Abstract
MRI of the small bowel is a new method for the assessment of inflammatory bowel diseases. However, inflammatory bowel disease can affect both the small and large bowel. Therefore, our goal was to assess the feasibility of displaying the small bowel and colon simultaneously by MR imaging. Eighteen patients with inflammatory bowel disease were studied. For small bowel distension, patients ingested a solution containing mannitol and locust bean gum. Furthermore, the colon was rectally filled with water. MR examinations were performed on a 1.5-T system. Before and after intravenous gadolinium administration, a T1w data set was collected. All patients underwent conventional colonoscopy as a standard of reference. The oral ingestion and the rectal application of water allowed an assessment of the small bowel and colon in all patients. By means of MRI (endoscopy), 19 (13) inflamed bowel segments in the colon and terminal ileum were detected. Furthermore, eight additional inflammatory lesions in the jejunum and proximal ileum that had not been endoscopically accessible were found by MRI. The simultaneous display of the small and large bowel by MRI is feasible. Major advantages of the proposed MR concept are related to its non-invasive character as well as to the potential to visualize parts of the small bowel that cannot be reached by endoscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease is a chronic inflammatory pathology potentially affecting the entire GI tract. The terminal ileum and proximal colon are the parts of the intestine that are mostly affected [1]. Different procedures, including ileocolonoscopy, ultrasound, CT imaging, conventional enteroclysis and/or surgical interventions in conjunction with biopsies are applied for the diagnosis of Crohn’s disease [2–4]. However, all these techniques are associated with certain drawbacks. While the diagnostic accuracy of ultrasonography is strongly operator-dependent, conventional enteroclysis and computed tomography expose patients to ionizing radiation [5]. Since most of the patients in question are young and the examinations often need to be repeated several times for therapeutic monitoring purposes, the radiation issue is of even higher concern. Therefore, ileocolonoscopy is mainly used to confirm the diagnosis of Crohn’s disease, but the poor patient acceptance because of the invasive character of this procedure, the potential risk of bowel perforation and the inability to reach the ileocecal valve in a high percentage of cases cast a shadow over the usefulness of endoscopy.
Advances in MRI, including the implementation of high gradient scanners and the availability of several contrast agents, have led to an increasing use of MRI for the evaluation of abdominal diseases. Thus, MRI for both the small and large bowel has become an established method for the assessment of inflammatory bowel diseases or the detection of colorectal masses [6]. A good distension of the intestine is crucial for bowel imaging. Regarding the colon, it can be easily accomplished by the administration of a rectal enema consisting of liquid or gasiform contrast agents [7]. For the visualization of the small bowel, however, this issue is much more complex. By applying contrast agents via a nasoduodenal tube, a sufficient bowel distension is achievable [8–10], but this procedure is often perceived as traumatizing by the patients due to its invasive character. The mere oral administration of water without intubation is non-invasive and well accepted. Unfortunately, water is rapidly reabsorbed in the small bowel, which diminishes the degree of bowel distension.
Certain additives can inhibit water resorption [11]. Recently, a solution containing mannitol and locust bean gum has been introduced as an oral contrast agent for small bowel MRI [12]. The oral application of this solution leads to a strong bowel distension without the need of duodenal intubation, which was verified in a volunteer trial. The aim of the present study was twofold: to evaluate the practicability of the mentioned oral contrast agent for MR imaging in patients with Crohn’s disease and to assess the feasibility of displaying the small bowel and colon simultaneously.
Materials and methods
Subjects
Twenty patients (13 men and seven women; mean age 43.5 years, age range 21–78 years) with known or suspected Crohn’s disease were enrolled in this study. Crohn’s disease was histologically proven in 18 patients. Four of these patients had undergone a resection of the terminal ileum and ascending colon. The two remaining patients presented symptoms of lower abdominal pain, diarrhea and leucocytosis. Informed consent was obtained prior to each examination. None of the patients showed contraindications to MR imaging, such as the presence of metallic implants.
Preparation and MR imaging
At first a standard preparation for bowel purgation was performed the evening before the MR examination. Patients ingested 3,500–4,000 ml of Golytely (Braintree Laboratories, Braintree, Mass.). All MR examinations were done the next morning. To provide sufficient small bowel distension, patients ingested 1,500 ml of the contrast solution containing 2.5% of mannitol (Merck, Darmstadt, Germany) and 0.2% of locust bean gum (LBG) (Roeper, Hamburg) over 45 min at a steady, evenly distributed rate. To that end, portions of 150 ml were given each 4–5 min. To enhance gastric emptying, 50 mg of erythromycin (Abbott Pharmaceutics Wiesbaden) was administered intravenously after the first 150 ml of the oral contrast compound.
