodium (Mn-DPDP) [4–6], and gadobenate dimeglumine (Gd-BOPTA) [13, 14] have been increasing in recent years.
The recent introduction of the multidetector computed tomography (MDCT) scanner for use in liver imaging has enabled the acquisition of optimum dynamic imaging, with both high temporal resolution and high longitudinal resolution [15, 16]. Several studies have reported on the potential advantages of the MDCT scanner over the single-detector row helical CT scanner, with regard to the detection and characterization of focal liver lesions [17–19]. Because high temporal and spatial resolution may be essential features of an imaging modality for the accurate detection and characterization of focal liver lesions, the improved longitudinal spatial resolution of MDCT may encourage expectations of further improvement in the diagnostic accuracy of liver CT imaging for the evaluation of hepatic metastases, up to a range comparable to that of liver MR imaging such as SPIO-enhanced imaging.
To the best of our knowledge, no comparative studies of 16-slice MDCT and SPIO-enhanced MR imaging have yet been conducted with state-of-the-art equipment, for the evaluation of small hepatic metastases. The objective of our study, then, was to compare the diagnostic performance of 16-slice MDCT and SPIO-enhanced MR imaging in the detection of small hepatic metastases and in the differentiation of hepatic metastases from cysts, using alternative-free response receiver operating characteristic (ROC) analysis, with multiple observers.
Materials and methods
Between September 2003 and April 2005, 31 consecutive patients, all of whom suffered from pathologically confirmed primary malignancy including gastrointestinal cancer and breast cancer, and hepatic metastases, underwent multiphasic contrast-enhanced MDCT and SPIO-enhanced MR imaging at the Chonbuk National University Hospital, a tertiary referral hospital. Written informed consent was provided by each of the patients before they were enrolled in the study, and the protocols of the study had previously been approved by the institutional review board of our hospital. At our institution, multiphasic dynamic abdominal CT scans are routinely performed as a component of the preoperative staging work-up for patients with gastrointestinal cancer. Also, for breast cancer patients, multiphasic dynamic abdominal CT scans are conducted for staging purposes, if the patients are found to be in an advanced stage (T3 N1 or above) [20], or in cases in which a suspicious liver mass is detected by sonography. In cases in which hepatic metastases are found to be suspicious on the dynamic CT scans of patients who are being considered for surgical resections for the resolution of primary cancer, SPIO-enhanced MR imaging is performed as soon as possible. The time intervals between the two imaging modalities were 3–10 days in all of the study patients (mean 5 days). We excluded eight of the 31 patients for the following reasons: four had neither histological proof of liver metastases nor sufficient follow-up examinations; four had in excess of ten metastases. Hence, 23 patients (17 men, six women; age range 36–71 years; mean age 54 years) with liver metastases were ultimately included in this study.
A total of 69 focal hepatic lesions were either histologically or clinically confirmed in 23 of the study patients. These lesions consisted of 55 hepatic metastases and 14 hepatic cysts. The primary cancer sites of patients exhibiting liver metastases were colorectal (n=9), stomach (n=8), and breast (n=6). The hepatic metastases ranged in size from 0.4 to 2.0 cm (mean 0.9 cm). Of these metastases, 34 lesions were 10 mm or less in diameter. The hepatic cysts ranged in size from 0.3 cm to 1.5 cm (mean 0.6 cm). Ten patients concomitantly had hepatic metastases and hepatic cysts. None of the study patients evidenced liver masses other than hepatic metastases and hepatic cysts. All of the patients were, upon initial examination, being considered for surgical resection of primary cancer. However, only 16 of the patients actually underwent surgical resections for primary cancer as well as hepatic metastases. With regard to the numbers of hepatic metastases, nine patients had solitary lesions, three patients had two lesions each, six patients had three lesions, four patients had four lesions, and one patient had six lesions. The final diagnosis of liver metastases in the remaining seven patients was made on the basis of a combination of sonographically guided percutaneous needle biopsy, characteristic MR or CT findings, and rapid progression on at least 8-month follow-up contrast-enhanced CT or MR imaging. The confirmation of hepatic cysts was predicated on typical imaging findings on MR imaging, including heavily T2-weighted images, as well as intraoperative sonography findings in the patients who underwent surgery.
