Abstract
The purpose was to evaluate a new semi-automated 3D region-growing segmentation algorithm for functional analysis of the left ventricle in multislice CT (MSCT) of the heart. Twenty patients underwent contrast-enhanced MSCT of the heart (collimation 16×0.75 mm; 120 kV; 550 mAseff). Multiphase image reconstructions with 1-mm axial slices and 8-mm short-axis slices were performed. Left ventricular volume measurements (end-diastolic volume, end-systolic volume, ejection fraction and stroke volume) from manually drawn endocardial contours in the short axis slices were compared to semi-automated region-growing segmentation of the left ventricle from the 1-mm axial slices. The post-processing-time for both methods was recorded. Applying the new region-growing algorithm in 13/20 patients (65%), proper segmentation of the left ventricle was feasible. In these patients, the signal-to-noise ratio was higher than in the remaining patients (3.2±1.0 vs. 2.6±0.6). Volume measurements of both segmentation algorithms showed an excellent correlation (all P≤0.0001); the limits of agreement for the ejection fraction were 2.3±8.3 ml. In the patients with proper segmentation the mean post-processing time using the region-growing algorithm was diminished by 44.2%. On the basis of a good contrast-enhanced data set, a left ventricular volume analysis using the new semi-automated region-growing segmentation algorithm is technically feasible, accurate and more time-effective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Different imaging modalities of the heart allow for the evaluation of different cardiac morphological and functional parameters. Besides imaging of the coronary arteries and an anatomical display of the myocardium, the heart valves and the pericardium, evaluation of cardiac function is often crucial to patients suffering from ischemic or other types of cardiomyopathy. The gold standard for the functional evaluation of the left ventricle (LV) is magnetic resonance imaging (MRI) [1, 2]. Contrast-enhanced retrospectively electrocardiogram (ECG)-gated multi-slice spiral computed tomography (MSCT) has been accepted as an efficient non-invasive tool for the detection of coronary artery stenosis, providing good sensitivity and specificity [3–5]. With ECG-gated image acquisition, cardiac MSCT provides images at different phases of the cardiac cycle and therefore allows for the assessment of the LV volume at end diastole and end systole, for example [6]. A high correlation of left and right ventricular end-systolic and end-diastolic volumes, stroke volume and ejection fraction comparing MSCT and MRI has been shown [7–10]. Therefore, every contrast-enhanced ECG-gated cardiac MSCT scan not only provides valuable information concerning coronary artery stenosis and cardiac morphology, but also allows for specific reliable functional analysis of the LV stroke volume and ejection fraction. Intense research and product development have been made to develop (semi-) automated dedicated analysis software for the measurement of LV volumes. Different segmentation algorithms including region-growing and contour-defined segmentation have been introduced, with most of them showing a good correlation of cardiac chamber volumes and ejection fraction in both cardiac MSCT and MRI [9–16]. However, most segmentation algorithms have the drawback of being very time-consuming. Boehm et al. reported an average time for LV ejection fraction analysis of 63±3 min [17]. However, semi-automated 3D multiphase image reconstruction was not available at that time. In a very recent study, Dewey et al. report a post-processing time of 16 min for a semi-automated volumetric analysis software tool [16]. But still “interactive contouring” of endocardial borders in multiple short-axis slices is very time-consuming [17].
The aim of this study was to evaluate a 3D region-growing-based semi-automated segmentation algorithm for the assessment of LV function. The applicability, accuracy and length of time for analysis of the new region-growing-based algorithm were determined and compared to a well-established manual contour-drawing algorithm.
Materials and methods
Image acquisition
Twenty consecutive patients (14 males; mean age: 58.8±13.3 years) with suspected coronary artery disease underwent contrast-enhanced MSCT with retrospective ECG-gating for evaluation of their coronary arteries. The study was approved by the institutional review board, and informed consent was obtained from all the patients. During the scan the ECG signal was digitally recorded; all patients included showed sinus rhythm throughout the scan. Image acquisition was performed in the cranio-caudal direction using a 16-slice CT scanner (SOMATOM Sensation 16; Siemens, Germany) during a single breath-hold of 20.3±2.8 s. The examination protocol included a tube voltage of 120 kV, an effective tube current-time product of 550 mAseff, a collimation of 16×0.75 mm, a table feed of 3.4 mm per rotation and a gantry rotation time of 420 ms. No ECG-pulsing, dose modulation or weight-adapted scan protocols were applied.