MR examinations were performed on a 1.5-T MR scanner (Magnetom Sonata, Siemens Medical Systems, Erlangen, Germany). For signal reception, a combination of two flex surface coils were employed, which were wrapped around the patient in the prone position. These two coils were used in conjunction with the built-in spine array coil to permit coverage of the entire abdomen. A spasmolytic agent (40 mg of scopolamine, Buscopan, Boehringer Ingelheim, Germany) was intravenously administered in order to reduce artifacts due to bowel motion. Following the placement of a rectal tube (E-Z-Em, Westbury, N.Y.), an enema consisting of 1,000 ml of warm tap water was administered. MR imaging was based on the acquisition of a 3D T1w gradient-echo sequence in the coronal plane under breath-hold conditions. The following scan parameters were used: TR/TE =3.08/1.13 ms, flip angle =12°, slice thickness =1.4 mm, matrix size =336×512. The acquisition time amounted to 20 s. In a first step, a “pre-contrast” data set was collected. Subsequently, paramagnetic contrast was injected intravenously (gadobenate dimeglumine, Gd-BOPTA, Multihance, Bracco, Italy) at a dosage of 0.2 mmol/kg body weight and a flow rate of 3.0 ml/s. Data acquisition was repeated with identical imaging parameters after a contrast delay of 75 s. Eventually, the rectal enema bag was placed on the floor in order to facilitate emptying of the colon.
Image interpretation and data analysis
All MR images were transferred to a post-processing workstation (Virtuoso, Siemens Medical Systems, Erlangen), which permitted coordinated multiplanar reconstructions of the coronal source data. Evaluations were made in a consensus mode by two radiologists who were blinded to any clinical patient information. MR data were classified regarding the following issues:
-
(1)
Increased bowel wall thickening
-
(2)
Narrowing of the bowel lumen
-
(3)
Increased contrast enhancement of the bowel wall following i.v. gadolinium
The first two parameters were determined by measuring the thickening of bowel wall and the bowel diameter, respectively, in non-affected parts of the intestinal tract as well as in the suspected lesions. The latter aspect was assessed by mean signal-to-noise-ratio (SNR) measurements performed in the normal ileum wall as well as in all thickened bowel wall segments. To that end, ellipsoid regions of interest (diameter of 2 mm) were placed in the according bowel wall and signal intensity (SI) values were determined. Image noise (standard deviation of signal intensities) was measured in a field of view outside the abdomen. SNR was calculated in the usual manner dividing SNR by image noise. This procedure was performed both for the pre-contrast as well as for the post-contrast data. As a second parameter, a so-called contrast-enhancing ratio (CER) for normal and thickened bowel wall segments was determined:
Both post-contrast SNR values and CER values of the normal and the thickened bowel wall were compared by applying a paired Student’s t-test. P-values < 0.05 were considered to prove statistical significance.
Besides, extra-intestinal findings were documented, such as inflammation-related pathologies (enlarged mesenteric lymph nodes, abscesses or fistulae) and non-inflammation-related lesions in parenchymal abdominal organs.
Conventional colonoscopy
As a standard of reference, all patients underwent conventional colonoscopy within 3–5 days after MRI, which was performed using conventional equipment (model CFQ 140, Olympus). The attending gastroenterologist was unaware of the MR findings. Sedatives (Dormicum, Roche, Germany) or analgesics (Dolantin, Hoechst, Germany) were optionally administered. Suspicious inflammatory lesions were recorded and biopsied. All biopsy materials were analyzed by histopathology.
Results
In 2 out of 20 patients, a complete colonoscopy was not possible due to an elongated sigmoid colon in 1 patient and a stenosis close to the left colonic flexure in another patient. Since a sufficient standard of reference was lacking, these two patients were excluded from the study analysis. Hence, the data of 18 patients were included. Two of the 18 patients were not able to ingest the entire 1,500 ml of the oral contrast agent, which led only to moderate small bowel distension. Slight respiratory motion artifacts were present in two different patients. However, the jejunum and ileum were visualized in all 18 examinations, and a simultaneous assessment of the small and large bowel was possible.
