Abstract
The accurate staging of primary bone tumors in children is critical for treatment planning. Limb salvage operations can now be performed with excellent outcomes in suitable patients. The purpose of this article is to review the current state of imaging techniques and their roles in enabling accurate staging and treatment planning to be performed in pediatric patients with primary bone tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accurate staging provides important information regarding the patient’s prognosis and helps clinicians decide on the best mode of therapy for their patients. This review highlights the role of imaging in the surgical staging and treatment planning of children with primary bone tumors.
Surgical staging of bone tumors
Wolf and Enneking [1] developed a system for the staging of bone and soft tissue tumors. This system is based on the grade (G), anatomic location or site of the tumor (T), and the presence or absence of metastases (M) (Table 1). The system has been adopted by the Musculoskeletal Tumor Society and is commonly known as the MTS system.
Grade
The grade of the tumor provides an assessment of the aggressiveness of a lesion and is based on histologic, radiographic, and clinical criteria [1]. Radiographic criteria are based on Lodwick’s radiographic grading system, which is discussed in detail later in this review. Clinical criteria take into consideration features such as growth rate, doubling time, size, temperature, biological markers, and symptoms such as pain and tenderness. Generally, the grade of a tumor follows the histologic grading but a higher surgical grade may be assigned to a tumor if it displays evidence of more aggressive radiographic features or clinical behavior [1].
Site
The anatomic location or site (T) of the tumor is classified according to whether the tumor is confined to its anatomic compartment of origin or has extended beyond its natural barriers. A tumor may be intracompartmental or extracompartmental. An intraosseous tumor is considered intracompartmental if it is confined to the intramedullary portion of the bone without breaching the cortex. It is considered extracompartmental if it has breached the cortex and has involved the adjacent soft tissue or joint. A lesion on the cortical surface is considered intracompartmental if it is still confined to the surface of the bone and extracompartmental if it has invaded the medullary cavity or outwardly to involve the adjacent soft tissue or joint.
Metastasis
In the Enneking system, tumors without metastasis are classified as M0. Lesions with metastatic disease are classified as M1. Skip lesions are considered M1 lesions because these lesions are caused by hematogenous spread and have a prognosis similar to that of distant metastasis [1].
Staging
The Enneking staging system categorizes malignant tumors into stages I–III (Table 2). Low-grade and high-grade tumors without metastases are classified as stage I and stage II lesions, respectively. Stage I and II lesions are further classified into subcategories A and B, depending on whether the tumor is intracompartmental or extracompartmental. Lesions with metastasis are considered stage III lesions.
Nonmesenchymal tumors, such as Ewing’s sarcoma, lymphoma, and leukemia, have different biological behavior and should not be staged under the Enneking system [1]. In Ewing’s sarcoma, the site, tumor volume, presence of metastases at diagnosis, and the tumor response to chemotherapy are the main prognostic factors [2].
Staging and limb salvage surgery
Limb salvage surgery aims to be curative as well as to preserve limb function. Malignant Enneking stage 1 tumors can generally be treated with wide excision and limb salvage surgery. Stage 2 lesions usually cannot be treated with a limb salvage operation alone, unless the tumor is highly responsive to chemotherapy. Stage 3 lesions that respond to adjuvant therapy can be treated with wide excision. Palliative resection is reserved for lesions that respond poorly to adjuvant therapy [1].
Generally, for limb salvage surgery to be successful, the following conditions must be met:
-
Tumor is located in the extremities and/or axial skeleton.
-
Tumor margins are amenable to surgery—this usually means at least 6 cm of normal bone around the tumor margins. Smaller margins may be accepted if the tumor is responsive to pre-operative chemotherapy [3].
-
Soft tissue tumor extension is localized—with approximately 2 cm of normal soft tissue around the soft tissue mass.
-
Neurovascular bundles are uninvolved, i.e., no encasement or invasion of the neurovascular structures by tumor tissue.
-
Metastases are not present or are resectable.
-
General physical condition of the patient is adequate [4].
Involvement of the vessels does not necessarily mean that limb salvage surgery cannot be performed, because arterial bypass and en-bloc resection can be performed in some cases. However, nerve involvement is more critical, e.g., encasement of the sciatic nerve usually precludes limb-sparing surgery. Contraindications to limb salvage surgery are tumors which do not satisfy the above criteria; lesions occurring in the distal lower extremities, where the functional results of the combination of amputation and prosthesis are good; and local recurrence after previous attempt at limb salvage [5].