Determination of the total number of metastases in the 16 patients who underwent hepatic surgeries was based on histopathological analyses of the surgical specimens, as well as intraoperative sonography. All the hepatic surgeries with intraoperative ultrasonography were conducted by one experienced hepatobiliary surgeon (H.C.Y). The average time interval between the surgeries and the final MR examinations was 9 days (6 days–15 days). Prior to surgery, the locations and numbers of liver metastases on the preoperative CT or MR imaging were carefully reviewed jointly by both the surgeon and the radiologist. After the resection of the liver metastases (enucleation n=13; segmentectomy n=3), the histopathological findings of the resected hepatic specimens were correlated with the preoperative imaging findings. In cases in which additional hepatic nodules suspected of being metastases were detected on intraoperative sonography, immediate frozen-section analysis was conducted by one hepatobiliary pathologist. For the frozen-section analyses, rapidly frozen surgical specimens at −20°C were stained with hematoxylin and eosin. In the remaining seven patients who did not undergo hepatic surgery, the standards of reference were based on the findings of image-guided biopsy and the consensus readings of all MR images, sonography, three phase spiral CT, CT during arterioportography, and the follow-up CT or MR images taken by the study coordinator (Y.K.K) and one radiologist (C.S.K) who was not involved in the image interpretation. For those patients exhibiting multiple liver metastases, if the imaging findings were identical in all lesions, an image-guided biopsy was performed for only one of the liver lesions.
MR imaging examination
All MR imaging was performed with a 1.5 T super-conducting imager (Magnetom Symphony; Siemens, Erlangen, Germany) with a phased array multicoil for signal reception. The liver was imaged in the axial plane in all of the following sequences. Baseline MR images, including the respiratory-triggered T2-weighted turbo-spin-echo (TSE) sequence, a breath-hold T2*-weighted fast imaging with steady-state precession (FISP) sequence, and a breath-hold T1-weighted fast low-angle shot (FLASH) sequence, were all acquired. Respiration-triggered T2-weighted TSE imaging was obtained using the following parameters: a TR/TE of 3,300–5,500 ms/85ms, an echo train length of 5, a 256×512 pixel matrix, and a signal average of two. Breath-hold T2*-weighted FISP imaging was acquired with the following parameters: a TR/TE of 180 ms/12 ms, 30° flip angle, a 96×256 pixel matrix, and a signal average of one. Breath-hold T1-weighted fast low-angle shot imaging was acquired with the following parameters: a TR/TE of 120 ms/4 ms, 70° flip angle, a 120×256 pixel matrix, and a signal average of one. For all of the sequences, we used a 6-mm slice thickness, with a 10% intersection gap, and a 35–40 cm field of view, depending on the size of the liver.
SPIO-enhanced MR imaging consisted of the respiratory-triggered T2-weighted TSE sequence and the breath-hold T2*-weighted FISP sequence, with the same parameters as those used in the baseline MR imaging. The SPIO agent (SHU-555-A; Resovist, Schering AG, Berlin, Germany), at a dose of 8 μmol of iron per kilogram of body weight, was rapidly intravenously injected through a 5 μm filter (1 ml/sec), followed by a 15 ml sterile saline solution flush; imaging commenced approximately 10 min after the intravenous injection of SPIO.
MDCT examination
CT examinations were performed on a 16-MDCT scanner (Sensation 16; Siemens, Forchheim, Germany). The images were acquired through the liver in a craniocaudal direction, with a 1.5×16 beam collimation. The other scanning parameters used were as follows: 160 mAs tube current; 120 kVp tube potential; 1.5 mm detector collimation; 24 mm per rotation table speed; and a 0.5 s gantry rotation time. A reconstruction slice thickness of 3.0 mm and a reconstruction interval of 3.0 mm were also established. Prior to the examinations, the patients were instructed to hold their breath, in order to prevent motion artifacts.
Unenhanced MDCT was initially performed starting at the top of the liver, in a cephalocaudal direction. After the unenhanced liver images had been acquired, contrast medium with an iodine concentration of 370 mg/ml (Ultravist 370; Schering, Berlin, Germany), was administered with a power injector (Multilevel CT; Medrad, Pittsburgh, Pa., USA). The contrast medium was then injected at 3 ml/s through an 18-gauge plastic i.v. catheter positioned in the antecubital vein. The volume of contrast medium delivered varied according to the body weight of each of the patients (2 ml per kilogram of body weight); therefore, the total contrast medium volume administered ranged between 110 ml and 150 ml (mean 120±10 ml). Scanning for the dynamic three-phase images, including the hepatic arterial phase, the portal venous phase, and the delayed phase, began at 35 s, 70 s, and 180 s, respectively, after the initiation of the contrast medium injection. The acquisition time for each of the phases was 5–7 s, according to the scanning range.
Imaging analysis
All the MR imaging was evaluated independently and separately by two gastrointestinal radiologists (S.W.K., S.B.H), both of whom were experienced in the interpretation of MR liver imaging in their daily clinical practice. All the acquired images were reviewed on a 2,000×2,000 picture archiving and communication systems (PACS; Marotech, Seoul, Korea) monitor. The readers were free to alter the window level and window width at their discretion. The two radiologists were uninvolved in the design of the present study, and were unaware of the results of all the other imaging findings as well as to the final diagnosis. However, they were made aware of the objective of the study. Two separate sets of images were analyzed in random order, i.e., the MDCT images (precontrast, arterial, portal, and equilibrium phases) and the two sequences of the unenhanced and SPIO-enhanced T2-weighted images (T2-weighted and respiratory-triggered T2-weighted turbo-spin-echo images). In order to minimize any learning biases, we allowed a 3-week interval between blind interpretations.