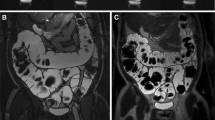
By means of MRI, 27 segments of bowel wall inflammation were diagnosed. All 18 patients at least presented one segment that was affected. Eight of these segments were located in the colon (Fig. 1), 11 in the terminal ileum (Fig. 2) and another 8 in the upper ileum or jejunum (Fig. 3). However, only 13 of these 27 segments of inflammation were detected by endoscopy and subsequent biopsy (3 in the colon, 10 in the terminal ileum and none in the upper small bowel). All segments rated by MRI to be inflammatorily changed showed a higher SNR value compared to the non-affected ileum (P<0.0001). The CER values also proved a statistically significant difference between normal and affected bowel segments (P<0.001). Table 1 provides an overview of all SNR and CER values, while Table 2 displays the bowel wall thickness and the luminal diameter of the according bowel segments.
The presence of an extra-mural abscesses was seen in three patients and inter-intestinal fistulae were detected in another three patients (Fig. 4). Furthermore, a conglomerate tumor formation (Fig. 5) was found within an affected segment of the terminal ileum by means of MRI, and enlarged mesenteric lymph nodes were displayed in five patients (Fig. 6). None of these extra-intestinal findings had been detected or even suspected by endoscopy. As for other parenchymal organs, minor finding such as hepatic or renal cysts, a hepatic hemangioma and cholecystolitiasis could be diagnosed by MR imaging in seven patients.
Discussion
MR imaging in conjunction with the oral application of a solution containing locust bean gum and mannitol is feasible. The additional rectal administration of water allows the visualization of both the small and large bowel at the same time. Not only inflammatory lesions in the colon and terminal ileum could be detected, but also affected bowel segments in the upper small bowel, which cannot be reached by endoscopic procedures. Hence, MRI is a promising technique for the diagnosis and follow-up of patients with inflammatory bowel diseases.
Up to now, most MR imaging techniques for the assessment of Crohn’s disease have focused on the display of small bowel loops. In a trial by Umschaden et al. [9], 30 patients with symptoms of inflammatory bowel disease were examined. After the completion of a conventional enteroclysis examination, which served as the standard of reference, patients were transferred to the MR unit. A methylcellulose solution was administered via a duodenal tube providing a good distension of the small bowel loops. The MR examinations proved a strong correlation with the results of conventional enteroclysis, thereby even permitting the detection of additional bowel lesions in six patients. In a study by Rieber et al. [8], a comparable technique for small bowel imaging was used. Instead of methylcellulose, either a paramagnetic oral contrast solution or a negative oral contrast agent was given after duodenal intubation. In spite of the excellent image quality due to strong bowel distension and the high diagnostic accuracy, there is one major drawback of this technique: the requirement of placing a duodenal tube, which has to be done under fluoroscopic guidance. Beyond the concerns of radiation exposure, which especially plays an important role since young patients are concerned, the use of two different diagnostic rooms is needed. In addition, patients often perceive the placement of the tube as traumatizing, which leads to a limited patient acceptance [13].
As for the mentioned limitations of MR enteroclysis, new approaches have been investigated considering the only oral application of contrast solutions for small bowel distension. In the first clinical settings, attempts were based on the oral administration of tap water [14, 15]. Due to the rapid resorption of water in the small intestine, the terminal ileum could not be adequately depicted in a high percentage of cases. Further investigations therefore focused on the development of oral contrast solutions imparting a decreased water resorption. To that end, different agents such as mannitol [16], Metamucil [17] and polyethylene glycol [18] have been proposed. In a recently published trial comparing different oral contrast agents in terms of bowel distension, a combination of locust bean gum and mannitol proved to be superior to the application of only one of the substances alone [12]. This solution meets nearly all the requirements of an oral contrast agent for small bowel imaging. It provides uniform bowel distension because of a reduced absorption. In addition, it has a good patient acceptance in terms of taste and lack of side effects. In addition, it is non-toxic and inexpensive. Although a non-invasive MR-based assessment of the small bowel without duodenal intubation seems to be possible, one problem has remained in the clinical routine. Beyond the affection of the small bowel, the colon as well can often be changed inflammatorily in patients with Crohn’s disease. MR imaging of the colon (MR colonography) has been shown to detect inflammatory lesions, e.g., ulcerative colitis, with high accuracy [19]. Therefore, we combined small bowel MR imaging with the display of the colon, which was accomplished by the additional rectal administration of tap water prior to the examination. Both the solution of LBG and mannitol in the small bowel as well as the water in the colon turned out to show nearly identical signal properties on the applied T1w sequence. Therefore, this procedure allowed a simultaneous assessment of the colon and small bowel, providing all advantages of MR imaging, such as the lack of ionizing radiation, non-invasiveness and the ability to display the parenchymal abdominal organs at the same time.
