Abstract
In MRI applications where short acquisition time is necessary, the increase of acquisition speed is often at the expense of image resolution and SNR. In such cases, the newly developed parallel acquisition techniques could provide images without mentioned limitations and in reasonably shortened measurement time. A newly designed eight-channel head coil array (i-PAT coil) allowing for parallel acquisition of independently reconstructed images (GRAPPA mode) has been tested for its applicability in neuroradiology. Image homogeneity was tested in standard phantom and healthy volunteers. BOLD signal changes were studied in a group of six volunteers using finger tapping stimulation. Phantom studies revealed an important drop of signal even after the use of a normalization filter in the center of the image and an important increase of artifact power with reduction of measurement time strongly depending on the combination of acceleration parameters. The additional application of a parallel acquisition technique such as GRAPPA decreases measurement time in the range of about 30%, but further reduction is often possible only at the expense of SNR. This technique performs best in conditions in which imaging speed is important, such as CE MRA, but time resolution still does not allow the acquisition of angiograms separating the arterial and venous phase. Significantly larger areas of BOLD activation were found using the i-PAT coil compared to the standard head coil. Being an eight-channel surface coil array, peripheral cortical structures profit from high SNR as high-resolution imaging of small cortical dysplasias and functional activation of cortical areas imaged by BOLD contrast. In BOLD contrast imaging, susceptibility artifacts are reduced, but only if an appropriate combination of acceleration parameters is used.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In MRI applications where short acquisition time is necessary, the increase of acquisition speed is often at the expense of image resolution and SNR. Typically, the acquisition speed is highly critical in cardiac MRI or contrast-enhanced MRA (CE MRA). In such cases, the newly developed parallel acquisition techniques could provide images without mentioned limitations and in a reasonably shortened measurement time. After successful application had been carried out in cardiac and pulmonary vascular imaging [1], we used parallel imaging in an eight-channel coil array for various types of cerebral imaging. This approach can potentially shorten measurement time without the loss of spatial resolution in time-of-flight (TOF) MRA, reduce susceptibility artifacts of measurement with EPI technique (BOLD fMRI, diffusion imaging) and perhaps introduce time-resolved CE MRA of intracranial vessels. On the other hand, some specific artifacts and SNR reduction can be expected, especially in case of overestimated demands for acquisition speed in particular applications.
The Partially Parallel Acquisition (PPA) technique uses spatial information from an array of simultaneously working coils to facilitate reconstructions either in k or image space [2–4]. In generalized autocalibrating partially parallel acquisitions (GRAPPA, [5]), measurement of a certain number of k-space lines can be omitted, and the missing lines are reconstructed from the a priori spatial information. The results of the technical evaluation of the coil and of first clinical examinations are reported.
Material and methods
i-PAT head coil and scanner
The new head coil array consists of eight surface coils and pre-amplifier elements (i-PAT head coil). This was applied in a 1.5-T Sonata scanner (Siemens, Erlangen). Both standard and GRAPPA modes were used to test image quality. In most applications (high resolution 3D imaging, TOF MRA, BOLD fMRI), the results were compared to those from a standard bird-cage head coil.
Phantom studies
These studies were carried out in a plastic bottle (sphere of 17 cm in diameter) containing a 0.9% saline solution.
-
(1)
Image homogeneity was tested using a 3D FLASH sequence with the following parameters: 3D-FLASH, TR=15 ms, TE=5 ms, flip angle=15°, matrix=256×256, FOV=256 mm, slice thickness=1 mm and 176 slices.
Three measurements were compared: (a) using the standard head coil and using the i-PAT coil, (b) without and (c) with a normalization filter.
-
(2)
Image quality expressed over artifact power was measured using a 3D FLASH sequence and an EPI-based GE sequence with the following parameters: 3D-FLASH: TR=15 ms, TE=3 ms, flip angle=15°, bandwidth=260 Hz/pixel, matrix=256×256, FOV=230 mm, slice thickness=5 mm and 32 slices;
EPI: TR=6 s, TE=59 ms, flip angle=90°, bandwidth=1,300 Hz/pixel, matrix=128×128, FOV=230 mm and slice thickness=4 mm.