Each of the observers recorded the presence and location of the lesions, assigning each of them a confidence level, scored on a 4-point scale, as follows: “1” as ‘probably not present’; “2” as ‘possibly present’; “3” as ‘probably present’; and “4” as ‘definitely present’. In order to prevent mismatches between the findings of the scored lesions and those of the gold standard for determining the total number of lesions, each of the observers recorded the individual image number, the segmental location of all the lesions, and the sizes of each of the lesions. For patients with multiple lesions in the same segment, the observers added further descriptions with regard to the sizes and locations of the masses within each of the segments, in order to avoid confusion in data analysis. Each of the observers also indicated the possibility of malignancy, assigning each of the lesions a confidence level on a 5-point scale, as follows: “1” as ‘probably benign’; “2” as ‘possibly benign’; “3” as ‘equivocal’; “4” as ‘probably malignant’; and “5” as ‘definitely malignant’. The decision by each observer as to whether lesions were benign or malignant was predicated on the subjective features of a lesion, including the margin sharpness, shape, homogeneity, attenuation, and the presence of enhancement; the diagnostic criteria for a cyst, i.e., smooth margins, homogeneous low attenuation similar to that of water, and no enhancements during contrast-enhanced CT. The two observers were aware that all the hepatic lesions included only metastases and cysts, benign at the time of interpretation. After the two reviewing sessions conducted by the two observers, the study coordinator (K.Y.K), and one radiologist (C.S.K) who was not involved in image interpretation, compared the scoring results of each of the observers with the gold standard and then devised a possible explanation for the false-positive and false-negative findings of each of the observers.
On the basis of the reviews submitted by the two observers, alternative-free response ROC curve analysis was performed on a lesion-by-lesion basis [21]. For each imaging set an alternative-free response ROC curve was fitted to each observer’s confidence rating data, using a maximum likelihood estimation program (ROCKIT 0.9 B; courtesy of Metz CE, University of Chicago, Ill., USA, 1998) [22]. The diagnostic accuracy of each imaging set and observer was assessed by calculating the area under the alternative-free response ROC curve. The differences between the imaging sets, with regard to the mean area under the alternative-free response ROC curves, were statistically analyzed using a two-tailed Student’s t test for paired data. The sensitivities of each of the image sets for all lesions and metastases alone were then calculated. The sensitivity of each observer and each set of images was evaluated according to the number of lesions assigned confidence levels of 3 or 4 from among the 69 lesions. Prior to image analysis, the observers were aware that only those lesions that had been assigned confidence levels of 3 or 4 would be included in separate sensitivity calculations. The sensitivities of the MR and CT images were then compared using the McNemar test. A two-tailed P value of less than 0.05 was considered to be a significant difference. In order to obtain a range of plausible sensitivity differences, we also calculated the 95% CIs [23].
In order to assess interobserver agreement for the evaluation of the two imaging modalities, we calculated the kappa statistic for multiple observers [24]. Agreement between the blind observers is reported below in terms of kappa values, those greater than 0 indicating positive correlations. Kappa values of less than 0.20 indicated positive but poor agreement; those of 0.21–0.40 indicated fair agreement; those of 0.41–0.60 indicated moderate agreement; those of 0.61–0.80 indicated good agreement, and those greater than 0.81 indicated excellent agreement. The significance of the difference between the kappa values assigned to the two imaging modalities was then tested using the z-tests. The statistical significance level was set at P<0.05. All statistical analyses were calculated with the SPSS 8.0 program (SPSS, Chicago, Ill., USA).
Results
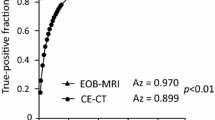
For all 69 lesions, including the 55 hepatic metastases, the calculated Az values for each of the observers with the SPIO-enhanced images and MDCT are provided in Table 1. For all observers, the Az values for SPIO-enhanced MR imaging were slightly higher than those for MDCT, but statistically significant differences in the areas under the ROC curves (Az) between both image sets were not found to exist, by any of the two observers, both for all 64 lesions overall and for the 55 hepatic metastases (mean Az on SPIO-enhanced MR imaging 0.955 for all lesions, 0.935 for metastases; mean Az on MDCT 0.925 both for all lesions and metastases) (P>0.5).
