Abstract
The aim of this study was to assess the reproducibility and anatomical accuracy of automated 3D CT angiography analysis software in the evaluation of carotid artery stenosis with reference to rotational DSA (rDSA). Seventy-two vessels in 36 patients with symptomatic carotid stenosis were evaluated by 3D CT angiography and conventional DSA (cDSA). Thirty-one patients also underwent rotational 3D DSA (rDSA). Multislice CT was performed with bolus tracking and slice thickness of 1.5 mm (1-mm collimation, table feed 5 mm/s) and reconstruction interval of 1.0 mm. Two observers independently performed the stenosis measurements on 3D CTA and on MPR rDSA according to the NASCET criteria. The first measurements on CTA utilized an analysis program with automatic stenosis recognition and quantitation. In the subsequent measurements, manual corrections were applied when necessary. Interfering factors for stenosis quantitation, such as calcifications, ulcerations, and adjacent vessels, were registered. Intraobserver and interobserver correlation for CTA were 0.89 and 0.90, respectively. (p<0.001). The interobserver correlation between two observers for MPR rDSA was 0.90 (p<0.001). The intertechnique correlation between CTA and rDSA was 0.69 (p<0.001) using automated measurements but increased to 0.81 (p<0.001) with the manually corrected measurements. Automated stenosis recognition achieved a markedly poorer correlation with MPR rDSA in carotids with interfering factors than those in cases where there were no such factors. Automated 3D CT angiography analysis methods are highly reproducible. Manually corrected measurements facilitated avoidance of the interfering factors, such as ulcerations, calcifications, and adjacent vessels, and thus increased anatomical accuracy of arterial delineation by automated CT angiography with reference to MPR rDSA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis of the carotid arteries is of a considerable clinical significance since it is associated with a high prevalence of serious complications, in particular ischemic brain infarction [1, 2]. It is considered as one of the major disorders which contributes to death due to atherosclerosis. It has been established that symptomatic patients with severe carotid stenosis (70–99%) benefit from carotid endarterectomy [3, 4, 5]. A clinical trial suggested that also patients with moderate stenosis (50–69%) could benefit from carotid endarterectomy [4]. Digital subtraction angiography is widely accepted as the standard method in the evaluation of carotid artery disease [6, 7]. The criteria from the North American Symptomatic Carotid Endarterectomy Trial (NASCET) are most commonly used in the evaluation of the degree of stenosis present in the carotid arteries [8, 9, 10, 11, 12]; however, previous studies have reported a total complication rate of 5% and permanent complication rate of 0.5% associated with digital subtraction angiography [13, 14, 15]. Other less invasive modalities are increasingly used as alternative methods to evaluate the degree of carotid stenosis [11, 12, 16, 17].
The rapid development of CT technology has resulted in exciting applications of CT angiography in the noninvasive evaluation of atherosclerotic manifestations. Both three-dimensional and two-dimensional postprocessing methods have been applied in the evaluation of carotid stenosis. Automated 3D CT angiography analysis software has recently become commercially available from various manufacturers. With the application of automated 3D CT angiography analysis software, it is possible to obtain both 3D visual evaluation and quantitation of the vessels with automatic detection of minimal diameters at the level of maximal stenosis.
The principle of automated 3D CT angiography analysis (Advanced Vessel Analysis, GE medical systems, Milwaukee, Wis.) relies on region-growing techniques from a start point determined by the observer. The trajectory is built in an iterative way, step by step, going into the direction of the end point. The algorithm will detect trajectories where the density along the trace remains high in amplitude. It also detects density changes perpendicular to the calculated trace, in order to create the vessel contours; thus, automated 3D CT angiography analysis is designed to make it easy to manipulate the vessels to be assessed by the observer. Most of the postprocessing work is thus done by the computer; however, the reproducibility and accuracy of this new image reconstruction technique in the evaluation of carotid artery stenosis still remains to be fully determined.
The main purpose of the present study was to evaluate the reproducibility and anatomical accuracy of automated 3D CT angiography analysis method. Since conventional planar DSA has its well-known weaknesses due to overlapping vessels and limited number of imaging projections, we used as the reference method the best currently available imaging modality for stenosis quantitation that is rotational digital subtraction angiography with multiplanar reconstruction technique (MPR rDSA).
Materials and methods
Patients
Thirty-six patients with symptomatic carotid artery stenosis confirmed by ultrasound and digital subtraction angiography were included in our patient series. The age of the patients ranged from 50 to 83 years (mean age 68.2 years). There were 21 men and 15 women. Informed consent was obtained from the patients. The study protocol was approved by the ethics committee of our institute.