Imaging techniques in surgical staging
Radiography
Evaluation of bone lesions begins with conventional radiographs. Lodwick et al. established a grading system based on the radiographic features of the lesions. In order of priority, the important radiographic signs are [6, 7]:
-
Margins of the lesion—geographic, moth-eaten, or permeative
-
Penetration of cortex
-
Presence or absence of sclerotic rim
-
Presence or absence, and extent (if present), of the expanded cortical shell
Lesions with well-defined or geographic margins are classified as grade 1 lesions. These are subcategorized depending on the margin characteristics into 1A (sclerotic), 1B (well-defined) or 1C (poorly-defined). Grade 2 lesions are moth-eaten lesions that always show cortical penetration. Grade 3 lesions are permeative lesions that preserve the outline of the bone but have numerous small, diffuse lytic lesions (Fig. 1). Although increasing the radiographic grade generally correlates well with the aggressiveness of the lesion, there will inevitably be some degree of overlap between the different grades of lesions, particularly those in grades 1C and 2. In these cases, histologic evaluation of the lesion is essential in determining the further management of these cases [1]. Furthermore, grade 2 and 3 lesions do not always correspond directly with low-grade and high-grade malignant lesions, respectively. Histologic evaluation is important for definitive diagnosis.
The radiological appearances of bone lesions are thus important in determining the subsequent management of the patient. This has given rise to another classification system relating the radiographic appearance of bone lesions to its subsequent management pathway. Group 1 lesions have definite radiographic benign features and do not require further investigation. Group 2 lesions are very likely to be benign, but should still be followed-up clinically and radiographically. Group 3 lesions are benign lesions that exhibit aggressive behavior or are at risk of pathological fracture and should be treated with elective surgery. Group 4 lesions with aggressive radiographic features should be considered malignant and require staging prior to biopsy and definitive therapy [8].
Computed tomography
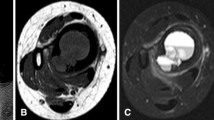
The two main objectives of computed tomography (CT) in children with primary malignant bone tumors are the assessment of primary disease and the evaluation of lung metastases. CT is very useful in evaluating tumors in complex-shaped bones such as the spine and pelvis. CT is also useful in evaluating the extent of cortical involvement and breakthrough (Fig. 2), as well as the presence of intratumoral calcification or ossification (Fig. 3).
A 12-year-old girl with Ewing’s sarcoma of the right scapula. a Frontal radiograph of the scapula shows marked sclerosis involving much of the bone. Evaluation of the body of the scapula was difficult due to the overlying ribs. b Axial CT scan shows the entire scapula, including the body to be involved with the tumor. The tumor has a mixed sclerotic and lytic appearance with a spiculated periosteal reaction (arrow). (Case courtesy of Dr. Tan and Prof. Stringer, National University Hospital, Singapore)
A 12-year-old boy with osteosarcoma of the left scapula. a Frontal radiograph of the left scapula shows an amorphous area of ossification projected over the left scapula. b Axial CT scan shows an aggressive sclerotic lesion arising from the scapula. There is anterior soft tissue extension. Biopsy confirmed an osteosarcoma
Although MR imaging is widely felt to be superior to CT in the evaluation of local tumor spread [9], a multicenter study concluded that CT is equally effective [10]. However, the higher contrast resolution of MR imaging makes it the favored modality and it is generally recognized as being superior to CT [9]. The choice between CT and MR imaging is influenced by local factors such as cost and availability of the different modalities, and the working understanding between diagnostic radiologists and referring clinicians. CT is better than MR imaging in the evaluation of subtle cortical lesions [11], matrix calcification, and periosteal new bone formation.
Unenhanced CT of the thorax is recommended for all patients during the surgical staging of bone tumors as it is more sensitive than chest radiographs for detection of pulmonary metastases.
Bone scintigraphy
Technetium (Tc)-99m-labeled diphosphonate scintigraphy is used in the staging of bone tumors to evaluate for the presence of metastases, skip lesions, and in recurrent disease. When an area of increased tracer uptake is noted on the bone scintiscans, radiographs of that region are required. If the radiographs are inconclusive, further evaluation with CT or MR imaging should be performed. Biopsy may also be considered if the CT and MR scans are negative, the patient is symptomatic, and there remains a strong clinical suspicion of metastasis.