In the present study, only 10% of the patients (2 out of 20) were not able to ingest the entire 1,500 ml because of abdominal pain. Although a loss of distension in the small bowel could be observed, MR imaging was diagnostic with a reliable display of the jejunum and ileum, anyway. In contrast, the endoscopic procedure was not fully diagnostic in two patients because the ileocecal valve and the terminal ileum could not be reached. We excluded those subjects from the data analysis because of the lacking standard of reference. However, this feature points out another advantage of the MR technique, because data acquisition and interpretation are possible even under suboptimal conditions such as the presence of stenosis in the GI tract.
As proved in other studies [16, 20], inflammation of the bowel wall led to an increased contrast uptake compared to unaffected bowel segments after i.v. administration of paramagnetic contrast. In a study by Schunk et al. [16] encompassing 82 patients, the percentage of increase of SI was shown to be significantly higher in the inflamed bowel wall compared to the normal bowel wall (80 vs. 43%). We could confirm these findings by two parameters, which both proved a statistically significant difference between normal and pathological bowel segments. Much to our surprise, we found the more complex CER to be a less reliable value for the determination of bowel inflammation (P<0.001) compared to the simple assessment of the SNR value of the bowel wall after i.v. contrast (P<0.0001). However, both parameters can be used for the assessment of the bowel wall. Furthermore, other parameters applied in the present trial such as bowel wall thickening [21] and narrowing of the bowel lumen [22] have been proved to strongly correlate with inflammatory changes of the bowel.
Our results proved a good correlation between the endoscopic and bioptic findings of the terminal ileum on the one hand and the MRI analysis on the other hand. However, there were large differences between the two modalities regarding the detection rate of inflammatory changes in the large bowel. By means of MRI, eight colonic segments were rated as inflamed, whereas colonoscopy only confirmed three of these lesions. A possible explanation could be the relative overestimation of MRI resulting in false positive findings or a truly higher sensitivity of MRI.
One of the main advantages of MRI turned out to be the additional display of the more proximal parts of the small bowel. By means of MRI, eight segments in the proximal ileum and the jejunum were rated to be inflamed. All these segments had not been reached by endoscopy. Apparently, the MRI features for bowel wall inflammation of the colon and terminal ileum (such as increased contrast enhancement and bowel wall thickening) can be employed as well for the assessment of the more proximal bowel segments [21]. However, a true gold standard is missing, and it is at least discussible whether one or more of these eight segments were false positively rated as inflammation. Furthermore, one can wonder whether there were some false positive findings within the higher number of inflamed colonic segments that were detected by MRI.
Certainly, there are other limitations of the present trial. We investigated a very selective, small patient cohort encompassing subjects with known or at least strongly suspected Crohn’s disease. Thus, our results need to be confirmed by examining larger patient numbers with a wider spectrum of pathologies. In addition, the examination protocol renders space for optimization. It should be assessed whether bowel purgation, which was considered most unpleasant by the majority of patients, can be obviated. A solution might be the application of fecal tagging protocols that have been proposed for MR colonography [23]. Anyhow, it might be possible that the assessment of bowel wall inflammation (in contrast to the display of bowel wall masses) can be done without any bowel preparation at all. Furthermore, the impact of other sequences amplifying the examination protocol should be assessed for the combined display of small and large bowel. Especially, additional TrueFISP imaging might be helpful. This sequence invokes both T1 and T2 contributions and has been shown to be an excellent tool for the evaluation of inflammatory lesions in Crohn’s disease [10, 21]. In spite of the outlined limitations, we are convinced that the proposed technique renders a very promising tool for the diagnosis and follow-up of inflammatory bowel diseases.