Angiography
Selective angiographies were carried out for 72 vessels in 36 patients using biplane DSA equipment (Siemens Neurostar Plus, Forchheim, Germany). Each bifurcation was imaged in four projections with 5–6 ml of nonionic contrast media (Visipaque 270mgI/ml, Nycomed, Oslo, Norway) per injection and a flow rate of 8 ml/s. In addition, rotational DSA was performed on one carotid artery from each patient, provided that the radiologist anticipated the patient was capable of staying motionless for the 24 s needed to complete the entire imaging protocol. Rotational angiography (rDSA) was made randomly on either the symptomatic or contralateral side to gather a wide range of all degrees of stenosis for statistical purposes. Altogether 33 vessels in 31 patients were imaged using rDSA. In rotational angiography, 23 ml of contrast media were injected at a flow rate of 2.5 ml/s. The imaging started in a lateral position and rotated 180° in 8 s around the carotid bifurcation to acquire 32 projections. A 512×512 image-intensifier matrix was used.
CT angiography
All patients underwent carotid artery scanning with a multislice CT (Siemens Volume Zoom, Forchheim, Germany). The scanning parameters included slice thickness of 1.5 mm (1-mm collimation, table feed 5 mm/s) and a reconstruction interval of 1.0 mm. The contrast agent (Ultravist 300 mgI/ml, Schering, Berlin, Germany) volume for CTA was 80 ml with a flow rate of 3 ml/s and a saline chaser bolus of 30 ml via an 1.3 mm (18-G) cannula through the antecubital vein. The bolus tracking method was used for detecting the optimal delay time for CT scanning to optimize the contrast enhancement in carotid arteries. Spiral scanning included the volume between the sixth cervical vertebra and the level above the circle of Willis. Patients were asked to breathe evenly and smoothly without swallowing or moving.
Evaluation of the imaging studies
The CT data were analyzed independently by two radiologists. The image quality of the axial source images was assessed on axial and reconstructed 3D images as good, suboptimal but diagnostic, or poor.
The axial images were created with the soft tissue algorithm. The axial raw data was loaded on a workstation (ADW 4.0, GE Medical Systems, Milwaukee, Wis.). Window width of 700 and window level of 200 were selected for the measurements. Firstly, the observer briefly viewed the axial scans to obtain a general overview of the vessel, such as the location of the common and the internal carotid arteries, the possible sites of stenosis, calcifications, ulcerations, and the relation of carotid artery with bypassing veins. Then, the automated 3D CT angiography analysis program was used to evaluate the stenosis degree of both carotid arteries.
Detailed diameter measurement values were registered on CTA by two observers both at the site of the maximal stenosis and at the site of the reference level in the ICA distal to the carotid bulb. Focal areas of very high density within the vessel wall were interpreted as calcification. Filling pouches of contrast media within the intima-media complex were interpreted as ulceration. Bypassing vessels, most commonly internal jugular veins, passing adjacent to the carotids at the level of measurements, were registered. The length of carotid stenosis was measured and stenotic segments shorter than 0.5 cm were classified as short-segment stenosis. Low intraluminal contrast media density was also registered visually.
The rotational DSA images were analyzed with a workstation (Siemens Virtuoso, Forchheim, Germany).
The image quality of both rotational DSA and MPR rDSA was assessed as good or poor by two independent observers. The quality of the rotational angiogram was considered to be diagnostic when contrast material filled the ICA with sufficient density to allow reliable measurements of diameter of the distal ICA and minimal residual lumen. The quality of the MPR rDSA images were registered as good when intraluminal contrast media was concentrated enough to visualize the morphology at the measured site.
Measurements
CTA
Automatic recognition
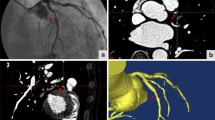
The workstation automatically displays MPR reconstructions on coronal, sagittal, and axial views. With the aid of those three views, the target vessel can be traced from the lower portion of the common carotid artery to the uppermost portion of the extracranial internal carotid artery. The workstation automatically displays the MIP images, lumen images, and oblique images of the full length of the extracranial carotid arteries. After manual selection of the level of reference and the lower and upper margins of the stenosis on the lumen image, the workstation automatically assesses the level of maximal stenosis. The workstation then reports the following measurements: reference diameter; minimal diameter; and stenosis degree according to the NASCET criteria (Fig. 1).