Angiography
Conventional angiography is occasionally performed to evaluate the blood supply and as a prelude to embolization for prophylactically decreasing hemorrhage before surgery [1]. It is now seldom used to diagnose neurovascular bundle involvement as this can be more accurately and less invasively assessed using CT or MR imaging.
Ultrasonography
Although the role of ultrasonography (US) is limited in patients with primary bone tumors, it may be used in conjunction with other modalities such as MR imaging or CT if artifacts from orthopedic hardware prevent proper evaluation. US can be used to assess the relationship of local tumor spread to the neurovascular bundle and to guide biopsy of lesions, if necessary. Color Doppler US may also be used to assess regression of tumor neovascularity during the course of treatment [12].
Image-guided biopsy
Image-guided biopsy of primary bone tumors is cheaper, less invasive, and has a lower complication rate compared to open biopsy [11]. Disadvantages include a suboptimal biopsy specimen, but this risk can be minimized by having a pathologist present at the time of biopsy to review the tissue samples obtained. Modalities for guiding biopsy include fluoroscopy, US, CT, and MR imaging. General anesthesia is usually necessary in children. In experienced hands, image-guided biopsy has a low complication rate and is diagnostic in the vast majority of cases [11].
MR imaging
Technical considerations
In large primary malignant or aggressive bone tumors, initial imaging using a volume coil to obtain a broader field-of-view is useful. The entire bone including the joints just above and below the affected bone must be imaged to detect skip lesions and joint involvement. After skip lesions are excluded, smaller surface coils may then be used to obtain higher resolution images that focus on the tumor itself. In subsequent examinations, smaller surface coils may be used. Parameters such as slice thickness, inter-slice gap, number of excitations, and matrix size depend on the lesion size, area of interest, and pulse sequence used. A surface marker (e.g., vitamin A capsule) should be placed over the lesion. In lesions involving the lower limb, a comparative examination is systematically performed with the opposite limb.
Conventional T1- and T2-weighted spin-echo (SE) sequences are still the main sequences used in MR imaging. Generally, pathological tissues are hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences. Sequences such as fat-saturated fast spin-echo (FSE) T2-weighted and short-T1 inversion recovery (STIR) sequences are also frequently used in determining the extent of malignant bone infiltration although most authors still prefer T1-weighted sequences for the evaluation of intramedullary tumor spread and skip lesions [13] (Fig. 4). Opinions vary regarding the comparative accuracy of T1-weighted and STIR sequences in the evaluation of intraosseous tumor spread [13, 14].
Fat-saturated T1-weighted sequences are obtained after the intravenous administration of gadolinium diethylene triamine penta-acetic acid (Gd-DTPA), in order to increase the signal intensity of pathological tissues. The use of Gd-DTPA has been found to be useful in [11, 15]:
-
Distinguishing cystic from solid areas within tumor tissue [11]
-
Distinguishing necrotic tissue and peritumoral edema from viable tumor tissue [11]
-
Adding specificity in tissue characterization [15]
-
Staging of local extent of tumor spread (Fig. 5) [15]
Fig. 5a–c An 8-year-old girl with osteosarcoma of the left lower femur. a Axial T1-weighted and b fat-saturated T2-weighted enhanced images show the tumor breaking through the cortex of the bone (arrows). The neurovascular bundle is not involved (arrowhead). c Coronal T1-weighted image shows the longitudinal extent of the tumor (arrows)
-
Biopsy planning [15]
-
Monitoring preoperative chemotherapy [15]
-
Detection of recurrence or residual tumor after surgery and other forms of treatment [15].
Evaluation of tumor vascularity is important in the preoperative assessment of primary bone tumors because it provides information regarding vascular invasion by the tumor and whether or not embolization is required in highly vascular tumors [16]. In some centers, MR angiography has replaced conventional angiography in the preoperative evaluation of tumor vascularity because it is noninvasive and provides good visualization of peripheral vascular branches and tumor neovascularity in patients [17].