Eleven measurements with various acceleration factors (AF=2, 3, 4) and numbers of reference lines (RL=8 or 12, 16, 32, 64) were performed with FLASH 3D and three with EPI sequence [(AF=2, RL=24), (AF=2, RL=64), (AF=3, RL=64)], and comparison with images measured using the same sequence parameters but without parallel technique were done.
Volunteer and patient studies
After elaboration of the most effective GRAPPA parameters, mainly the acceleration factor, the following imaging techniques were tested in volunteers recruited among the authors:
-
(1)
Intracranial TOF angiography (MRA): 3D-FISP: TR=40 ms, TE=7.15 ms, flip angle=25°, bandwidth=65 Hz/pixel, matrix=512×256, FOV=210 mm, slice thickness=1 mm, 80 slices and voxel size 0.8×0.4×1.0 mm. Three volunteers were examined.
-
(2)
3D high resolution imaging: MP-RAGE: TR=1,900 ms, TI=1,100 ms, TE=3.93 ms, flip angle=15°, bandwidth 130 Hz/pixel, FOV=250 mm, slice thickness=1.25 mm and 72 slices. Two volunteers were examined.
-
(3)
Cerebral activation with motor stimulation using finger tapping with BOLD contrast: EPI sequence: TR=3 s, TE=60 ms, flip angle=90°, bandwidth=1,300 Hz/pixel, matrix=64×64, FOV=192 mm, slice thickness=5 mm and 23 slices. Each volume of slices was acquired 160 times with an alternation of periods of rest (ten volumes) and stimulation (ten volumes). Standard deviation maps of the signal change in time were calculated to compare the effective SNR and CNR of measurements performed in the head coil, i-PAT coil and also measured with the GRAPPA technique. Usual fMRI evaluation was done using SPM99 (realignment, smoothing with FWHM=8 mm, normalization and f-test statistic with corrected P=0.001). Six volunteers were examined.
-
(4)
In patients with suspected sinus thromboses and uncertain MR angiographic results in standard phase contrast angiography, 3D contrast-enhanced (CE) MRA with high temporal resolution was performed using the i-PAT coil: FLASH 3D: TR=3.14 ms, TE=1.35 ms, flip angle=20°, bandwidth 690 Hz/pixel, FOV=230 mm, slice thickness=2 mm, 64–80 slices, voxel size 1.2×0.6×2.0 mm, AF=3, RL=48, eight consecutive repetitive measurements, time/measurement 6.6–8.3 s, total time about 1 min. Measurement was started together with intravenous 4 ml/s bolus injection of 20 ml contrast agent (Magnevist). Five patients were examined.
Results
Phantom studies
RF homogeneity
Maps of relative signal homogeneity were created from 3D FLASH sequence data sets acquired by the standard head coil and the i-PAT coil with and without a normalization filter by calculation of the intensity difference of each voxel against a reference value from a small region of interest (ROI) from the center of the phantom. As can be seen from Fig. 1, homogeneity of the signal was best preserved in the use of the standard head coil (a) as compared to the i-PAT coil (b), but improved by the application of the normalization filter (c).
Power of artifacts
To compare the influence of the AF and the number of RLs on 3D FLASH images, these two parameters were varied and artifact power was calculated as the sum of the absolute difference of all GRAPPA images through the total volume to the reference measurements with the i-PAT coil without GRAPPA mode over the entire volume. AFs of 2, 3, 4 and the number of RLs of 8, 16, 32, 64 were used and resulted in an eventual 70% shortening of the acquisition time, but an increase of artifact power and image degradation at the same time (Fig. 2). As can be seen from the plot in Fig. 3, there is no linear relation between these parameters, but some combinations (higher AC and lower number of RLs) are more effective than others in preserving image quality within a reduced repetition time.