a Conventional digital subtraction angiography (DSA) demonstrates left internal carotid stenosis (arrow). b After rotational DSA, the multiplanar reconstruction technique (MPR) rDSA image was used for diameter measurements. c Reconstructed 3D CTA image excellently reveals the lesion. In d “straightened” vessel demonstrates sites of reference, start of stenosis, end of stenosis and the maximal stenosis. The histographic curve in d also demonstrates the site of maximal stenosis. e, f Diameter measurements are performed automatically at the level of maximal stenosis and at the level of reference in the internal carotid artery. Note the existence of the external carotid artery in the corresponding slices (arrowheads)
Manual correction
Two months after the first measurements, the same observer performed the measurements for the second time; however, after automatic recognition, manual corrections were now applied to correct for the measurements from automatic recognition when the ROI indicator was visually estimated to be out of the range of the true vessel lumen.
The manually corrected 3D CTA analysis was independently performed by two observers, and one observer repeated the manually corrected 3D CTA measurements with an interval of 1 month.
The stenosis degree was estimated to be 95% if no lumen was seen at the level of stenosis, but contrast media filling was noted in the distal internal carotid artery.
MPR rDSA
For the measurements on rDSA, the MPR technique (MPR rDSA) was used. The site of maximal stenosis, and the reference point distal to the carotid bulb were determined. Double oblique views of the MPR images were used to make the longitudinal axis of the measured vessel segment perpendicular to the measured slice. The shortest diameters at both the maximal stenosis points and at the reference points were used to calculate the final stenosis degree according to the NASCET criteria. Two radiologists performed the measurements independently.
Statistical analysis
Pearson correlation coefficient was used to assess the intertechnique, intraobserver and interobserver reproducibility of automated 3D CT angiography analysis, and MPR rotational DSA. The intertechnique correlation was separately evaluated between the automatic recognition and manual correction on CT angiography and rDSA. The intraobserver and interobserver reproducibilities were evaluated on CT angiography. Interobserver reproducibility was also evaluated on MPR rDSA. Statistic kappa was used to analyze the intraobserver and interobserver agreement in measurements on 3D CTA. Coefficient of variation (CV) was used to quantitatively estimate the variability of the measurements on 3D CTA.
Results
Image quality
Rotational digital subtraction angiography with multiplanar reconstruction was obtained in all 33 carotids with good diagnostic quality. All 3D CTA images in the 72 carotids evaluated were diagnostic with 67 vessels being of good quality (Fig. 1). The image quality of five vessels was suboptimal but still diagnostic, the poor quality being due to high densities caused by high concentrations of contrast media in the adjacent jugular vein (n=2), a low intraluminal low concentration of contrast media in both carotid arteries (n=2), or a motion artifact (n=1) in one carotid artery bifurcation, whereas the motion artifact was avoided on the other side by the upper location of the contralateral carotid bifurcation.
Agreement and reproducibility
The categorical NASCET system was used in the agreement and reproducibility assessment. The percentages of 0–29, 30–49, 50–69, 70–99, and 100 were used to categorize the degree of stenosis. Altogether 12 of 72 carotid arteries changed from their original stenosis category in the five-point grading scale to another stenosis category after manual correction; however, only one stenosis changed across 70% cut-off point. In the comparison between the two manually corrected 3D CT angiography measurements by the same radiologist, 49 (68%) of the 72 vessels exactly agreed with each other according to NASCET categories. In the comparison between two observers, 43 (64%) of 67 vessels agreed with each other according to NASCET categories. There was good intraobserver agreement and satisfactory interobserver agreement on automated 3D CTA measurements (Table 1). There was satisfactory interobserver correlation for MPR rDSA measurements, and excellent intraobserver and interobserver correlation for 3D CTA measurements (Table 2). The interobserver correlation of 3D CTA was equal to that of MPR rDSA. Correlation further improved after manual corrections were performed on the 3D CTA measurements. Diagnostic performance by sensitivity, specificity, and overall accuracy for automated CTA with reference to conventional DSA (n=72) were 0.52 (12 of 23), 0.94 (46 of 49), and 0.81 (58 of 72), the corresponding values for manually corrected CTA being very similar at 0.47 (11 of 23), 0.94 (46 of 49), and 0.79 (57 of 72).
Interfering factors
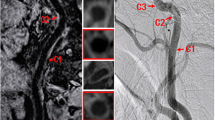
Intramural calcifications were found to be the most common interfering factors (Fig. 2). There were 14 (42%) calcifications and 5 (15%) ulcerations at the level of measurements. The other interfering factors included bifurcating vessels (n=2), adjacent bypassing vessels (n=2), partial-volume effects from short segment stenosis (n=2), and intraluminal low contrast media density (n=4). An interfering factor was registered in 27 arteries at the level of the stenosis and in 6 cases at the reference level.