Role of MR imaging in surgical staging
The main role of MR imaging is in the evaluation of whether a tumor is located in the extramedullary or intramedullary compartments. MR imaging is able to detect tumor involvement of the adjacent muscle compartments, neurovascular structures, growth plate, and joints [9, 11, 18, 19]. Evaluation of neurovascular involvement can be accurately assessed on T1-weighted images because of the contrast between the tumor (low signal intensity) and the fat surrounding the neurovascular bundle (high signal intensity) [20]. Other sequences with fat-saturation such as T2-weighted FSE, proton-density weighted, and contrast-enhanced axial T1-weighted sequences have also been used to evaluate neurovascular bundle involvement [13, 21, 22] (Fig. 6). MR imaging features of neurovascular bundle involvement include complete encasement and/or infiltration of the vessels [13]. Involvement of the vascular structures may require vascular reconstruction while involvement of the lumbosacral plexus or sciatic nerve may preclude limb salvage [16].
A 13-year-old girl with osteosarcoma of the right upper tibia. a Axial unenhanced T1-weighted image shows a soft tissue mass displacing the neurovascular bundle (arrow). The fatty rim surrounding the neurovascular bundle is displaced but preserved. b Axial enhanced fat-saturated T1-weighted image confirms the neurovascular bundle to be intact (arrow)
Although some authors have found MR imaging to be sensitive in the evaluation of joint involvement [19], tumor over-staging was a problem in some cases [23]. Enhanced T1-weighted sequences are the most useful sequence in the evaluation of joint involvement by tumor, but synovial enhancement may mimic tumor involvement [24] (Fig. 7). The presence of joint effusion is not diagnostic of tumor involvement, as joint effusion may be reactive in nature. The absence of joint effusion, however, has a high negative predictive value of 92% [23].
A 9-year-old boy with osteosarcoma of the right humerus. a Frontal radiograph of the right upper humerus shows a destructive osteolytic lesion involving the upper metaphysis. The epiphyseal plate is eroded and the inferomedial epiphysis is involved. b Axial T1-weighted MR image of the right humeral head shows total tumor replacement of normal marrow within the humeral epiphysis. The glenohumeral joint is involved (arrow). c Coronal enhanced fat-saturated T1-weighted MR image shows the heterogeneously enhancing tumor with shoulder joint involvement and marked synovial enhancement (arrowheads). Diffusion of contrast into the joint space has occurred due to the slight delay in obtaining this sequence (arrow)
Assessment of the intramedullary extent of primary bone tumors includes longitudinal medullary extent, epiphyseal involvement, and skip metastases [13]. Growth plate and epiphyseal involvement is more accurately assessed on MR imaging than on radiographs [25] (Fig. 8).
A 9-year-old boy with osteosarcoma of the right distal femur. a Frontal radiograph shows the typical appearance of an osteosarcoma in the distal metaphysis. Involvement of the epiphyseal plate is difficult to ascertain. b Coronal unenhanced T1-weighted, c coronal, and d sagittal enhanced fat-saturated T1-weighted MR images show the heterogeneously enhancing primary tumor within the distal metaphysis extending up to the mid-shaft of the femur. The tumor has crossed the epiphyseal plate and involved the epiphysis. The joint was not involved. A large soft tissue component is present
Whole-body MR imaging using echo planar, STIR, and T1-weighted spin-echo sequences to detect patients with suspected skeletal metastases has shown a high sensitivity and specificity in the detection of metastases [26, 27, 28]. There is also evidence that whole-body MR imaging is more accurate than conventional planar Tc-99m-MDP scintigraphy [26].
Assessment of treatment response
The aim of preoperative chemotherapy is to eradicate microscopic metastases and reduce the size of the primary tumor, making limb salvage surgery possible in some cases [29]. The response of tumors to preoperative chemotherapy is an important prognostic factor for indicating the relapse-free interval and survival in osteosarcoma and Ewing’s sarcoma [30, 31].
