Abstract
The purpose of this study was to evaluate the detection rate of tumor vessels and vascularity in hepatocellular carcinoma (HCC) by contrast-enhanced coded US using Levovist, and to compare with conventional color/power Doppler US (CDUS) and dynamic CT. Ninety nodules (72 hypo/isoechoic nodules, 18 hyperechoic nodules) in 61 patients were studied. We observed tumor vessels by continuous transmission at the early vascular phase (40 s following administration of Levovist) and vascularity by intermittent transmission (intervals of 2–3 s) at the late vascular phase (40 to approximately 120 s). The detection rate of tumor vessels at the early vascular phase was 97% in hypo/isoechoic nodules and 70% in hyperechoic nodules with high density in dynamic CT being higher than that by CDUS. Tumor vascularity at the late vascular phase in hypo/isoechoic and hyperechoic nodules was hyper-enhancement in 78 and 40%, iso-enhancement in 19 and 40%, and hypo-enhancement in 3 and 0%, respectively. The detection rates of tumor vessels and vascularity in hyperechoic nodules were similar to those by CDUS. The detection rates of tumor vessels and vascularity were not affected by the tumor size in HCC tumors with high density in dynamic CT. Contrast-enhanced US with Levovist was superior to CDUS and equal to dynamic CT to assess tumor vessels in hypo/isoechoic nodules. Although it was equal to CDUS for hyperechoic nodules, this modality is useful in evaluating tumor hemodynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of imaging modalities has enabled easy detection and diagnosis of hepatocellular carcinoma (HCC). The characterization of tumor vascularity is important for the differential diagnosis, the choice of treatment, and the assessment of therapeutic response. Hepatocellular carcinoma tumor vascularity has been evaluated extensively with various imaging modalities, e.g., color or power Doppler ultrasonography [1, 2, 3, 4] angiography, dynamic CT, CT during angiography [5, 6, 7] and MRI. [8]. Computed tomography during arteriography and CT during arterial portography are not currently used as gold standard for the detection and evaluation of liver tumor vascularity, since these imaging techniques are invasive for the patients. Dynamic CT using a spiral technique is noninvasive and provides information on arterial or portal supplies by scanning at different time delays after an injection of contrast agent; therefore, dynamic CT is clinically used as a standard modality for the assessment of tumor vascularity.
Microbubble contrast agents have been available for clinical use with sonography [9, 10, 11, 12, 13, 14]. Real-time gray-scale harmonic US imaging by combination of phase-inversion harmonic US and coded technology (Coded Harmonic Angio, CHA; General Electric, Milwaukee, Wis.) allows signals to be displayed from microbubbles in slow flow with the use of contrast agents [15, 16]; therefore, contrast-enhanced coded harmonic US using a microbubble contrast agent offers the potential of providing high quality and detailed vascular information. We performed this study to compare contrast-enhanced real-time gray-scale US with dynamic CT and color Doppler US in the assessment of HCC vascularity.
Materials and methods
Subjects
Between September 2000 and December 2001, 61 patients (35 men and 26 women; mean age 66 years, age range 28–81 years) with HCC were enrolled in this study (Table 1). After the subjects were given a complete description of the study, written informed consent was obtained from all of them. Sixteen patients had hepatitis B surface antigen, 37 had hepatitis C antibody, and two had both. Both hepatitis B surface antigen and hepatitis C antibody were negative in the remaining 6 patients. Diagnosis of HCC was based on histological findings from fine-needle (21-G) biopsy in 42 nodules, and the diagnosis in the remaining tumors was based on imaging findings and tumor markers (the elevation of serum alpha-fetoprotein or serum protein-induced vitamin K absence or antagonist II). In summary, we defined HCC as a positively enhanced lesion in the arterial phase of dynamic CT. When it was a negatively enhanced lesion, it was always diagnosed by liver biopsy.
US examination
Fundamental B-mode US, color Doppler, and power Doppler US were performed before contrast-enhanced coded US. Color/power Doppler US were performed by one sonologist (M.K.) with US equipment (Aloka 5500, Aloka, Tokyo, Japan) and a 3- to 5-MHz curved-array probe. The color gain was manipulated until the color noise first became apparent at the region of interest in the image background on the color/power Doppler US scans. A low pulse repetition frequency and a medium or low wall filter were used to detect tiny color signals in the small nodules. An analysis of the Doppler spectrum using pulsed Doppler waveform analysis for color/power Doppler signals was performed to evaluate the presence of pulsatile or continuous waveform.