Contrast material was administered via the right cubital vein. The scan delay was determined using the bolus tracking technique: when a threshold of 140 HU was reached in the ascending aorta at the level of the origin of the coronary arteries, a delay of 8 s was applied before the scan was initiated. A biphasic contrast injection protocol with the injection of 30 ml of non-ionic contrast material (Ultravist 370, Schering, Berlin, Germany) at a flow rate of 4 ml/s followed by 50 ml at a flow rate of 3 ml/s was used. A saline chaser bolus of 30 ml injected at a flow rate of 3 ml/s was applied immediately after the contrast material injection was finished.
Image reconstruction
From the raw data of each scan, 20 axial image series were reconstructed every 5% (0–95%) of the RR interval with an effective slice thickness of 1 mm and an increment of 0.6 mm. A field of view of 180×180 mm2, a 512×512 matrix and a medium smooth convolution kernel (B30f) were applied. Window settings were individually adapted using the half contour principle described elsewhere [17]. For image analysis with manual contour drawing, double-oblique 8-mm multiplanar reformats (MPR) without an interslice gap were calculated along the left ventricular short axis.
Cardiac volume analysis
Image analysis was performed by a radiologist with 3-year experience in cardiac imaging; a time period of 4 weeks between analyses using the two different algorithms was preserved in order to blind the reader to the results of the first algorithm.
Prior to volume analysis, all 20 axial image series were loaded into the CIRCULATION software implemented on an external Leonardo workstation (Siemens, Forchheim, Germany); a 20-phase cine loop of the left ventricle in the vertical long axis view (two chamber view) was displayed, and in each patient the phase of end systole and end diastole were determined visually.
Short-axis MPRs of the end-systole and end-diastole phases were analyzed using ARGUS (Siemens, Forchheim, Germany) software implemented on the same Leonardo workstation [3 GHz XEON (Intel) processor with 2 GB RAM]. In accordance with other studies, the first slice with a visible left ventricular lumen was defined as the left ventricular apex, while basal slices were carefully reviewed and the left atrium was excluded [19]. Endocardial borders of the left ventricle were traced manually in end systole and end diastole. As the region-growing algorithm based on the CT values of the contrast-enhanced ventricular lumen excludes the soft tissue-dense papillary muscles, papillary muscles in the ARGUS analysis were also manually excluded. The length of time from the beginning of the manual contour drawing to the display of the left ventricular volume results was recorded.
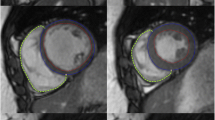
For use of the new region-growing algorithm (CIRCULATION), the 1-mm axial slices of end systole and end diastole were loaded. The only manually added preparation needed before automated left ventricular volume analysis was the setting of a short axis plane at the base of the left ventricle going through the mitral valve and a single mouse click into the lumen of the left ventricle in both end systole and end diastole (Fig. 1). After automated calculation of the LV volumes, the segmentation result was checked visually and the upper segmentation threshold could be adapted individually, depending on the contrast enhancement and the initial segmentation result. The length of time from the setting of the short axis plane at the base of the left ventricle to the display of the left ventricular volumes also was recorded.
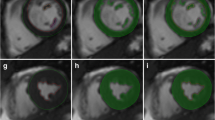
A 54-year-old man with coronary artery disease who was referred for cardiac CT evaluation. Images a–d show the segmentation results of the region-growing algorithm (CIRCULATION) in systole (a, b) and diastole (c, d) in two long-axis cardiac planes each. Images e and f show representative mid-ventricular short axis images of the same patient with manually drawn endocardial contours in systole (e) and diastole (f) as required for the ARGUS evaluation
To assess the overall image quality and the opacification in each patient, the CT density in the septal wall of the heart and the contrast-enhanced left ventricular chamber were measured in three consecutive slices at a mid-ventricular level. Image noise was determined with the standard deviation of the CT value in a 0.30-cm2-large region of interest in the mid-septum or left ventricle lumen. Contrast enhancement in each patient was assessed as the difference of the mean CT values in the left ventricle and the septum. The patient dose was calculated using CT-Expo, version 1.4 [20].