MR imaging can be used to assess tumors after chemotherapy [14, 32]. Static unenhanced sequences showing postchemotherapeutic signal changes and changes in tumor size may not correlate well with the histopathologic response in some tumors because postchemotherapy MR signal changes can be complex and may represent necrosis, hemorrhage, edema, granulation tissue, and/or fibrosis [31]. Some indicators of good response include the following:
-
Decrease in signal intensity on T2-weighted images [10]
-
Combination of a circumferential hypointense rim together with a reduction in size of the soft tissue component in Ewing’s sarcoma [21]
-
Increased homogeneous T2-weighted signal within the intramedullary portion of Ewing’s sarcoma which indicates tumor replacement by the hypocellular mucomyxoid matrix [21]
Contrast-enhanced MR imaging is useful in assessing remnant tumor in patients after treatment. Viable remnant tumor enhances to a greater degree than nonviable tissue [33]. Necrotic tumor areas do not enhance on post-Gd-DTPA images [34]. Differentiation between viable and nonviable tumor tissue is better made on dynamic scans compared to static scans because viable residual tumor is characterized by earlier and more rapid uptake of the contrast agent (Fig. 9) [35]. Differentiation may be difficult on static scans because vascularized granulation tissue, neovascularity in necrotic areas, and reactive hyperemia may also occur after the administration of contrast medium. In patients with osteosarcoma and Ewing’s sarcoma, good responders to chemotherapy show a relative reduction of the enhancement uptake slopes after chemotherapy compared to the prechemotherapy slopes, while poor responders show little or no reduction [36]. Parametric first-pass imaging and subtraction MR imaging have also been used to increase the detection of early arterial enhancement within viable residual tumor [36].
A 15-year-old boy with osteosarcoma of the distal femur. a Axial fat-saturated T1-weighted MR image of the right distal femur shows region of interests (ROIs) drawn over normal muscle (8), a peripheral vein (9), tumor within the femur (6), soft tissue tumor mass (5), and a nonenhancing area of soft tissue (7). b Dynamic time-intensity curves show a steep rise in tumor tissue as seen in ROIs (5) and (6). A similar but delayed steep rise is also seen in the peripheral vein (9). The ROI is drawn over nonenhancing soft tissue (7) and normal muscles (8) show only mild rise in their respective time-intensity curves
MR spectroscopy (MRS) using phosphorus-31 (31P) to provide indicators of response to chemotherapy in musculoskeletal tumors has been studied [37]. There are early spectral changes in human extremity sarcomas after therapy. Sijens has shown that the 31P MR spectra measured before treatment, and the changes in phosphate metabolites measured shortly after treatment, correlate with the clinical response after 2 or 3 months [38]. Limitations of MRS include the technical difficulty in obtaining representative spectra from all locations within the tumor, contamination of tumor spectra from phosphorus in adjacent normal tissues, and its insensitivity to tumor heterogeneity due to low spatial resolution that can be achieved at 1.5 or 2 T with 31P MRS [33].
Detection of tumor recurrence
Theoretically, recurrence is suspected when a lesion that is hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences is seen at the previous tumor site [39–41]. Areas of T2-weighted hyperintensity may, however, be caused by radiation-induced tissue changes, postsurgical seroma, hematoma, fat necrosis, surgical hemostatic packing material, soft tissue expanders, and intercalary bone allograft [25, 40]. Lack of high signal on T2-weighted images in a nonnodular lesion with low to intermediate signal intensity on T1-weighted images is said to be typical of chronic posttherapeutic changes and not recurrence [42].
Dynamic contrast-enhanced MR imaging may be helpful in detecting early enhancement that is not seen in posttherapeutic changes [18]. Reconversion of fatty marrow to hematopoietic marrow may occur in children with osteosarcoma treated with chemotherapy and granulocytic colony-stimulating factor. This may resemble recurrent tumor on MR imaging although reconverted marrow usually occurs bilaterally and symmetrically, and is usually isointense to skeletal muscle [43].
Positron emission tomography
Evaluation of treatment response and tumor recurrence
FDG-PET is a promising tool for the evaluation of adjuvant chemotherapy response in osteosarcoma [44]. Schulte et al. found that the decrease of FDG uptake in osteosarcomas expressed as a ratio of posttherapeutic and pretherapeutic tumor-to-background ratios (TBR) showed a close correlation to the amount of tumor necrosis induced by chemotherapy (P<0.001; Spearman). With a TBR ratio cut-off level of 0.6, all responders and eight of ten nonresponders could be identified by FDG-PET.
FDG-PET can be used in conjunction with MR imaging to distinguish viable tumor from posttherapeutic changes in patients with bone and soft-tissue sarcomas [45]. Although occasional persistent uptake has been seen in therapy-related fibrous tissue [46], FDG-PET remains a promising and useful modality in the detection of postchemotherapy recurrence in bone sarcomas.