The sensitivities from each observer for each image set are shown in Table 2. Overall, there was a trend toward increased sensitivity for SPIO-enhanced MR imaging as compared with MDCT, and the sensitivities of SPIO-enhanced MR imaging in all observers were significantly higher than those of MDCT, both for all 69 lesions overall, and for the 55 hepatic metastases (Fig. 1) (observer 1, P=0.008, P=0.016; observer 2, P=0.002, P=0.004). Among the 55 hepatic metastases, SPIO-enhanced MR imaging allowed for the depiction of 52 lesions (sensitivity 94.5%; 95% CI 84.9%, 98.8%) by both observers, and MDCT allowed for the depiction of 45 lesions (sensitivity 81.8%; 95% CI 69.1%, 90.9%) to be discerned by observer 1 and 43 lesions (sensitivity, 78.2%; 95% CI: 65.0%, 88.2%) to be discerned by observer 2. There were ten metastases (0.4–1.0 cm) in eight patients, which were not detected by any observers on MDCT but were clearly revealed on the SPIO-enhanced MR imaging (Figs 1 and 2) as confidence rating 3 or 4 by two observers. Seven of these ten lesions were confirmed via surgery. On the retrospective reviewing of the MDCT images, all lesions appeared as iso-attenuation or faint low attenuation (Figs. 1 and 2). On the other hand, there was only one metastasis, which was not detected by any observers on SPIO-enhanced MR imaging but which was detected on the MDCT images as a confidence rating 4 by both observers. At SPIO-enhanced MR imaging, each of the two observers made one false-positive diagnosis that was attributed to a vessel; at MDCT imaging, no observer made false-positive diagnoses.
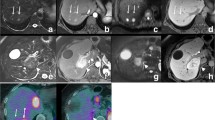
Two liver metastases from gastric carcinoma in a 55-year-old man. The presence of liver metastases was confirmed at surgery. a Portal venous phase image of multi-detector row CT scanning with 1.5 mm collimation and 3 mm reconstruction interval shows only one low-attenuated liver mass (arrow) clearly. Note faint small low attenuation (small arrow) at posterior subcapsular area in right liver. Two reviewers missed this lesion during image interpretation. b SPIO-enhanced respiratory-triggered T2-weighted turbo-spin echo image clearly shows two high signal intensity masses (arrows) at the same location as in a. c SPIO-enhanced breath-hold T2*-weighted fast images obtained with steady-state procession also shows two high signal intensity masses (arrows). The conspicuity of these lesions on SPIO--enhanced images was superior to that obtained on portal phase CT images
Single liver metastasis in a 59-year-old man with colon carcinoma. a The portal venous phase image of multi-detector row CT scanning with 1.5 mm collimation and 3 mm reconstruction interval shows no liver masses. b SPIO--enhanced respiratory-triggered T2-weighted turbo-spin echo images shows a tiny high signal intensity mass (arrow)
With regard to the differentiation of liver metastases from cysts, the calculated Az values for each observer with the SPIO-enhanced images and MDCT are shown in Table 3. For all observers, the Az values for the SPIO-enhanced MR imaging were slightly higher than those for MDCT, but no statistically significant differences in the area under the ROC curves (Az) between both image sets were demonstrated by either of the two observers (mean Az on SPIO-enhanced MR imaging 0.999; mean Az on MDCT 0.982) (P>0.1). There were three metastases (0.4–1.0 cm) in three patients, which were misinterpreted as cysts on the MDCT images by both observers but were accurately characterized by both observers on the SPIO-enhanced MR imaging (Fig. 3). One hepatic cyst was misinterpreted as a metastasis on both the MDCT and SPIO-enhanced MR imaging by both observers. We did not perform a separate image analysis of non-enhanced MR imaging and combination of non-enhanced MR imaging and SPIO-enhanced imaging. However, non-enhanced MR images, including respiratory-triggered T2-weighted TSE sequences, showed no additional role in SPIO-enhanced images for the detection of lesions and in the differentiation of liver metastases from cysts on reviewing sessions by two observers.