Statistical analysis
Statistical analysis was performed using Med Calc, version 7.4.2.0 (MedCalc Software, Mariakerke, Belgium). Continuous data are expressed as the mean±standard deviation (SD). Agreement for LV function was determined by Pearson’s correlation coefficient and Bland-Altman analysis [21]. Differences in the length of time needed for volume analysis comparing ARGUS and CIRCULATION were compared applying a paired Student’s t-test. Differences in image noise and contrast enhancement were compared applying an unpaired Student’s t-test. The significance level was chosen as 5%.
Results
The mean heart rate during the scan was 68.3±14.3 bpm. With a mean scan range of 127.0±16.7 (125.4±13.5) mm in male (female) patients, the calculated CTDIvol was 42.8 (42.8) mGy; the dose length product was 614 (606) mGy*cm, and the effective radiation dose was calculated as 8.5 (12.4) mSv for male (female) patients.
Applying the new region-growing algorithm in 13/20 patients (65%), proper segmentation of the left ventricle was technically feasible. In the remaining patients, segmentation, regardless of the manual adaptation of the upper segmentation threshold, either did not include the full lumen of the left ventricle or exceeded the left ventricle and included parts of the adjacent right ventricular lumen. Comparing contrast enhancement differences between the left ventricle and the ventricular septum in these 7 patients (the remaining 13 patients), there was a difference of 120.2±25.9 (205.8±72.4) HU. This equals a significant difference of the mean values of 85.6 HU (P=0.0078; unpaired t-test). In the 13 patients with proper segmentation, the signal-to-noise ratio was higher than in the remaining patients (3.2±1.0 vs. 2.6±0.6; P=0.20; unpaired t-test). With image noise levels of 39.8±12.3 (36.3±11.5), no significant difference (P=0.5282; unpaired t-test) comparing image noise between the two groups was found.
In the remaining 13 patients, left ventricular segmentation applying the CIRCULATION algorithm was easily performed and delivered visually reliable segmentation results (for sample images, see Fig. 1a–d). ARGUS evaluation with manually drawn endocardial contours could be completed in all patients (Fig. 1e,f). For further analysis of left ventricular volumes and function, only the results of the 13 patients with proper ARGUS and CIRCULATION evaluation were included into the statistical analysis.
There was an excellent correlation of end-systolic volume, end-diastolic volume, stroke volume and ejection fraction comparing the two segmentation algorithms. Absolute values are given in Table 1. Fig. 2 visualizes the correlation of the left ventricular volumes and functional parameters applying Blant-Altman plots.
The time needed for manual contour drawing was 310.0±52.3 s; the evaluation with the automated region-growing algorithm with setting of the mitral valve axis and the left ventricular volume only took 173.1±38.0 s, including the time for segmentation calculation. Looking at the time reduction in each patient, the use of the region-growing algorithm resulted in a significant reduction of the mean evaluation time of 137.0±57.2 s (−44.2%) compared to the contour-drawing algorithm (P<0.0001, paired t-test).
Discussion
Volume analysis of the left ventricle from contrast-enhanced ECG-gated MSCT scans can be very time-consuming, even with the use of semi-automated segmentation algorithms [17]. The aim of this study was to evaluate the accuracy and time efficiency of a new region-growing-based segmentation algorithm (CIRCULATION).
In accordance with the study by Schlosser et al., the end-systolic volume, end-diastolic volume, stroke volume and ejection fraction derived from automated segmentation algorithms showed a good correlation with data derived from manual contour drawing [11]. In the study by Ehrhard et al., a comparison of the threshold-value-supported 3D volume reconstructions of the left ventricle and manual volume analysis of the left ventricular was not made.
In all patients where the new algorithm was applicable, it led to a reduction of the time needed for the LV evaluation of more than 2 min, preserving an excellent correlation of LV volumes, stroke volume and ejection fraction. Compared to the contour-drawing algorithm, additional potentially time-saving changes are present using the automated region-growing algorithm: (1) automated segmentation is performed on multiphase transaxial image series, which are mandatory for evaluation of the coronary arteries; no separate image reconstruction, saving and loading/importing of individually adapted short axis image series are required. (2) The evaluation time measured in this study also included the time needed for calculation of the LV segmentation, without any reader interaction. Whereas calculation of the Simpson’s method following intensive contour drawing is completed by the computer in less than a second, a rather intensive segmentation calculation is performed using the automated region-growing algorithm; this time requires no reader interaction and can be used differently. (3) It can be expected that less experienced readers profit even more from a tool that requires less reader interaction compared to an experienced reader who was been trained on contour drawing in the past.