Detection of metastases
An added attraction of PET is its ability to evaluate for the presence of metastasis at the same time as evaluating the primary tumor. FDG-PET is more sensitive than bone scintigraphy in the detection of osteolytic metastatic lesions, but the detection of osteoblastic metastases is less than satisfactory [47]. There are reports that high-quality tomographic PET bone scans are possible with 18F–fluoride with possible advantages in sensitivity and specificity over conventional bone scintigraphy [48].
Imaging protocol and conclusion
Imaging has an important role in the staging of bone tumors. Accurate staging enables an appropriate choice of therapy for affected patients. An appropriate imaging protocol should always begin with radiography. Lesions that are clearly benign do not require further treatment and may be followed up clinically and radiologically. Suspicious aggressive appearing or clearly malignant lesions require further evaluation and local staging with cross-sectional imaging such as CT or MR imaging. CT is useful for detailed assessment of subtle bony lesions and complex-shaped bones. CT of the thorax should be performed to evaluate for the presence of lung metastases. Currently, MR imaging is the modality of choice in the local staging of the primary tumor. Dynamic contrast-enhanced MR imaging may be useful in determining the absence or presence of recurrent or residual tumor after treatment. Evaluation of bone metastases and the detection of viable local recurrent or residual tumor after treatment are usually performed using bone scintigraphy, although whole-body MR imaging and PET are possible future replacements. It is likely that in the future PET imaging will have an increasing role in the staging of primary bone tumors in children.
References
Wolf RE, Enneking WF (1996) The staging and surgery of musculoskeletal neoplasms. Orthop Clin North Am 27:473–481
Grier HE (1997) The Ewing family of tumors. Ewing’s sarcoma and primitive neuroectodermal tumors. Pediatr Clin North Am 44:991–1004
Veth RP, van Hoesel QGCM, Bökkerink JPM, Hoogenhout J, Pruszczynski M (1995) The art of limb salvage in musculoskeletal oncology. Crit Rev Oncol/Hematol 21:77–103
Veth RP, Nielsen HKL, Oldhoff J, Schraffordt Koops H, Mehta D, Oosterhuis JW, Kamps WA, Goeken LN (1989) Megaprostheses in the treatment of primary malignant and metastatic tumors in the hip region. J Surg Oncol 40:214–218
McDonald DJ (1994) Limb-salvage surgery for treatment of sarcomas of the extremities. Am J Roentgenol 163:509–513
Lodwick GS, Wilson AJ, Farrell C, Virtama P, Dittrich F (1980) Determining growth rates of focal lesions of bones from radiographs. Radiology 134:577–583
Lodwick GS, Wilson AJ, Farrell C, Virtama P, Smeltzer FM, Dittrich F (1980) Estimating rate of growth in bone lesions: observer performance and error. Radiology 134:585–590
Sundaram M, McLeod RA (1990) MR imaging of tumor and tumorlike lesions of bone and soft tissue. Am J Roentgenol 155:817–824
Bloem JL, Taminiau AHM, Eulderink F, Hermans J, Pauwels EK (1988) Radiologic staging of primary bone sarcoma: MR imaging, scintigraphy, angiography, and CT correlated with pathologic examination. Radiology 169:805–810
Panicek DM, Gatsonis C, Rosenthal DI, Seeger LL, Huvos AG, Moore SG, Caudry DJ, Palmer WE, McNeil BJ (1997) CT and MR imaging in the local staging of primary malignant musculoskeletal neoplasms: report of the radiology diagnostic oncology group. Radiology 202:237–246
Peh WCG (1999) The role of imaging in the staging of bone tumors. Crit Rev Oncol Hematol 31:147–167
Bodner G, Schocke MF, Rachbauer F, Seppi K, Peer S, Fierlinger A, Sununu T, Jaschke WR (2002) Differentiation of malignant and benign musculoskeletal tumors: combined color and power Doppler US and spectral wave analysis. Radiology 223:410–416