Various interfering factors on 3D CTA analysis. a, b Intramural calcification (curved arrow) causing region-of-interest (ROI) indicator mismatching. There are multiple interfering factors in b, including the coexistence of ulceration (open arrow) and carotid bifurcation (arrowhead) at the level of stenosis. Intramural calcification (curved arrow) is also detected in this slice. A bypassing internal jugular vein (long arrow in b, c) also interferes with the measurement (c). d The low contrast media concentration in internal carotid artery decreases the density difference between the internal carotid artery and peripheral soft tissue, leading to mismatching of ROI indicator. Note the ipsilateral external carotid artery in the same slice (arrowhead). The internal jugular vein is lateral to the internal carotid artery (long arrows in a–c)
Vessels with and without interfering factors had marked differences both on coefficient of variation and on correlation (Table 3).
Discussion
Computed tomography angiography is a relatively new and minimally invasive technique where the patient is given an intravenous bolus injection of contrast media followed by high-speed spiral scanning and computer-assisted generation of images of the arteries in the region to be scanned. In the cerebral circulation, CTA has been involved in many disease entities, such as cerebral aneurysm and carotid stenosis [18, 19]. The 2D CTA techniques include axial CT and MPR CT. The 3D CT angiographic techniques include maximal intensity projection (MIP), surface-shaded display (SSD), and volume rendering (VR). Recent studies have demonstrated that there are good correlations between CTA and DSA [14, 20]; however, there are still some problems in the CT angiographic evaluation. For example, 2D CTA lacks a 3D display and 3D CTA is time-consuming [17].
Conventional DSA has been widely accepted as the standard of reference in the evaluation of carotid stenosis with good reproducibility [7, 21]; however, a recent publication indicated that DSA has a tendency to underestimate the degree of stenosis [22]. The rDSA may be used as an alternative standard in the evaluation of carotid stenosis. In our study, we used the MPR reconstruction of rotational DSA as the reference technique. The reliability of the reference method was verified by the excellent interobserver correlation for MPR rDSA measurements (r=0.90).
The main purpose of the present study was to evaluate the reproducibility of the techniques; thus, intraobserver and interobserver correlation and agreement were analyzed with correlation coefficient, coefficient of variation, and categorical kappa test. A review of the literature revealed several reproducibility studies in CTA (Table 4) [16, 22, 23, 24, 25], but no reports on automated 3D CT angiography analysis methods. We obtained good correlation coefficients for interobserver (r=0.90) and intraobserver (r=0.89) measurements on 3D CT angiography analysis, which are not only similar to other reported data for other CTA techniques, but also identical to the intratechnique correlation of MPR rDSA in present study (Table 2). By dividing the vessels into groups according to the clinical use, using the cut-off point of 50% and the cut-off point of 70%, the results demonstrated a trend toward better agreement (k=0.78 for intraobserver, k=0.59 for interobserver) at the cut-off point of 70%. During automated measurements, the ROI indicators sometimes matched poorly with the actual contour of the measured vessels. When we manually corrected the observations by ROI indicators during measurements, the correlation coefficient increased from 0.69 to 0.81 (Table 2). Based on excellent intra- and interobserver reproducibility and good anatomical accuracy (correlation) with reference to MPR rDSA, automated CTA (manually corrected, if necessary) seems clinically useful, and a detailed analysis of the diagnostic performance of this technique in comparison with fully manual stenosis quantification techniques of CTA is warranted.
In the previous literature on carotid stenosis assessment with CTA, calcifications and ulcerations have been found to be responsible for most of the discrepancies in the measurement of degree of stenosis [25, 26, 27, 28]. In our study, other interfering factors were also included, such as bifurcating vessel branches, bypassing vessels (usually veins), short segment stenosis, and a low concentration of contrast media in measured vessels (Fig. 2). Intramural calcification was the main interfering factor also in our patient series, accounting for 14 of 33 carotids. A calcification with high density leads to decreases in the density difference between intraluminal contrast media and calcification, making it difficult for the software to detect the exact boundary of enhanced carotids (Fig. 2a). It is sometimes difficult to determine the true contour of carotid even by manual correction of the ROI indicator because of partial-volume effects. On the other hand, three carotids with intramural calcifications agreed well with MPR rDSA, having NASCET difference of <10% between 3D CTA and MPR rDSA. That probably means that the calcification provided a high enough density difference to demarcate the internal lumen diameter as opposed to soft tissue plaque, enabling accurate automated measurements on 3D CTA.