Very few other studies comment on the time needed for left ventricular volume analysis. Boehm et al. reported a much higher post-processing time of 63±3 min in a study including 20 patients [17]. However, short axis images with a width of 5 mm were used, leading to an increased number of images requiring contour drawing. Additionally, semi-automated 3D multiphase image reconstruction was not available at that time, a fact that dramatically increased the time consumption for image reconstruction and reformation. Dewey et al. reported a post-processing time of 15.9±2.8 min using improved hardware and software tools and measuring the cardiac chamber volume of multiple cardiac phases [16]. In our study, the time needed for image reconstruction and image upload has not been included into the analysis because (1) it is mandatory for both segmentation algorithms, (2) it can be done semi-automated, as mentioned above, and (3) because it is very much dependent on the hardware performance of the workstation, which potentially varies from site to site.
In 35% of all patients included in the study, the region-growing algorithm did not reveal a proper LV segmentation result: either not the entire LV lumen or parts of the also contrast-enhanced right ventricle were included in the segmentation volume. These patients, compared to the group with proper LV segmentation, showed significantly poorer contrast enhancement of the left ventricle. This proves that visual control of the segmentation result still is mandatory and that an optimized contrast bolus planning, ideally including a saline chaser, is crucial. Especially the use of a saline chaser will lead to reduced contrast enhancement of the right ventricular lumen, which will facilitate automated LV segmentation. From our data, we can recommend to evaluate patients with a difference in CT values of the left ventricular lumen and the myocardial septum of at least 150 HU primarily with the automated 3D region-growing algorithm. With the continuing development of new segmentation algorithms and the combination of region-growing and shape-based algorithms, the performance of auto-segmentation software for LV volume and function will further improve in the future.
LV volume and function parameters in this study were compared applying two different segmentation algorithms on ECG-gated cardiac MSCT scans only; the gold standard for LV volume analysis, cardiac MRI, has not been included. As the automated segmentation algorithm CIRCULATION for LV volume and not the modality MSCT was going to be evaluated, we focused on the comparison of CIRCULATION to a well-established contour-drawing algorithm (ARGUS). A good correlation of LV volume parameters comparing cardiac MRI and CT has already been shown [7, 12]. Moreover, looking at the same MSCT scan for both segmentation algorithms ensures that the heart rate, heart rhythm, breathing or other motion artifacts and other hemodynamically important parameters of the patients are kept constant.
No randomization as to which reconstruction algorithm was applied first and which second has been performed. The cardiac phases of end systole and end diastole were determined visually based on a long-axis cine loop and not based on the left ventricular volume measured in all 20 cardiac phases, an approach that might be more accurate. All patients included showed sinus rhythm throughout the examination. It is to be expected that the region-growing algorithm will be challenged, especially in arrhythmic patients; higher patient numbers including patients with, e.g., premature beats during the scan are required.
The effect of different dose-reduction features on the segmentation algorithm like weight-adapted scan protocols, dose modulation in the z-direction or ECG pulsing has not been evaluated. It is to be expected that weight-adapted scan protocols will lead to more constant image noise levels, facilitating both the manual and automated segmentation of cardiac volumes. ECG pulsing, however, with decreased tube current in systole will hamper image quality in this cardiac phase and may potentially influence segmentation results.
Summarizing the results of this study, we can state that the new semi-automated region-growing algorithm (CIRCULATION) in ECG-gated MSCT of the heart is able to assess LV volume parameters in selected patients and significantly reduces post-processing time compared to an established contour-drawing algorithm (ARGUS). However, optimized contrast bolus planning is crucial in order to facilitate automated segmentation.