Saifuddin A (2002) The accuracy of imaging in the local staging of appendicular osteosarcoma. Skeletal Radiol 31:191–201
Onikul E, Fletcher BD, Parham DM, Chen G (1996) Accuracy of MR imaging for estimating intraosseous extent of osteosarcoma. Am J Roentgenol 167:1211–1215
Verstraete KL, Lang P (2000) Bone and soft tissue tumors: the role of contrast agents for MR imaging. Eur J Radiol 34:229
Nomikos GC, Murphey MD, Kransdorf MJ, Bancroft LW, Peterson JJ (2002) Primary bone tumors of the lower extremities. Radiol Clin North Am 40:971–990
Lang P, Grampp S, Vahlensieck M, Johnston JO, Honda G, Rosenau W, Matthay KK, Peterfy C, Higgins CB, Genant HK (1995) Primary bone tumors: value of MR angiography for preoperative planning and monitoring response to chemotherapy. Am J Roentgenol 165:135–142
Vanel D, Verstraete KL, Shapeero LG (1997) Primary tumors of the musculoskeletal system. Radiol Clin North Am 35:213–237
Van Trommel MF, Kroon HM, Bloem JL, Hogendoom PC, Taminiau AH (1997) MR imaging based strategies in limb salvage for osteosarcoma of the distal femur. Skeletal Radiol 26:636–641
Pettersson H, Gillespy T 3rd, Hamlin DJ, Enneking WF, Springfield DS, Andrew ER, Spanier S, Slone R (1987) Primary musculoskeletal tumors: examination with MR imaging compared with conventional modalities. Radiology 164:237–241
Van der Woude HJ, Bloem JL, Holscher HC, Nooy MA, Taminiau AH, Hermans J, Falke TH, Hogendoorn PC (1994) Monitoring the effect of chemotherapy in Ewing sarcoma of bone with MR imaging. Skeletal Radiol 23:493–500
Gronemeyer SA, Kauffman WM, Rocha MS, Steen RG, Fletcher BD (1997) Fat-saturated contrast-enhanced T1-weighted MRI in evaluation of osteosarcoma and Ewing sarcoma. J Magn Reson Imaging 7:585–589
Schima W, Amann G, Stiglbauer R, Windhager R, Kramer J, Nicolakis M, Farres MT, Imhof H (1994) Preoperative staging of osteosarcoma: efficacy of MR imaging in detecting joint involvement. Am J Roentgenol 163:1171–1175
Murphey MD, Robbin MR, McRae GA, McRae GA, Flemming DJ, Temple HT, Kransdorf MJ (1997) The many faces of osteosarcoma. Radiographics 17:1205–1231
Panuel M, Gentet JC, Scheiner C, Jouve JL, Bollini G, Petit P, Bourliere-Najean B, Devred P (1993) Physeal and epiphyseal extent of primary malignant tumors in childhood: correlation of preoperative MRI and the pathological examination. Pediatr Radiol 23:421–424
Eustace S, Tello R, DeCarvalho V, Carey J, Wroblicka JT, Melhem ER, Yucel EK (1997) A comparison of whole-body turboSTIR MR imaging and planar 99mTc-methylene diphosphonate scintigraphy in the examination of patients with suspected skeletal metastases. Am J Roentgenol 169:1655–1661
Daldrup-Link HE, Franzius C, Link TM, Laukamp D, Sciuk J, Jurgens H, Schober O, Rummeny EJ (2001) Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. Am J Roentgenol 177:229–236
Mazumdar A, Siegel MJ, Narra V, Luchtman-Jones L (2002) Whole-body fast inversion recovery MR imaging of small cell neoplasms in pediatric patients: a pilot study. Am J Roentgenol 179:1261–1266
Pastorino U, Gasparini M, Tavecchio L, Azzarelli A, Mapelli S, Zucchi V, Morandi F, Bellani FF, Valente M, Ravasi G (1991) The contribution of salvage surgery to the management of childhood osteosarcoma. J Clin Oncol 9:1357–1362
Meyers PA, Heller G, Healy J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G (1992) Chemotherapy for nonmetastatic osteogenic sarcoma: the memorial Sloan–Kettering experience. J Clin Oncol 10:5–15
Picci P, Bougraff BT, Bacci G, Neff JR, Sangiorgi L, Cazzola A, Baldini N, Ferrari S, Mercuri M, Ruggieri P (1993) Prognostic significance of histopathologic response to chemotherapy in nonmetastatic Ewing’s sarcoma of the extremities. J Clin Oncol 11:1763–1769