The image quality expressed over the artifact power against the relative shortening of the acquisition time. All points plotted with an asterisk were measured with AF=2, square points with AF=3 and triangle points with AF=4. The number of RL is decreased sequentially from left to right, which leads to shortened acquisition time, but also reduced image quality. The plot also shows that for example the combination of AF=3 and RL=64 is more effective than AF=2 and RL=8 because in almost the same measurement time we got lower artifact power. (Points with the same AF are being placed to the right with decreasing RL in the graph because the lower RL leads to increased shortening of measurement time)
Susceptibility artifacts
These artifacts were measured in T2*-weighted EPI-based GE sequences as they are used for perfusion or BOLD contrast measurements. Again, only i-PAT coil measurements were performed with and without application of the GRAPPA mode and variations of the AFs and number of RLs from (1) AF=2, RL=64 to (2) AF=2, RL=24 and (3) AF=3, RL=64. Susceptibility artifacts were reduced only by the combination of AF=2 and RL=64 as in condition (1), but increased with a further reduction of RLs or increase of AF (Figs. 4 and 5). Artificial signal variations calculated as artifact power grew from 2.27 (1) to 2.66 (2) and 4.48 (3). This means that the last combination of parameters leads to an almost two times higher standard deviation of signal intensity as compared to the reference image.
Volunteer and patient studies
TOF MRA
According to the results of our phantom studies, we chose the most effective set of GRAPPA parameters with (1) AF=2, RL=40 and (2) AF=3, RL=64 for intracranial TOF MRA and compared them to a standard i-PAT coil 3D FISP sequence. The measurement time was reduced from 9 min 48 s (standard sequence) to 70% (6 min 51 s) in (1) and to 50% (4 min 53 s) in (2). As can be seen from Fig. 6, there is no visible loss of quality, mainly of peripheral artery “enhancement” in condition (1) in spite of an important time advantage, whereas with further reduction of measurement time as in condition (2), image quality was severely impaired.
Axial MIP from TOF MRA of the same volunteer with i-PAT coil and a standard measurement, b GRAPPA with AF=2, RL=40 (70% of standard acquisition time) and c GRAPPA with AF=3, RL=64 (50% of standard acquisition time). This comparison shows that whereas the difference between a and b is almost invisible, the measurement with higher AF as in c is diagnostically inferior
3D high resolution imaging
To evaluate the i-PAT coil performance in high-resolution imaging, a 3D MP RAGE data set was acquired (176 sagittal slices, no GRAPPA mode), and the images were compared to those of a standard head coil. This evaluation was done by visual inspection of main parts of the sagital image separately (middle part, brain convexity). Although some cortical areas clearly showed better differentiation of the cortex from the subcortical white matter using the i-PAT coil (Fig. 7), this improvement of image quality was confined to the brain surface and the enlargement of grey-white matter contrast.
BOLD imaging
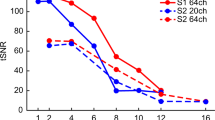
To compare the influence of the i-PAT coil and GRAPPA technique in fMRI studies, we performed six measurements with healthy volunteers using simple motor stimulation (finger tapping). General noise levels were expressed as the standard deviation of images acquired with the standard head coil and the i-PAT coil during rest and stimulation for each voxel. We found that an average level of the standard deviation (stdv) in the i-PAT coil images is 0.39 of that measured with the standard head coil (Fig. 8).
Standard deviation maps (two selected slices) from images acquired with head coil (left) and i-PAT coil (right) showing lower noise level in i-PAT coil. Standard deviation averaged over all pixels containing brain tissue in images acquired with i-PAT coil was only 38% of the value measured in identical ROI in images from standard head coil. All maps are displayed with the same window
Two identical fMRI experiments with simple right hand finger tapping using either the standard head coil or i-PAT coil were evaluated with SPM (realignment, smoothing with FWHM=8 mm, f-test, corrected P=0.001). Also, correlation maps were calculated on realigned, smoothed data (uncorrected P=0.00001) and used to compare both coils. Comparison of the signal amplitude from the same ROI in the primary motor cortex shows a clear increase of signal change in cases of i-PAT coil. Results are shown in Fig. 9. A clearly larger extent of activation expressed over the higher number of active voxels can be seen on fMRI performed in the i-PAT coil (Fig. 11).