Single liver metastasis in a 61-year-old man with gastric carcinoma a The portal venous phase image of multi-detector row CT scanning with a 1.5 mm collimation and a 3 mm reconstruction interval shows a small low-attenuated round lesion (arrow). This lesion was misinterpreted as a cyst by both reviewers. b SPIO-enhanced respiratory-triggered T2-weighted turbo-spin echo image shows a slightly high signal intensity mass (arrow) at the same location as in a, which was correctly interpreted as a malignant mass by the two reviewers
For lesion detection, the kappa value for SPIO-enhanced MR imaging (0.870) was similar to that of MDCT (0.847) (P=0.93), which indicated excellent inter-observer agreement. With regard to the differentiation of the lesions as metastases or cysts, however, the kappa value of the SPIO-enhanced MR imaging (0.867) was significantly higher than that of MDCT (0.763) (P<0.05), which also indicated good-to-excellent inter-observer agreement
Discussion
With the technical breakthroughs in liver imaging modalities, considerable attention has been focused on the determination of which liver imaging modality has the most potential to fulfill the dual requirement of early detection and accurate characterization of focal liver lesions. With regard to the detection of hepatic metastases, there has been extensive research, including meta-analyses, that has compared different imaging modalities for the determination of imaging modality with better accuracy [1–6, 11–14, 25]. Until recently, there appeared to be consensus that MR imaging with liver-targeted contrast agents, including SPIO and Mn-DPDP, was the most sensitive non-invasive technique for the detection of hepatic metastases. However, comparative studies between MR images using liver-targeted agents have been limited to a small number of studies that have shown similar accuracies of SPIO-enhanced MR images versus Mn-DPDP-enhanced MR images or Gd-BOPTA-enhanced delayed-phase images for the detection of hepatic metastases [14, 26]. Also, with regard to the characterization of focal liver lesions, direct comparative study between SPIO-enhanced MR images and MR images with hepatobiliary agents has been limited [26], although usefulness of these liver-targeted agents for the characterization of focal liver lesions has been shown in many studies [4–6,10–13]. For differentiation of hepatic metastases from cysts, SPIO-enhanced MR images might be advantageous over MR images with hepatobiliary agents because of the inherent ability of T2-weighted TSE sequence to differentiate solid and non-solid liver lesions as well as T1-shortening effect of SPIO particles that could show typical rim enhancement of hepatic metastases on SPIO-enhanced T1-weighted images [11]. In consideration of these two kinds of characteristics of SPIO particles, the diagnostic performance of 16-slice MDCT for the detection of hepatic metastases and differentiation of hepatic metastases from cysts, in our study, was estimated via comparison with SPIO-enhanced MR imaging.
In our present study, we hypothesized that the diagnostic capability of 16-slice MDCT for hepatic metastases might be improved over that of single-detector row spiral CT, or might be comparable to that of SPIO-enhanced MR imaging. There has been only one comparative study of SPIO-enhanced MR imaging and four-slice MDCT for the detection of hepatic metastases in which accuracy with SPIO-enhanced MR imaging was better than with CT [27]. To the best of our knowledge, ours is the first comparative study conducted with 16-slice MDCT and SPIO-enhanced MR imaging, specifically with regard to the ability of these techniques to evaluate small (2.0 cm or less; mean 0.9 cm) hepatic metastases, using the most recently established techniques. We used two kinds of T2-weighted imaging for the SPIO-enhanced MR imaging: T2-weighted TSE with a 512 matrix for high-resolution imaging and differentiation between malignancy and benignity such as cysts, and T2*-weighted gradient-recalled echo (GRE) sequences for maximum lesion-to-liver contrast. The T2*-weighted gradient-recalled echo technique has been generally considered to be the optimal pulse sequence, due to its greater susceptibility, the result of local field inhomogeneities [28, 29]. For the MDCT imaging, three-phasic dynamic images, including arterial, portal venous, and equilibrium phases, were acquired at 3 mm reconstruction thickness, using a detector collimation of 1.5 mm. Recent study about determining the optimal CT protocol for detection of hepatic metastases using four-slice MDCT [30] has shown that a thin section of 2.5 mm or an increased table speed are less efficient in detecting small metastases, even though MDCT with a different detector configuration from ours was used in that study. Moreover, we utilized a highly concentrated iodine contrast agent [31, 32] to maximize the lesion-to-liver contrast. With regard to the delineation of hypovascular hepatic metastases, portal venous phase imaging is generally considered to be the most effective technique. However, we acquired arterial phases and equilibrium phases as well, for their potential additional roles in lesion detection and lesion characterization [33].
This study revealed that, in alternative-free response ROC analysis regarding the presence of lesions, both observers achieved slightly higher diagnostic accuracy with the SPIO-enhanced MR imaging than with the MDCT imaging, both for all hepatic lesions and for the hepatic metastases alone. However, the diagnostic accuracy obtained with the two image sets was not statistically significantly different, for either of the two observers (P>0.05). However, the sensitivities for the detection both of all hepatic lesions and of hepatic metastases with SPIO-enhanced MR imaging, were determined to be significantly higher than those achieved with MDCT, for both observers (P<0.05). When we retrospectively reviewed the ten metastases (size range 0.5–0.8 cm) commonly missed by both observers on the MDCT, we found that all ten of the metastases had not been depicted or had appeared as faint low attenuation, probably because the low-attenuated portions of the small hepatic metastases were obscured by their marginal enhancement, as well as by the surrounding enhancing liver parenchyma (Figs. 1 and 2). However, the majority of hepatic cysts that were similar in size to those of the metastases missed on MDCT were clearly depicted on the MDCT. This finding is consistent with the results of a previous study, in which it was demonstrated that small cysts are more likely to be seen than small metastases on CT, even when a thinner collimation is employed [9]. The slice thickness used in our MDCT was one half of the 6 mm slice thickness used in the SPIO-enhanced MR imaging. Moreover, in the MDCT, there is no interslice gap, as there is in the two-dimensional SPIO-enhanced MR imaging technique. Therefore, given that SPIO-enhanced MR imaging exhibits the highest degree of contrast between the liver parenchyma and liver malignancies among the variety of available image modalities, our results may indicate that higher longitudinal resolution is not the only criterion for the depiction of hypovascular hepatic lesions, but that lesion-liver contrast may, indeed, be more important with regard to lesion detection. Acquisition of T2-weighted images with increased longitudinal resolution compared with the ones utilized in this study could show better diagnostic accuracy of SPIO-enhanced MR imaging than was in our study.