The SH U 508A (Levovist, Schering, Berlin, Germany) was used as the US contrast agent. This agent was prepared by shaking it with 7 ml of water for 5–10 s. After standing for 2 min for equilibration, 8 ml of the contrast agent suspension (300 mg/ml concentration) was injected manually through a 21-G cannula inserted in an antecubital vein at a speed of 1 ml/s followed by an additional 10 ml of physiological saline to flush. Contrast-enhanced coded harmonic US was performed using US equipment (Logiq 700MR Expert, General Electric, Milwaukee, Wis.) with a 2- to 4-MHz curved-array wide-band transducer, which transmitted an ultrasound beam at 2.4 MHz and received 4.8 MHz in harmonic B-mode. The acoustic power of the coded harmonic US was set at the setting with a mechanical index of 0.6–0.8. The protocol for the contrast-enhanced coded harmonic US is shown in Fig. 1. We confirmed a scanning area displaying both the tumor and surrounding hepatic parenchyma before injection of the contrast agent. After injection of the contrast agent, the patient held his breath. The US images at the early vascular phase were obtained in a real-time manner by slowly changing the scanning plane to observe the whole area of the nodule 40 s after the injection. Intermittent contrast harmonic images at the late vascular phase were obtained for 40–120 s after the injection. Because these intermittent images are obtained at 2- to 3-s intervals, bubbles can flow into the small parenchymal vessels or the sinusoids, and thereafter they are destroyed; therefore, these images depict the vascularity as large numbers of bubbles being destroyed. There were no complications in contrast-enhanced US. We used two different types of US equipment, Aloka 5500 and General Electric Logiq 700MR Expert. Because we found that color/power Doppler US using Aloka 5500 equipment was more sensitive for the detection of tumor vessels than that of General Electric Logiq 700MR Expert, Aloka 5500 was used to examine color/power Doppler US.
CT examination
Computed tomography (Somatom Plus, Siemens, Forchheim, Germany) was performed using a spiral technique. Unenhanced images were acquired first and were followed by dual-phase contrast enhancement during the power injection of 100 ml of iopamidol (Iopamiron, Nihon-Schering, Osaka, Japan) at the rate of 2.7 ml/s. The entire liver was scanned twice. Arterial phase imaging was initiated at 30 s, and portal venous phase imaging at 120 s after initiation of the injection. All scans were obtained with 7-mm collimation. Five nodules in 5 cases could not evaluated by enhanced CT due to allergy. Vascularity of the tumor on CT was classified as follows: “hypervascular” or “hypovascular” was defined as higher density, or lower density than that of the surrounding liver parenchyma at the arterial phase, respectively. “Isovascular” was defined as the same density as that of the surrounding liver parenchyma.
Image analysis
To minimize the procedural variations, color/power Doppler and contrast-enhanced coded US were performed using the same examination protocol by the same sonologist (M.K.). The imaging data were recorded on videotapes for subsequent review by two other sonologists (Y.M., M.U.). The two readers were blinded to each other’s findings. Vascular findings at the early vascular phase were classified into two patterns: presence of tumor vessels; or absence of tumor vessels (Fig. 2). Vascular findings at the late vascular phase were classified into three patterns: hyper-enhancement, iso-enhancement, or hypo-enhancement depending on the tumor vascularity relative to the surrounding liver parenchyma (Fig. 3). Hyper-enhancement was defined as tumor perfusion flow of a higher degree than that of the surrounding liver parenchyma. Iso- or hypo-enhancement was defined as tumor perfusion flow of the same degree as or a lower degree than that of the surrounding liver parenchyma. Because the background echogenicity affects the evaluation of tumor vascularity, we divided HCC tumors by fundamental B-mode US into two groups: hypo/isoechoic nodules and hyperechoic nodules.
Vascular findings on color/power Doppler US scans were evaluated according to the modified classification proposed by Tanaka et al. [3]. In brief, a basket pattern, a fine blood-flow network surrounding the tumor, intratumoral pattern, blood-flow runs into the tumor from the periphery, and branches within the tumor were defined as the presence of tumor vessels. No color signal or small color spot, which cannot be confirmed as a waveform analysis, were considered as no detection of tumor vessels. Statistical analysis was performed using the chi-square test or Fisher’s exact probability test. A p value of <0.05 was considered significant.