a Comparison of BOLD activation map (group statistic over all volunteers) by right hand finger tapping scanned with head coil (left) and i-PAT coil (right). Larger extent of activation is demonstrated with i-PAT coil; a clear improvement is seen mainly in primary sensory-motor cortex and basal ganglia. b Signal course in the basal ganglia measured in head coil (left) and i-PAT coil is compared, and the lower signal fluctuation level makes the activation in this region much easier to detect
Similar comparison for i-PAT coil with and without GRAPPA technique was done. The reduction of the number of acquired k-space lines using GRAPPA leads to slight improvement of image distortion. This is particularly true at the brain edges. On the contrary, a higher number of artifacts decreased SNR in the image center (Fig. 10). The absolute difference of the signal intensity during the time course from the same ROI in the primary motor cortex is almost of the same level. The graph in Fig. 11 shows a quantitative comparison of the primary motor cortex activation. Each of three columns represents the number of statistically significant voxels (correlation map threshold of P=0.00001) found in the head coil, i-PAT coil and a combination of the i-PAT with GRAPPA; results are shown for all six volunteers.
Comparison of BOLD activation by right hand finger tapping for all our volunteers is shown. In all cases, a clear increase of the number of statistically significant voxels is demonstrated if the i-PAT coil is used (middle column). However, in four cases a reduction of the number of active voxels was observed using i-PAT and GRAPPA combination (right column)
3D CE MRA
Using the i-PAT coil and GRAPPA mode, CE MRA allows the depiction of cerebral sinuses and venous vessels with good quality within a few seconds. We used this method in patients with suspected sinus thrombosis, in whom our standard phase contrast MRA showed a severe asymmetry of the transverse sinus and occlusion could not be excluded. The repetitive application of a 3D sequence with GRAPPA parameters AF=3 and RL=48 shows the cerebral flow through of contrast blood in different phases. Whereas imaging of venous vessels is excellent (Fig. 12), time resolution is not high enough for a systematic demonstration of a pure arterial phase.
Discussion
Although the robustness of reconstruction has been pointed out recently in parallel imaging techniques such as SMASH and SENSE to accelerate the acquisition of high-quality images [9, 10], our testing of the Siemens i-PAT coil in combination with the GRAPPA mode revealed some advantages, but also some limitations for its routine application in neuroradiology: Due to the array concept of the i-PAT head coil, a considerable image inhomogeneity with a relative signal drop in the central caudal part of the coil is typically observed. High-resolution imaging therefore appeared promising only if it was confined to superficial, cortical structures showing a better S/N and so better differentiation of gray and white matter. This is well known also from other reports about surface coil imaging in patients with extratemporal epilepsy [11]. On the other hand, such signal variation is used in the process of image reconstruction of any PPA technique. Therefore, one of the main advantages of the array concept should be in the use of PPA.
However, despite the “autocalibrating” nature of the GRAPPA mode [5], our phantom measurements using a 3D FLASH sequence showed an important artifact growth towards the center of the image. Since the amount of these artifacts depends on the successful estimation of the coil sensitivity factors and complex weights, the S/N of acquired reference lines can probably have a strong influence on the final image quality (artifact power) and control not only the overall image SNR. Therefore, the artifact power can depend on many usual sequence parameters determining SNR, but also on image orientation in the case of PPA reconstruction. It is even difficult to accept any general conclusion about the dependence of the image quality on the main GRAPPA parameters such as AF and RL; we used one sequence with relevant clinical parameters to test the influence of both parameters. This function helped us to choose GRAPPA parameters for typical clinical examination with the realistic chance of acceptable image quality. Of course, it does not necessarily mean that exactly the same function of the artifact power would be observed with other sequence parameters. Generally, sequence parameters including slice orientation influence the SNR of reference lines and together with the used reconstruction algorithm (GRAPPA in our case) they set the final amount of artifacts in the image. Since dramatic progress has been seen in the field of reconstruction algorithms for parallel imaging during the last years, we can expect a certain dependence just on the program used (even the software version) and the manufacturer. Our results of the phantom study should mainly emphasize the nonlinear and more complex behavior of the artifact power function with the choice of PPA parameters.
Confirming previous examinations, we also found a decrease in SNR and an increase in artifact power with enlargement of the AF. As the artifact power also depends on the number of RLs, a combination of a higher AF with a higher number of RL measurements seems to be more effective in artifact reduction than a lower AF with a lower number of RLs in the case of a similar reduction of measurement time.