With regard to the differentiation of metastases form liver cysts in our study, although the radiologists’ performance was found to be slightly better with SPIO-enhanced MR imaging than with MDCT, neither of the observers exhibited statistically significantly differences in performance between the two techniques (observer 1, P=0.25; observer 2, P=0.14). The higher capability of SPIO-enhanced MR imaging in differentiating between hepatic metastases or hepatic cysts might be attributable to the use of two kinds of SPIO-enhanced imaging, that is, unenhanced and SPIO-enhanced T2-weighted TSE sequences facilitate the differentiation between hepatic metastases and hepatic cysts, as well as lesion detection, although the T2*-weighted GRE sequence was used only for lesion detection, as the sequence which provides the highest degree of lesion-liver contrast [28, 29]. Three small hepatic metastases (0.4 cm, 0.5 cm, 0.7 cm) were misinterpreted as hepatic cysts on MDCT commonly by both observers, because all of them mimicked hepatic cysts, showing well-defined clear low attenuation on the portal venous phase, without rim enhancement. Additionally, only one small hepatic cyst was misinterpreted as a hepatic metastasis on the MDCT. All these lesions were correctly characterized by all observers on SPIO-enhanced MR imaging. There have been some reports that have indicated limitations of the single-detector row helical CT and MDCT with regard to the characterization of small hepatic metastases [5, 7–9]. However, our study results showed that the diagnostic capability of 16-MDCT in differentiating between hepatic metastases and hepatic cysts might be superior to previous CT techniques, even though the majority of lesions in our study were less than 1.0cm in size. This could be the result of the reduced partial volume averaging achieved by using thinner collimation and thinner reconstruction thicknesses than have been used in previous studies [8]. However, the criteria established for the differentiation of small hepatic metastases from small hepatic cysts inevitably depended on the observer’s subjective decisions. Therefore, interobserver variability in the differentiation of metastases form liver cysts using MDCT was actually found to be significantly higher than that achieved with SPIO-enhanced MR imaging in this study (P=0.047).
Our study has limitations. First, not all of the lesions were confirmed surgically. This may have resulted in some degree of overestimation of the actual sensitivity of both image modalities, by reducing the number of false-negative lesions. The objective of our study, however, was not designed to determine the absolute diagnostic accuracy and sensitivity of liver imaging modalities with regard to the detection and characterization of lesions, but to compare diagnostic accuracy and sensitivity between two different imaging modalities. In addition, the advent of liver imaging techniques may reduce the chances of hepatic surgery for metastases, as it shows a relatively larger number of hepatic metastases than did previous liver imaging modalities. Nevertheless, in our study, we have included only patients who had undergone liver surgery or who did not undergo liver surgery but had undergone pathological confirmation of hepatic metastases by biopsy and who had attended at least an 8-month follow-up MDCT or MR imaging session, which possibly ascertained the liver segment to be free of clinically relevant lesions. A second criticism of this study would involve the fact that we did not use overlapping reconstruction in our MDCT imaging. Many authors have claimed that 50% overlapping reconstructions should be a routine part of liver CT imaging, in order to improve the sensitivity and conspicuity of focal liver lesions [8, 34]. However, in our institution, sections thinner than 3 mm are not routinely taken with overlapping reconstruction, due to excessive image storage in the PACS. Third, selection bias may exist with regard to the initial imaging referrals, because the majority of the patients included in our study were already suspected of having hepatic metastases, as evidenced by MDCT taken during routine preoperative diagnostic work-ups. Therefore, lesions not apparent on MDCT may have been under-represented in our study. Also, since only patients with metastases were included, the observers were aware that all patients were likely to have at least one metastatic lesion before the image interpretation. This could introduce a bias in the accuracy calculation, but they had no information about the number and location of metastases as well as coexisting cysts. Fourth, the small number of patients involved in the present study is another possible limitation. Finally, in our study, 14 of 23 patients exhibited multiple hepatic metastases and ten patients had hepatic cysts concomitantly with metastases. Therefore, a problem might exist in data clustering in the alternative-free response ROC analysis, and there is a risk that the statistical significance may have been overestimated [35, 36]. Also, the high prevalence of hepatic cysts coexisting in the same patients with metastases may have introduced a bias resulting in poor performance of lesion characterization. However, there were no noticeable patterns or clustering of false-negative lesions or misinterpreted lesions for any patients, and a relatively even distribution of them in many patients was shown. For clustered data, we also calculated 95% CIs of sensitivity, via the method of Rao and Scott [23]. Also, with regard to the characterization of lesions, the majority of small hepatic cysts (except one) were accurately characterized on MDCT, although hepatic metastases of similar size coexisted in these patients.