Results
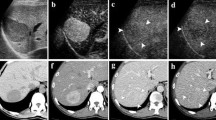
Ninety nodules in 61 patients were divided by the background echogenicity of fundamental B-mode US into 72 hypo/isoechoic nodules and 18 hyperechoic nodules (Table 1). Because four nodules could not be evaluated by dynamic CT, 68 hypo/isoechoic nodules were divided into 62 nodules with high density, 4 nodules with isodensity, and 2 nodules with low density at the arterial phase of dynamic CT (Table 2). In 62 hypo/isoechoic nodules with high density by dynamic CT, tumor vessels at the early vascular phase of contrast-enhanced coded US were demonstrated in 60 (97%; Fig. 4), whereas tumor vessels by color/power Doppler US were demonstrated in 49 (79%), and pulsatile flow was seen in 41 (69%). Three HCCs adjacent to the heart or large vein could not be evaluated for tumor vessels by color/power Doppler US due to motion artifact. Hyper-enhancement at the late vascular phase of contrast-enhanced coded US was demonstrated in 48 (77%) of 62 HCCs and iso-enhancement was seen in 12 (19%) of 62 HCCs. Two nodules in which tumor vessels were not demonstrated at the early vascular phase and hypo-enhancement was demonstrated at the late vascular phase, were located at 8- or 11-cm depth from the abdominal wall.
Case 1. A 65-year-old woman with a 1.8-cm hepatocellular carcinoma in liver segment VIII. a Arterial phase of dynamic CT scan reveals a hypervascular mass (arrow). b Conventional B-mode US shows a hypoechoic nodule (arrow). c Contrast-enhanced coded US at the early vascular phase clearly shows a feeding artery (black arrow) into the tumor nodule (white arrow). d Contrast-enhanced coded US at the late vascular phase shows hyper-enhancement (arrow) indicating tumor perfusion flow
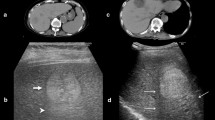
In six hypo/isoechoic nodules in which the arterial phase of dynamic CT showed iso/low density, tumor vessels at the early vascular phase of contrast-enhanced coded US were demonstrated in 5 (83%) of 6 nodules. Four (80%) of these 5 HCCs were proved to be hypervascular by CT during arteriography (Fig. 5); therefore, in these cases contrast-enhanced coded US is more sensitive than the arterial phase of dynamic CT.
Case 2. A 67-year-old woman with a 1.8-cm hepatocellular carcinoma in liver segment VIII. a Arterial phase of dynamic CT scan reveals no tumor (arrow). b Portal phase of dynamic CT scan reveals a perfusion defect (arrow). c Computed tomography scan during arteriography reveals a small stain (black arrow). d Contrast-enhanced coded US at the early vascular phase shows a tiny flow into the tumor (arrow). e Contrast-enhanced coded US at the late vascular phase (interval of 2 s) shows a vascular spot (black arrow) in hypo-enhancement (white arrow). f Contrast-enhanced coded US at the late vascular phase (interval of 3 s) shows a hypervascular lesion (black arrow) in hypo-enhancement (white arrow)
When the detection of tumor vessels in 72 hypo/isoechoic nodules was compared between contrast-enhanced coded US and color/power Doppler US (Table 3), contrast-enhanced coded US detected tumor vessels in all 53 nodules in which tumor vessels were demonstrated by color/power Doppler US, and detected tumor vessels in 13 of 16 nodules in which tumor vessels were not demonstrated by color/power Doppler US.
In 10 hyperechoic HCCs with high density at the arterial phase of dynamic CT (Table 4), tumor vessels at the early vascular phase of contrast-enhanced coded US were demonstrated in 7 (70%), whereas tumor vessels by color/power Doppler US were found in 5 (50%) and pulsatile flow was seen in 4 (40%). Hyper-enhancement at the late vascular phase of contrast-enhanced coded US was demonstrated in 4 (40%) of 10 HCCs and iso-enhancement was seen in 6 (60%).
In 7 hyperechoic HCC nodules with iso/low density by dynamic CT, tumor vessels at the early vascular phase of contrast-enhanced coded US were demonstrated in one (14%). Six (86%) of the 7 HCCs showed iso-enhancement at the late vascular phase of contrast-enhanced coded US; however, we could not accurately determine whether this finding is the result of enhancement in hyperechoic nodules due to their background echogenicity (Fig. 6).