The loss of image quality can be critical, especially in clinical applications dealing with fine structures such as MRA, and therefore we chose this method to find a realistic set of PPA parameters to shorten the measurement time while still keeping an acceptable diagnostic quality. Using the GRAPPA mode and a combination of AF=2 and RL=40, we were able to achieve high-resolution TOF MRAs of a similar quality as in conventional imaging, but with a 30% reduction of measurement time. A similar result was reported using a SENSE 3D TOF technique [12]. As in the phantom studies, a further time reduction severely increased the artifact power, and diagnostic intracranial TOF MRAs with a 50% time reduction could not be achieved.
Because of the optimistic reports concerning CE MRA quality in cardiac and peripheral artery imaging [1, 13], we examined four patients with suspected sinus thrombosis with a 3D FLASH sequence, i-PAT coil and GRAPPA mode, but a rather low time resolution of 6–9 s per data set. The resulting venograms were superior in contrast and space resolution to our usual phase contrast angiograms and allowed the diagnosis of a hypoplasia versus occlusion of a transverse sinus in one case. This application may turn out to be a further true i-PAT coil/GRAPPA advantage.
Scan time requirements to visualize cerebral artery dynamics similar to DSA still remain a great challenge, and we were not able to achieve a clinically useful compromise between time and spatial resolution. An exception may be the imaging of slow flow, e.g., within (partially coiled) aneurysmal sacs [14] or the demonstration of high-grade stenoses with turbulent flow as in carotid bifurcation imaging using SENSE [15] in the extra-cranial region.
Acquisition speed is also very important in such applications as fMRI, diffusion or perfusion imaging. Since these methods use mostly EPI sequences today, there is an intrinsic limitation in spatial resolution, and also images are strongly influenced by the susceptibility effect. This is unfortunately in strong contradiction to clinical demands for diffusion tensor imaging and MR tractography where high spatial resolution and no image distortions are needed. One approach could be to use the HASTE sequence instead of EPI [16]. However, parallel imaging can overcome these limitations or at least improve the situation, because shorter echo-train length (ETL) of EPI sequence leads to susceptibility artifact reduction. But the reduction of the susceptibility sensitivity because of shorter ETL can modify the BOLD sensitivity in fMRI studies and also the reduction of SNR, and an increase of the amount of artifacts proper to PPA can eventually diminish other advantages of parallel imaging in combination with fMRI.
As predicted by the authors in [5], susceptibility artifacts were decreased in EPI-based GE sequences as they are used for perfusion and BOLD imaging. However, we could only confirm this expectation if the combination of AF=2, RL=64 was used, but higher AF paradoxically did not have such a positive influence. In our “in vivo” studies, we could observe a clear reduction of susceptibility artifacts and image distortions when a SE EPI sequence with GRAPPA was used for diffusion tensor imaging. Obviously, BOLD imaging should also profit from this improvement, but we can report a strong increase of activity detection mainly because of the surface coil property of the i-PAT array. On the contrary, the use of the GRAPPA technique also led to a slight decrease of the activation. We can speculate that there could be perhaps two reasons explaining our results: (1) a decreased SNR and maybe also (2) the fact that all reference lines are acquired before fMRI measurements (during rest condition). Therefore, a major field of future application might be imaging of cerebral activation in an event-related design where high temporal resolution is needed. A similar successful application of the SENSE variant (PRESTO-SENSE) of the parallel acquisition technique with reduction of measurement time has been reported by Golay et al. [17] and de Zwart et al. [18].
Conclusion
Apart from cardiac and peripheral vascular imaging, the newly designed i-PAT coil offers some advantages also in neuroradiological applications. Being an eight-channel surface coil array, peripheral cortical structures profit from high SNR as high-resolution imaging of small cortical dysplasias and functional activation of cortical areas imaged by BOLD contrast. The additional application of a parallel acquisition technique such as GRAPPA can decrease measurement time in the range of about 30% without the clinically unacceptable expense of SNR. This technique performs best in conditions in which imaging speed is important, such as CE MRA, and also in BOLD contrast imaging in which susceptibility artifacts are reduced, but only if an appropriate combination of acceleration parameters is used.