On the contrary, in case of complex stenosis with ulcerations, the outpouching ulcer enlarges the diameter of the measured vessels and this leads to an underestimation of the NASCET values (Fig. 2b). In a case of a “tunneled ulcer,” the ulcer goes to the subendothelium layer of the vessel, creating a septum between the carotid lumen and the ulcer. The “in between” location of the septum may lead to unsatisfactory demarcation from the intraluminal contrast media because of partial-volume effects or small thickness of the septum. That may also cause the mismatching of ROI indicators to the outer margin of the “tunnel,” leading to an underestimation of the degree of stenosis. In the present study, 14 calcifications and 5 ulcerations were detected and caused underestimation by CTA with the reference of rotational DSA.
When the stenosis is located close to a bifurcating vessel branch, e.g., at the flow divider of the carotids, the bifurcating vessel may also decrease the density difference, leading to mismatching of ROI indicators and discrepancies in measurements (two cases). The same reason can account for the mismatching of ROI indicator in carotids with bypassing vessels (2 cases), most commonly seen in internal jugular veins (Fig. 2c). A relatively decreased intraluminal density in the carotids may also lead to discrepancies. Extraluminal structures, such as carotid walls and perivascular soft tissues, are usually moderate in density. An insufficient concentration of contrast media in the carotid lumen may produce a similar density with the extraluminal structures, thus causing inaccurate ROI indicator matching (Fig. 2d). Four of 72 carotids were involved in this situation.
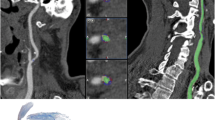
Short-segment stenosis was defined as a stenosis of <5 mm. In the case of short segment stenosis, it is sometimes difficult for the computer to recognize a surrounding overlapping vessel lumen from the normal vessel lumen (Fig. 3). The slice thickness of the CT scans was 1.5 mm, which is much shorter than the defined short-segment stenosis. The reason for the overlapping of the surrounding vessel lumen to the normal vessel lumen may partly be due to the relatively high density in the adjacent vessel wall and also partly due to the pouching of the vessel lumen to the level of the measurements. Two vessels with short-segment stenosis were involved in our patient series.
a Conventional DSA demonstrates a short-segment stenosis (arrow) at left internal carotid artery (arrowhead). b The MPR rDSA image also reveals the stenosis. c The corresponding 3D CTA image displays the stenosis; however, the measurement on 3D CTA was disrupted by partial-volume effect (curved arrows in d, e) due to short-segment stenosis (d), causing underestimation of the stenosis. Correction was made on ROI indicator to fit the contour of the measured vessel lumen (e). Note the external carotid artery (arrowheads in d, e) and internal jugular vein (straight arrows in d, e) at the slice of measurement
Stenosis percentages according to the NASCET criteria are obtained from the ratio between maximal stenosis diameter and the reference diameter. It should be stressed that not only the mismatching at the level of maximal stenosis, but also any mismatch at the level of reference will lead to interference on the final NASCET stenosis values; thus care must be taken to check the ROI indicator match at both the point of reference and the point of maximal stenosis. A bypassing internal jugular vein could be possible source of interference at the level of the reference point. A low contrast media concentration may also be one of the reasons for ROI indicator mismatching at the level of the reference point.
We selected those vessels with any of the above-mentioned interfering factors to compare them with those vessels without any interfering factors. The correlation between measurements on 3D CTA and MPR rDSA was poor (r=0.42) in vessels (n=20) with interfering factors. In contrast, the correlation became excellent (r=0.93) in vessels (n=13) without interfering factors (Table 3). There was also remarkable increase in coefficient of variation, indicating poorer agreement in vessels with interfering factors. Manual correction is recommended to avoid interference from those factors.
Conclusion
The results of the present study demonstrated that automated 3D CT angiography analysis was highly reproducible, but interfering factors, such as calcification, ulceration, bifurcating and bypassing vessels, short-segment stenosis, and low contrast concentration in vessel lumen, could lead to discrepancies in the assessment of the degree of carotid stenosis.