Case 3. A 67-year-old woman with a 1.6-cm hepatocellular carcinoma in liver segment V. a Arterial phase of dynamic CT scan reveals a hypervascular mass (arrow). b Portal phase of dynamic CT scan reveals perfusion a defect (arrow). c B-mode US shows a hyper-echoic mass (arrow). d Contrast-enhanced coded US at the early vascular phase shows a tiny flow into the tumor (arrow). e Contrast-enhanced coded US at the late vascular phase shows iso-enhancement (arrow), but it is difficult to evaluate the enhancement due to the hyperechoic tumor
The detection rates of tumor vessels and vascularity according to tumor size by contrast-enhanced coded US or color/power Doppler US in 72 nodules (62 hypo/isoechoic nodules and 10 hyperechoic nodules) with high density by the arterial phase of dynamic CT are shown in Table 5. There was no significant difference in the detection rates of tumor vessels and vascularity between nodules <2 cm and those ≥2 cm in diameter.
Discussion
It is important to study intratumoral hemodynamics for the differential diagnosis of hepatic focal lesions. In fact, dynamic CT, CT during angiography, dynamic MRI, and angiography are widely performed as useful modalities for the diagnosis of HCC. Contrast-enhanced coded US enables clear visualization of blood flow into the tumor from the artery to very tiny vessels after bolus injection of Levovist (Fig. 4). The hemodynamics of classical HCC are characterized by tumor vessels, hypervascularity, and the absence of portal blood flow [5, 6, 7]. Although conventional color/power Doppler US is able to show tumor vessels, it is not always satisfactory for evaluating hypervascularity quantitatively [3, 4]. In this study, contrast-enhanced coded US clearly demonstrates tumor vessels at the early vascular phase and hypervascularity at the late vascular phase. The detection rate of tumor vessels by contrast-enhanced coded US was apparently higher than that by conventional color/power Doppler US in agreement with a previous report [15]. Motion artifact of conventional color/power Doppler US inhibits the evaluation of tumor hemodynamics in the lesions located adjacent to the heart or large vessels. In fact, 3 of 72 nodules could not be evaluated for tumor hemodynamics due to motion artifact. On the other hand, contrast-enhanced coded US is not affected by tissue motion. Furthermore, the detection rate of tumor vessels by contrast-enhanced coded US in hypervascular nodules was not affected by the size of the lesions; however, the evaluation of tumor hemodynamics by contrast-enhanced coded US was affected by the depth from the abdominal wall like with color/power Doppler US [16]. In fact, two nodules, which did not demonstrate tumor vessels by contrast-enhanced coded US, were located deeper than 8 cm from the abdominal wall. These findings indicated that contrast-enhanced coded US is superior to conventional color/power Doppler as previous articles have reported [10, 14], at least in hypo/isoechoic nodules.
Tumor vessels at the early vascular phase were obtained even in nodules with iso- or low density by the arterial phase of dynamic CT. Contrast-enhanced coded US is able to display arteries in more detail than dynamic CT. Practically, tortuous tiny tumor vessels could be demonstrated in real-time by contrast-enhanced coded US (Fig. 4). The limitation of contrast-enhanced coded US is that only one nodule of interest can be observed in a dynamic image at each injection of Levovist, whereas dynamic CT can scan the total liver in a dynamic study; therefore, contrast-enhanced coded US is suitable to observe a specified nodule in detail.
We proposed our analysis method to estimate tumor vascularity objectively, i.e., enhancement findings at the late vascular phase were classified into three patterns: hyper-, iso-, and hypo-enhancement compared with the surrounding liver tissue. Although some investigators estimated tumor vascularity as positive enhancement or tumor stain [15, 16, 17, 18], they did not distinguish between hyper- and iso-enhancement. Intermittent harmonic imaging transmits a US beam at a constant interval to destroy most of the microbubbles in the scan area and refreshes the bubble flow into the plane with refreshed blood at the next interval. Intermittent images at the late vascular phase show blood inflow volume into the nodule at intervals of 2 or 3 s. Hyper-enhancement indicates that blood inflow into the nodules increases compared with the surrounding liver tissue; therefore, there is no doubt that nodules with hyper-enhancement are hypervascular. In contrast, 12 of 62 nodules with high density by the arterial phase of dynamic CT were iso-enhancement at the late vascular phase. All of these 12 nodules had tumor vessels at the early vascular phase. In general, hypervascular HCCs are only fed by the hepatic artery and have no portal inflow, whereas the surrounding liver tissue is supplied by both the hepatic artery and portal vein. Therefore, nodules with tumor vessels at the early vascular phase and with iso-enhancement at the late vascular phase are indicated to have the same blood inflow from only the artery as the surrounding liver tissue supplied by both the artery and portal vein; thus, these nodules appear to derive their blood supply increase from the hepatic artery.