In summary, SPIO-enhanced MR imaging and multiphasic 16-MDCT showed comparable diagnostic accuracy with regard to the detection of small hepatic metastases, as well as for the differentiation between hepatic metastases and hepatic cysts on alternative-free response ROC analysis. However, the sensitivity of detection of small hepatic metastases with SPIO-enhanced MR imaging was superior to that with 16-MDCT.
References
Hagspiel KD, Neidel KFW, Eichenberger AC, Weder W, Marincek B (1995) Detection of liver metastases: comparison of superparamagnetic iron-oxide-enhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology 196:471–478
Ward J, Naik KS, Guthrie JA, Wilson D, Robinson PJ (1999) Hepatic lesion detection: comparison of MR imaging after the administration of superparamagnetic iron oxide with dual-phase CT by using alternative free response receiver operating characteristic analysis. Radiology 210:459–466
Semelka RC, Brown ED, Ascher SM, Patt RH, Bagley AS, Li W, Edelman RR, Shoenut JP, Brown JJ (1994) Solitary hepatic metastasis: comparison of dynamic contrast-enhanced CT and MR imaging with fat-suppressed T2-weighted, breath-hold T1-weighted FLASH, and dynamic gadolinium-enhanced FLASH sequences. J Magn Reson Imaging 4:319–323
Bartolozzi C, Donati F, Cioni D, Procacci C, Morana G, Chiesa A, Grazioli L, Cittadini G, Cittadini G, Giovagnoni A, Gandini G, Maass J, Lencioni R (2004) Detection of colorectal liver metastases: a prospective multicenter trial comparing unenhanced MRI, MnDPDP-enhanced MRI, and spiral CT. Eur Radiol 14:14–20
Kim KW, Kim AY, Kim TK, Park SH, Kim HJ, Lee YK, Park MS, Ha HK, Kim PN, Kim JC, Lee MG (2004) Small (≤ 2 cm) hepatic lesions in colorectal cancer patients: detection and characterization on mangafodipir trisodium-enhanced MRI. AJR Am J Roentgenol 182:1233–1240
Torres CG, Lundby B, Sterud AT, McGill S, Gordon PB, Bjerknes HS (1997) MnDPDP for MR imaging of the liver: results from the European phase III studies. Acta Radiol 38:631–637
Jones EC, Chezmar JL, Nelson RC, Bernardino ME (1992) The frequency and significance of small hepatic lesions (<15 mm) detected by CT. AJR Am J Roentgenol 158:535–539
Haider MA, Amitai MM, Rappaport DC, O’Malley ME, Hanbidge AE, Redston M, Lockwood GA, Gallinger S (2002) Multi-detector row helical CT in preoperative assessment of small (≤ 1.5 cm) liver metastases: is thinner collimation better? Radiology 225:137–142
Krakora GA, Coakley FV, Williams G, Yeh BM, Breiman RS, Qayyum A (2004) Small hypoattenuating hepatic lesions at contrast-enhanced CT: prognostic importance in patients with breast cancer. Radiology 233:667–673
Tsushima K, Nishie A, Yoshimitsu K, Taketomi A, Honda H (2005) Liver metastasis with apparent intratumoral superparamagnetic iron oxide uptake. Eur Radiol 15:2203–2204
Takahama K, Amano Y, Hayashi H, Ishihara M, Kumazaki T (2003) Detection and characterization of focal liver lesions using superparamagnetic iron oxide-enhanced magnetic resonance imaging: comparison between ferumoxides-enhanced T1-weighted imaging and delayed-phase gadolinium-enhanced T1-weighted imaging. Abdom Imaging 28:525–530
Reimer P, Jahnke N, Fiebich M, Schima W, Deckers F, Marx C, Holzknecht N, Saini S (2000) Hepatic lesion detection and characterization: value of nonenhanced MR imaging, superparamagnetic iron oxide-enhanced MR imaging, and spiral CT-ROC analysis. Radiology 217:152–158
Kim YK, Lee JM, Kim CS, Lee YH (2004) Gadobenate dimeglumine-enhanced liver MR imaging: value of delayed imaging for the characterization and detection of focal liver lesions. Eur Radiol 14:5–13
Kim YK, Lee JM, Kim CS, Chung GH, Kim CY, Kim IH (2005) Detection of liver metastases: gadobenate dimeglumine-enhanced three-dimensional dynamic phases and one-hour delayed phase MR imaging versus superparamagnetic iron oxide-enhanced MR imaging. Eur Radiol 15:220–228
Hu H, He HD, Foley WD, Fox SH (2000) Four multidetector-row helical CT: image quality and volume coverage speed. Radiology 215:55–62