References
Dietrich O, Nikolaou K, Wintersperger BJ, Flatz W, Nittka M, Petsch R, Kiefer B, Schoenberg SO (2002) i-PAT: Applikationen für schnelle kardiovaskuläre MRT. Electro Med (Siemens/Erlangen) 70:149–162
Carlson JW, Minemura T (1993) Imaging time reduction through multiple receiver data acquisition and image reconstruction. Magn Reson Med 29:681–688
Sodickson DK, Manning WJ (1997) Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med 38:591–603
Pruessmann KP, Weiger M, Scheidegger MB, Boesinger P (1999) SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42:952–962
Griswold MA, Jakob PM, Heidemann RM, Nitta M, Jellus V, Wang J, Kiefer B, Haase A (2002) Generalized autocalibrating partially acquisitions (GRAPPA). Magn Reson Med 47:1202–1210
Sodickson DK, Griswold MA, Jakob PM, Edelman RR, Manning WJ (1999) Signal-to-noise ratio and signal-to-noise efficiency in SMASH imaging. Magn Reson Med 41:1009–1022
Griswold MA, Jakob PM, Heidemann PM, Haase A (2000) Parallel imaging with localized sensitivities (PILS). Magn Reson Med 44:602–609
Heidemann RM, Griswold MA, Haase A, Jakob PM (2001) VD-AUTO-SMASH imaging. Magn Reson Med 45:1066–1074
Kyriakos WE, Panych LP, Kacher DF, Westin CF, Bao SM, Mulkern RV, Jolesz FA (2000) Sensitivity profiles from an array of coils for encoding and reconstruction in parallel (SPACE RIB). Mag Reson Med 44:301–308
Sodickson DK, McKenzie CA (2001) A generalized approach to parallel magnetic resonance imaging. Med Phys 28:1629–1643
Grant PE, Barkovich AJ, Wald LL, Dillon WP, Laxer KD, Vignero DB (1997) High-resolution surface-coil MR of cortical lesions in medically refractory epilepsy: a prospective study. Am J Neuroradiol 18:302–306
King KF, Reynolds HG, Bernstein MA, Estkowski L, Angelos L, Feo DC, Monski W, Wagner M (2001) SENSE 3D time of flight angiography using a 4-channel head coil. Proc Int Soc Magn Res 9:1392
Wintersperger BJ, Nikolaou K, Dietrich O, Rieber J, Nittka M, Reiser MF, Schoenberg SO (2003) Single breath-hold real-time cine MR imaging: improved temporal resolution using generalized autocalibrating partially parallel acqusition (GRAPPA) algorithm. Eur Radiol 13:1931–1936
Gottschalk S, Gaebel C, Haendler C, Gellissen J, Missler U, Seidel G, Nowak G, Petersen D (2002) Contrast-enhanced intracranial 3D MR angiography (CE-MRA) in assessing arterial stenoses and aneurysms. Fortschr Röntgenstr (RöFo) 174:704–713
Golay X, Brown SJ, Itoh R, Melhem ER (2001) Time-resolved contrast-enhanced carotid MR angiography using sensitivity encoding (SENSE). Am J Neuroradiol 22:1615–1619
Lovblad KO, Jakob PM, Baird AE, Chen Q, Laubach HJ, Schlaug G, Warach S, Edelman RR (1998) Turbo-spin echo diffusion-weighted MR of human stroke. Am J Neuroradiol 19:201–208
Golay X, Pruessmann KP, Weiger M, Crelier GR, Folkers PJ, Kollias SS, Boesiger P (2000) PRESTO-SENSE: an ultrafast whole-brain fMRI technique. Magn Res Med 43:779–786
De Zwart JA, van Gelderen P, Kellman P, Duyn JH (2002) Application of sensitivity-encoded echo-planar imaging for blood oxygen level-dependent functional brain imaging. Magn Res Med 48:1011–1020
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tintera, J., Gawehn, J., Bauermann, T. et al. New partially parallel acquisition technique in cerebral imaging: preliminary findings. Eur Radiol 14, 2273–2281 (2004). https://doi.org/10.1007/s00330-004-2427-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2427-9