We encountered a problem in estimating the vascularity of hyperechoic nodules at the late vascular phase by contrast-enhanced coded US. It might be difficult to distinguish echogenicity by contrast agents from background echogenicity of tissue images because contrast-enhanced coded US is in a gray-scale format; therefore, in our study there was no hypo-enhancement at the late vascular phase in hyperechoic nodules, and there may have been some false-positive nodules due to this.
Some benign hepatic nodules, mainly focal nodular hyperplasia (FNH) and hemangioma, are reported to show arterial hypervascularity [14, 19, 20, 21, 22]. All FNH show marked homegenous enhancement in the late vascular phase and moreover half of FNH show a “central stellate” or “spoke-wheel” pattern of vascularity within the nodule in the early vascular phase [19]. While hemangioma shows a subtle nonspecific enhancement in the early vascular phase and peripheral globular enhancement in the late vascular phase and the progressive centripetal fill-in pattern [21]; however, these images are observed only in low percentage of cases of liver hemangioma; therefore, FNH without a central stellate pattern and hemangioma without peripheral globular enhancement may be difficult to distinguish from HCC. While a minority of HCCs, especially early-stage well-differentiated HCCs, are hypovascular, they often have fatty metamorphosis and are visualized as hyperechoic nodules. In this study, 7of 17 hyperechoic HCCs were iso or hypoenhancement at the arterial phase of dynamic CT. These hypovascular HCCs should be differentiated from many other hypovascular lesions [23]. Differentiation from other hepatic nodular lesions associated with liver cirrhosis, such as adenomatous hyperplasia, is most important clinically; however, because the arterial blood supply in these nodules decreases or does not change, these nodules can not be differentiated by estimation of the arterial phase. Recently, in post-vascular imaging (beyond 5 min) following administration of contrast agents, HCCs are reported to appear as echo-poor filling defects in a background of bright liver [24]. This imaging may be useful to differentiate the hypovascular HCCs from other hepatic nodules
Transcather arterial embolization, percutaneous ethanol injection, or percutaneous radio-frequency ablation are performed as nonsurgical treatments for HCC. Complete necrosis of HCC needs to be achieved with these therapeutic procedures; therefore, it is important to determine by imaging modalities whether the treated tumor is completely necrotic or not. Dynamic CT or MR imaging is generally used to assess the effectiveness of the treatment. Even though these methods can depict residual areas in tumors after treatment, they do not enable real-time guidance of percutaneous therapy. Absence of tumor vessels at the early vascular phase and absence of enhancement at the late vascular phase in contrast-enhanced coded US indicate successful treatment. When tumor vessels or enhancement remain, residual areas in tumors can be accurately treated repeatedly under US guidance [16, 25, 26]; therefore, assessment of intratumoral hemodynamics is also useful to evaluate therapeutic efficacy.
Conclusion
In conclusion, contrast-enhanced coded harmonic US detects individual liver masses within the liver away from the major liver veins and within 8–10 cm from the abdominal wall with each injection of contrast agent and may provide the most detailed information on tumor vessels and vascularity, at least in hypo/isoechoic nodules.
References
Winter TC III, Takayasu K, Muramatsu Y, Furukawa H, Wakao F, Koga H, Sakamoto M, Hirohashi S, Freeny PC (1994) Early advanced hepatocellular carcinoma: evaluation of CT and MR appearance with pathologic correlation. Radiology 192:379–387
Takayasu K, Furukawa H, Wakao F, Muramatsu Y, Abe H, Terauchi T, Winter TC III, Sakamoto M, Hirohashi S (1995) CT diagnosis of early hepatocellular carcinoma: sensitivity, findings, and CT-pathologic correlation. Am J Roentgenol 164:885–890
Tanaka S, Kitamura T, Fujita M, Nakanishi K, Okuda S (1990) Color Doppler flow imaging of liver tumors. Am J Roentgenol 154:509–514
Lencioni R, Pinto F, Armillotta N, Bartolozzi C (1996) Assessment of tumor vascularity in hepatocellular carcinoma: comparison of power Doppler US and color Doppler US. Radiology 201:353–358
Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M (1991) Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology 178:493–497