Berland LL, Smith JK (1998) Multidetector-array CT: once again, technology creates new opportunities. Radiology 209:327–329
Murakami T, Kim T, Takamura M, Hori M, Takahashi S, Federle MP, Tsuda K, Osuga K, Nakamura H, Kudo M (2001) Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology 218:763–767
Foley WD, Mallisee TA, Hohenwalter MD, Wilson CR, Quiroz FA, Taylor AJ (2000) Multiphase hepatic CT with a multirow detector CT scanner. AJR Am J Roentgenol 175:679–685
Wong K, Paulson EK, Nelson RC (2001) Breath-hold three-dimensional CT of the liver with multidetector helical CT. Radiology 219:75–79
National Comprehensive Cancer Network. Practice guidelines in oncology—v.1 (2005) Invasive breast cancer/clinical staging. Available at http://www.nccn. org/. Accessed 27 February 2005
Chakraborty DP, Winter LH (1990) Free-response methodology: alternate analysis and a new observer-performance experiment. Radiology 74:873–881
Metz CE (1986) ROC methodology in radiologic imaging. Invest Radiol 21:720–733
Rao JN, Scott AJ (1992) A simple method for the analysis of clustered binary data. Biometrics 48:577–585
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Bipat S, van Leeuwen MS, Comans EFI, Piji ME, Bossuyt PM, Zwinderman AH, Stoker J (2005) Colorectal liver metastases: CT, MR imaging, and PET for diagnosis-meta-analysis. Radiology 237:123–131
Kim MJ, Kim JH, Lim JS, Oh YT, Chung JJ, Choi JS, Lee WJ, Kim KW (2004) Detection and characterization of focal hepatic lesions: mangafodipir vs. superparamagnetic iron oxide-enhanced magnetic resonance imaging. J Magn Reson Imaging 20:612–621
Ward J, Robinson PJ, Guthrie JA, Downing S, Wilson D, Lodge JP, Prasad KR, Toogood GJ, Wyatt JI (2005) Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology 237:170–180
Fretz CJ, Elizondo G, Weissleder R, Hahn PF, Stark DD, Ferrucci JT Jr (1989) Superparamagnetic iron oxide-enhanced MR imaging: pulse sequence optimization for detection of liver cancer. Radiology 172:393–397
Kim SH, Choi D, Lim JH, Lee WJ, Jang HJ, Lim HK, Lee SJ, Cho JM, Kim SK, Kim GC (2002) Optimal pulse sequence for ferumoxides-enhanced MR imaging used in the detection of hepatocellular carcinoma: a comparative study using seven pulse sequences. Korean J Radiol 3:87–97
Abdelmoumene A, Chevallier P, Chalaron M, Schneider F, Verdun FR, Frascarolo P, Meuli R, Schnyder P, Denys A (2005) Detection of liver metastases under 2 cm: comparison of different acquisition protocols in four row multidetector-CT (MDCT). Eur Radiol 15:1881–1887
Furuta A, Ito K, Fujita T, Koike S, Shimizu A, Matsunaga N (2004) Hepatic enhancement in multiphasic contrast-enhanced MDCT: comparison of high- and low-iodine-concentration contrast medium in same patients with chronic liver disease. AJR Am J Roentgenol 183:157–162
Yagyu Y, Awai K, Inoue M, Watai R, Sano T, Hasegawa H, Nishimura Y (2005) MDCT of hypervascular hepatocellular carcinomas: a prospective study using contrast materials with different iodine concentrations. AJR Am J Roentgenol 184:1535–1540
Sica GT, Ji H, Ros PR (2000) CT and MR imaging of hepatic metastases. AJR Am J Roentgenol 174:691–698
Urban BA, Fishman EK, Kuhlman JE, Kawashima A, Hennessey JG, Siegelman SS (1993) Detection of focal hepatic lesions with spiral CT: comparison of 4- and 8-mm interscan spacing. AJR Am J Roentgenol 160:783–785
Obuchowski NA (2003) Receiver operating characteristic curves and their use in radiology. Radiology 229:3–8
Zweig MH, Campbell G (1993) Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39:561–577
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y.K., Ko, S.W., Hwang, S.B. et al. Detection and characterization of liver metastases: 16--slice multidetector computed tomography versus superparamagnetic iron oxide-enhanced magnetic resonance imaging. Eur Radiol 16, 1337–1345 (2006). https://doi.org/10.1007/s00330-005-0140-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-0140-y