Cohen IJ, Hadar H, Schreiber R, Horev G, Katz K, Meller I, Yosipovitch Z, Zaizov R (1994) Primary bone tumor resectability: the value of serial MR studies in the determination of the feasibility, timing, and extent of tumor resection. J Pediatr Orthop 14:781–787
Van der Woude HJ, Bloem JL, Hogendoorn PCW (1998) Preoperative evaluation and monitoring chemotherapy in patients with high-grade osteogenic and Ewing’s sarcoma: review of current imaging modalities. Skeletal Radiol 27:57–71
Erlemann R, Reiser MF, Peters PE, Vasallo P, Nommensen B, Kusnierz-Glaz CR, Ritter J, Roessner A (1989) Musculoskeletal neoplasms: static and dynamic Gd-DTPA-enhanced MR imaging. Radiology 171:767–773
Lang P, Honda G, Roberts T, Vahlensieck M, Johnston JO, Rosenau W, Mathur A, Peterfy C, Gooding CA, Genant HK (1995) Musculoskeletal neoplasm: perineoplastic edema versus tumor on dynamic postcontrast MR images with spatial mapping of instantaneous enhancement rates. Radiology 197:831–839
De Baere T, Vanel D, Shapeero LG, Charpentier A, Terrier P, di Paola M (1992) Osteosarcoma after chemotherapy: evaluation with contrast material-enhanced subtraction MR imaging. Radiology 185:587–592
Moller HE, Vermathen P, Rummeny E, Wortler K, Wuisman P, Rossner A, Wormann B, Ritter J, Peters PE (1996) In vivo 31P NMR spectroscopy of human musculoskeletal tumors as a measure of response to chemotherapy. NMR Biomed. 9:347–358
Sijens PE (1998) Phosphorus MR spectroscopy in the treatment of human extremity sarcomas. NMR Biomed 11:341–353
Fletcher BD, Hanna SL, Fairclough DL, Gronemeyer SA (1992) Pediatric musculoskeletal tumors: use of dynamic, contrast-enhanced MR imaging to monitor response to chemotherapy. Radiology 184:243–248
Glazer HS, Lee JKT, Levitt RG, Heiken JP, Ling D, Totty WG, Balfe DM, Emani B, Wasserman TH, Murphy WA (1985) Radiation fibrosis: differentiation from recurrent tumor by MR imaging. Radiology 156:721–726
Griffiths HJ, Thompson RC, Nitke SJ, Olson PN, Thielen KR, Amundson P (1997) Use of MRI in evaluating postoperative changes in patients with bone and soft tissue tumors. Orthopedics 20:215–220
Lang P, Johnston JO, Arenal-Romero F, Gooding CA. (1998) Advances in MR imaging of pediatric musculoskeletal neoplasms. MRI Clin North Am 6:579–604
Ryan SP, Weinberger E, White KS, Shaw DW, Patterson K, Nazar-Stewart V, Miser J (1995) MR imaging of bone marrow in children with osteosarcoma: effect of granulocytic colony-stimulating factor. Am J Roentgenol 165:915–920
Schulte M, Brecht-Krauss D, Werner M, Hartwig E, Sarkar MR, Keppler P, Kotzerke J, Guhlmann A, Delling G, Reske SN (1999) Evaluation of neoadjuvant therapy response of osteogenic sarcoma using FDG PET. J Nucl Med 40:1637–1643
Bredella MA, Caputo GR, Steinbach LS (2002) Value of FDG positron emission tomography in conjunction with MR imaging for evaluating therapy response in patients with musculoskeletal sarcomas. Am J Roentgenol 179:1145–1150
Jones DN, McCowage GB, Sostman HD, Brizel DM, Layfield L, Charles HC, Dewhirst MW, Prescott DM, Friedman HS, Harrelson JM, Scully SP, Coleman RE (1996) Monitoring of neoadjuvant therapy response of soft-tissue and musculoskeletal sarcoma using fluorine-18-FDG PET. J Nucl Med 37:1438–1444
Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I (1998) Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol 16:3375–3379
Cook GJ, Fogelman I (2000) The role of positron emission tomography in the management of bone metastases. Cancer 15(12 Suppl):2927–2933
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teo, H.E.L., Peh, W.C.G. The role of imaging in the staging and treatment planning of primary malignant bone tumors in children. Eur Radiol 14, 465–475 (2004). https://doi.org/10.1007/s00330-003-2211-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-2211-2