References
Alfakih K, Reid S, Jones T, Sivananthan M (2004) Assessment of ventricular function and mass by cardiac magnet resonance imaging. Eur Radiol 14:1813–1822
Rerkpattanapipat O, Mazur W, Link KM, Hundley WG (2003) Assessment of cardiac function with MR imaging. Magn Reson Imaging Clin N Am 11:67–80
Ropers D, Baum U, Pohle K, Anders K, Ulzheimer S, Ohnesorge B, Schlundt C, Bautz W, Daniel WG, Achenbach S (2003) Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation 107:664–666
Nieman K, Cademartiri F, Lemos PA, Raaijmakers R, Pattynama PMT, de Feyter PJ (2002) Reliable noninvasive coronary angiography with fast sub-millimeter multislice spiral computed tomography. Circulation 106:2051–2054
Kuettner A, Beck T, Drosch T, Kettering K, Heuschmid M, Burgstahler C, Claussen CD, Kopp AF, Schroeder S (2005) Diagnostic accuracy of noninvasive coronary imaging using 16-detector slice spiral computed tomography with 188 ms temporal resolution. J Am Coll Cardiol 45:123–127
Juergens KU, Grude M, Fallenberg EM, Opitz C, Wichter T, Heindel W, Fischbach R (2002) Using ECG-gated multidetector CT to evaluate global left ventricular myocardial function in patients with coronary artery disease. Am J Roentgenol 179:1545–1550
Mahnken AH, Koos R, Katoh M, Spuentrup E, Busch P, Wildberger JE, Kühl HP, Günther RW (2005) Sixteen-slice spiral CT versus MR imaging for the assessment of left ventricular function in acute myocardial infarction. Eur Radiol 15:714–720
Mahnken AH, Katoh M, Bruners P, Spuentrup E, Wildberger JE, Gunther RW, Buecker A (2005) Acute myocardial infarction: assessment of left ventricular function with 16-detector row spiral CT versus MR imaging-study in pigs. Radiology 236:112–117
Koch K, Oellig F, Oberholzer K, Bender P, Kunz P, Mildenberger P, Hake U, Kreitner KF, Thelen M (2005) Assessment of right ventricular function by 16-detector-row CT: comparison with magnetic resonance imaging. Eur Radiol 15:312–318
Juergens KU, Fischbach R (2005) Left ventricular function studied with MDCT. Eur Radiol DOI 10.1007/s00330-005-2888-5
Schlosser T, Pagonidis K, Herborn CU, Hunold P, Waltering KU, Lauenstein TC, Barkhausen J (2005) Assessment of left ventricular parameters using 16-MDCT and new software for endocardial and epicardial border delineation. Am J Roentgenol 184:765–773
Lynch M, Ghita O, Whelan PF (2005) Automatic segmentation of the left ventricle cavity and myocardium in MRI data. Comput Biol Med May 27; [Epub ahead of print]
Kaus MR, von Berg J, Weese J, Niessen W, Pekar V (2004) Automated segmentation of the left ventricle in cardiac MRI. Med Image Anal 8:245–254
Ehrhard K, Oberholzer K, Gast K, Mildenberger P, Kreitner KF, Thelen M (2002) Multi-slice CT (MSCT) in cardiac function imaging: threshold-value-supported 3D volume reconstructions to determine the left ventricular ejection fraction in comparison to MRI. Rofo 174:1566–1569
Juergens KU, Grude M, Maintz D, Fallenberg EM, Wichter T, Heindel W, Fischbach R (2004) Multi-detector row CT of left ventricular function with dedicated analysis software versus MR imaging: initial experience. Radiology 230:403–410
Dewey M, Muller M, Teige F, Hamm B (2005) Evaluation of a semiautomatic software tool for left ventricular function analysis with 16-slice computed tomography. Eur Radiol. DOI 10.1007/s00330-005-2817-7
Boehm T, Alkadhi H, Roffi M, Willmann JK, Desbiolles LM, Marincek B, Wildermuth S (2004) Time-effectiveness, observer-dependence, and accuracy of measurements of left ventricular ejection fraction using four-channel MDCT. Rofo 176:529–537
Thompson BH, Stanford W (1994) Evaluation of cardiac function with ultrafast computed tomography. Radiol Clin N Am 32:537–551
Schalla S, Nagel E, Lehmkuhl H, Klein C, Bornstedt, A, Schnackenburg B, Schneider U, Fleck E (2001) Comparison of magnetic resonance real-time imaging of left ventricular function with conventional magnetic resonance imaging and echocardiography. Am J Cardiol 87:95–99
Stamm G, Nagel HD (2002) CT-expo-a novel program for dose evaluation in CT. Rofo 174:1570–1576
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mühlenbruch, G., Das, M., Hohl, C. et al. Global left ventricular function in cardiac CT. Evaluation of an automated 3D region-growing segmentation algorithm. Eur Radiol 16, 1117–1123 (2006). https://doi.org/10.1007/s00330-005-0079-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-0079-z