Takayasu K, Muramatsu Y, Furukawa H, Wakao F, Moriyama N, Takayama T, Yamasaki S, Sakamoto M, Hirohashi S (1995) Early hepatocellular carcinoma: appearance at CT during arterial portography and CT arteriography with pathologic correlation. Radiology 194:101–105
Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Nonomura A, Nakanuma Y (1999) Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. Am J Roentgenol 172:969–976
Ward J, Robinson PJ (2002) How to detect hepatocellular carcinoma in cirrhosis. Eur Radiol 12:2258–2272
Fujimoto M, Moriyasu F, Nishikawa K, Nada T, Okuma M (1994) Color Doppler sonography of hepatic tumors with a galactose-based contrast agent: correlation with angiographic findings. Am J Roentgenol 163:1099–1104
Kim AY, Choi BI, Kim TK, Han JK, Yun EJ, Lee KY, Han MC (1998) Hepatocellular carcinoma: power Doppler US with a contrast agent—preliminary results. Radiology 209:135–140
Choi BI, Kim TK, Han JK, Kim AY, Seong CK, Park SJ (2000) Vascularity of hepatocellular carcinoma: assessment with contrast-enhanced second-harmonic versus conventional power Doppler US. Radiology 214:381–386
Lencioni R, Cioni D, Bartolozzi C (2002) Tissue-harmonic and contrast-specific imaging: back to gray scale in ultrasound. Eur Radiol 12:151–165
Jakobsen JA (2001) Ultrasound contrast agents: clinical applications. Eur Radiol 11:1329–1337
Harvey CJ, Albrecht T (2001) Ultrasound of focal liver lesions. Eur Radiol 11:1578–1593
Hirai T, Ohishi H, Tokuno E, Takahashi M, Sakaguchi H, Anai H, Nishimoto Y, Hirohashi S, Kichikawa K (2002) Qualitative diagnosis of hepatocellular carcinoma by contrast-enhanced ultrasonography using coded harmonic angio with Levovist. J Med Ultrasonics 29:3–9
Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Chung H, Kawasaki T, Maekawa K (2001) Evaluation of post-treatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology 221:721–730
Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Maekawa K (2001) Hepatocellular carcinoma: depiction of tumor parenchymal flow with intermittent harmonic power Doppler US during the early arterial phase in dual-display mode. Radiology 220:349–356
Ding H, Kudo M, Maekawa K, Suetomi Y, Minami Y, Onda H (2001) Detection of tumor parenchymal blood flow in hepatic tumors: value of second harmonic imaging with a galactose-based contrast agent. Hepatol Res 21:242–251
Leen E (2001) The role of contrast-enhanced ultrasound in the characterisation of focal liver lesions. Eur Radiol 11:E27–E34
Dill-Macky MJ, Burns PN, Khalili K, Wilson SR (2002) Focal hepatic masses: enhancement patterns with SH U 508A and pulse-inversion US. Radiology 222:95–102
Quaia E, Bertolotto M, Dalla Palma L (2002) Characterization of liver hemangiomas with pulse-inversion harmonic imaging. Eur Radiol 12:537–544
Lencioni R, Cioni D, Crocetti L, Donati F, Franchini C, Giusti S, Bartolozzi C (2002) Ultrasound imaging of focal liver lesions with a second-generation contrast agent. Acad Radiol 9:S371–S374
Fracanzani AL, Burdick L, Borzio M, Roncalli M, Bonelli N, Borzio F, Maraschi A, Fiorelli G, Fargion S (2001) Contrast-enhanced Doppler ultrasonography in the diagnosis of hepatocellular carcinoma and premalignant lesions in patients with cirrhosis. Hepatology 34:1109–1112
Blomley MJ, Sidhu PS, Cosgrove DO, Albrecht T, Harvey CJ, Heckemann RA, Butler-Barnes J, Eckersley RJ, Basilico R (2001) Do different types of liver lesions differ in their uptake of the microbubble contrast agent SH U 508A in the late liver phase? Early experience. Radiology 220:661–667
Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Maekawa K (2001) Contrast-enhanced subtraction harmonic sonography for evaluating treatment response in patients with hepatocellular carcinoma. Am J Roentgenol 176:661–666
Cioni D, Lencioni R, Rossi S, Garbagnati F, Donati F, Crocetti L, Bartolozzi C (2001) Radiofrequency thermal ablation of hepatocellular carcinoma: using contrast-enhanced harmonic power Doppler sonography to assess treatment outcome. Am J Roentgenol 177:783–788
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koda, M., Matsunaga, Y., Ueki, M. et al. Qualitative assessment of tumor vascularity in hepatocellular carcinoma by contrast-enhanced coded ultrasound: comparison with arterial phase of dynamic CT and conventional color/power Doppler ultrasound. Eur Radiol 14, 1100–1108 (2004). https://doi.org/10.1007/s00330-003-2172-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-2172-5