References
Gore RM, Balthazar EJ, Ghahremani GG, Miller F (1996) CT features of ulcerative colitis and Crohn’s disease. Am J Roentgenol 167:3–15
Glickman RM (1991) Inflammatory bowel disease, ulcerative colitis and Crohn’s disease. Harrison’s principles of internal medicine, 12th edn. McGraw Hill, New York, pp 1268–1281
Low RN, Sebrechts CP, Politoske DA, Bennett MT, Flores S, Snyder RJ, Pressman JH (2002) Crohn disease with endoscopic correlation: single-shot fast spin-echo and gadolinium-enhanced fat-suppressed spoiled gradient-echo MR imaging. Radiology 222:652–660
Debatin JF, Patak MA (1999) MRI of the small and large bowel. Eur Radiol 9:1523–1534
Thoeni RF, Gould RG (1991) Enteroclysis and small bowel series: comparison of radiation dose and examination time. Radiology 178:659–662
Shoenut JP, Semelka RC, Magro CM, Silverman R, Yaffe CS, Micfli AB (1994) Comparison of magnetic resonance imaging and endoscopy in distinguishing the type and severity of inflammatory bowel disease. J Clin Gastroenterol 19:31–35
Lubolt W, Bauerfeind P, Wildermuth S, Debatin JF (1999) Contrast optimization for assessment of the colonic wall and lumen in MR colonography. J Magn Reson Imaging 9:745–750
Rieber A, Aschoff A, Nüssle K, Wruk D, Tomczak R, Reinshagen M, Adler G, Brambs H-J (2000) MRI in the diagnosis of small bowel disease: use of positive and negative oral contrast media in combination with enteroclysis. Eur Radiol 10:1377–1382
Umschaden HW, Szolar D, Gasser J, Umschaden M, Haselbach H (2000) Small bowel disease: comparison of MR enteroclysis images with conventional enteroclysis and surgical findings. Radiology 215:717–725
Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, Prassopoulos P (2002) MR enteroclysis: technical considerations and clinical applications. Eur Radiol 12:2651–2658
Schunk K, Metzmann U, Kersjes W, Schadmand-Fischer S, Kreitner KF, Duchmann R, Protzer U, Wanitschke R, Thelen M (1997) Follow-up of Crohn’s disease: can hydro-MRI replace fractionated gastrointestinal passage examination? Rofo Fortschr 166:389–396
Lauenstein TC, Schneemann H, Vogt FM, Herborn CU, Rühm SG, Debatin JF (2003) Optimization of oral contrast agents for MR imaging of the small bowel. Radiology 228:279–283
Maglinte DD, Lappas JC, Kelvin FM, Rex D, Chernish SM (1987) Small bowel radiography: how, when, and why? Radiology 163:297–305
Lomas DJ, Graves MJ (1999) Small bowel MRI using water as a contrast medium. Br J Radiol 72:994–997
Minowa O, Ozaki Y, Kyogoku S, Shindoh N, Sumi Y, Katayama H (1999) MR imaging of the small bowel using water as a contrast agent in a preliminary study with healthy volunteers. Am J Roentgenol 173:581–582
Schunk K, Kern A, Oberholzer K, Kalden P, Mayer I, Orth T, Wanitschke R (2000) Hydro-MRI in Crohn’s disease: appraisal of disease activity. Invest Radiol 35:431–437
Patak MA, Froehlich JM, von Weymarn C, Ritz MA, Zollikofer CL, Wentz K (2001) Non-invasive distention of the small bowel for the magnetic-resonance Imaging. Lancet 358:987–988
Laghi A, Borrelli O, Paolantonio P, Dito L, Buena de Mesquita M, Falconieri P, Passariello R, Cucchiara S (2003) Contrast enhanced magnetic resonance imaging of the terminal ileum with Crohn’s disease. Gut 52:393–397
Hansmann HJ, Hess T, Hahmann M, Erb G, Elsing C, Richter GM (2001) MRI in chronic inflammatory bowel disease. Rofo Fortschr 173:4–11
Maccioni F, Viscido A, Broglia L, Marrollo M, Masciangelo R, Capri R, Rossi P (2000) Evaluation of Crohn’s disease with magnetic resonance imaging. Abdom Imaging 25:219–228
Gourtsoyiannis N, Papanikolaou N, Grammatikalis J, Papamastorakis G, Prassopoulos P, Roussomoustakaki M (2004) Assessment of Crohn’s disease activity in the small bowel with MR and conventional enteroclysis: preliminary results. Eur Radiol 14:1017–1024
Schmidt S, Lepori D, Meuwly J-Y, Duvoisin B, Meuli R, Michetti P, Felley C, Schnyder P, Melle G, Denys A (2003) Prospective comparison of MR enteroclysis with multidetector spiral-CT enteroclysis: interobserver agreement and sensitivity by means of “sign by sign” correlation. Eur Radiol 13:1303–1311
Lauenstein TC, Goehde SC, Ruehm SG, Holtman G, Debatin JF (2002) MR colonography with barium-based fecal tagging: initial clinical experience. Radiology 223:248–254
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Narin, B., Ajaj, W., Göhde, S. et al. Combined small and large bowel MR imaging in patients with Crohn’s disease: a feasibility study. Eur Radiol 14, 1535–1542 (2004). https://doi.org/10.1007/s00330-004-2364-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2364-7