References
Peiper C, Nowack J, Ktenidis K, Hopstein S, Keresztury G, Horsch S (2001) Prophylactic urgent revascularization of the internal carotid artery in the symptomatic patient. Vasa 30:247–251
Levinson MM, Rodriguez DI (1999) Endarterectomy for preventing stroke in symptomatic and asymptomatic carotid stenosis: review of clinical trials and recommendations for surgical therapy. Heart Surg Forum 2:147–168
(1991) Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North american symptomatic carotid endarterectomy trial collaborators. N Engl J Med 325:445–453
Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE (1998) Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American symptomatic carotid endarterectomy trial collaborators. N Engl J Med 339:1415–1425
Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, Barnett HJ (2000) The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American symptomatic carotid endarterectomy trial collaborators. N Engl J Med 342:1693–1700
Alvarez-Linera J, Benito-Leon J, Escribano J, Campollo J, Gesto R (2003) Prospective evaluation of carotid artery stenosis: elliptic centric contrast-enhanced MR angiography and spiral CT angiography compared with digital subtraction angiography. Am J Neuroradiol 24:1012–1019
Elgersma OE, Buijs PC, Wust AF, van der Graaf Y, Eikelboom BC, Mali WP (1999) Maximum internal carotid arterial stenosis: assessment with rotational angiography vs conventional intraarterial digital subtraction angiography. Radiology 213:777–783
Mattos MA, Sumner DS, Bohannon WT, Parra J, McLafferty RB, Karch LA, Ramsey DE, Hodgson KJ (2001) Carotid endarterectomy in women: challenging the results from ACAS and NASCET. Ann Surg 234:438–446
Gray WA, White HJ Jr, Barrett DM, Chandran G, Turner R, Reisman M (2002) Carotid stenting and endarterectomy: a clinical and cost comparison of revascularization strategies. Stroke 33:1063–1070
Rotstein AH, Gibson RN, King PM (2002) Direct b-mode NASCET-style stenosis measurement and Doppler ultrasound as parameters for assessment of internal carotid artery stenosis. Australas Radiol 46:52–56
Staikov IN, Nedeltchev K, Arnold M, Remonda L, Schroth G, Sturzenegger M, Herrmann C, Rivoir A, Mattle HP (2002) Duplex sonographic criteria for measuring carotid stenoses. J Clin Ultrasound 30:275–281
Sundgren PC, Sunden P, Lindgren A, Lanke J, Holtas S, Larsson EM (2002) Carotid artery stenosis: contrast-enhanced MR angiography with two different scan times compared with digital subtraction angiography. Neuroradiology 44:592–599
Link J, Brossmann J, Penselin V, Gluer CC, Heller M (1997) Common carotid artery bifurcation: preliminary results of CT angiography and color-coded duplex sonography compared with digital subtraction angiography. Am J Roentgenol 168:361–365
Anderson GB, Ashforth R, Steinke DE, Ferdinandy R, Findlay JM (2000) CT angiography for the detection and characterization of carotid artery bifurcation disease. Stroke 31:2168–2174
Nederkoorn PJ, Mali WP, Eikelboom BC, Elgersma OE, Buskens E, Hunink MG, Kappelle LJ, Buijs PC, Wust AF, van der Lugt A, van der Graaf Y (2002) Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing. Stroke 33:2003–2008
Berg MH, Manninen HI, Rasanen HT, Vanninen RL, Jaakkola PA (2002) CT angiography in the assessment of carotid artery atherosclerosis. Acta Radiol 43:116–124
Phillips CD, Bubash LA (2002) CT angiography and MR angiography in the evaluation of extracranial carotid vascular disease. Radiol Clin North Am 40:783–798
Carvi Y, Nievas MN, Haas E, Hollerhage HG, Drathen C (2002) Complementary use of computed tomographic angiography in treatment planning for posterior fossa subarachnoid hemorrhage. Neurosurgery 50:1283–1289
Villablanca JP, Jahan R, Hooshi P, Lim S, Duckwiler G, Patel A, Sayre J, Martin N, Frazee J, Bentson J, Vinuela F (2002) Detection and characterization of very small cerebral aneurysms by using 2D and 3D helical CT angiography. Am J Neuroradiol 23:1187–1198
Cinat M, Lane CT, Pham H, Lee A, Wilson SE, Gordon I (1998) Helical CT angiography in the preoperative evaluation of carotid artery stenosis. J Vasc Surg 28:290–300
Vanninen R, Manninen H, Koivisto K, Tulla H, Partanen K, Puranen M (1994) Carotid stenosis by digital subtraction angiography: reproducibility of the European carotid surgery trial and the North American symptomatic carotid endarterectomy trial measurement methods and visual interpretation. Am J Neuroradiol 15:1635–1641
Hirai T, Korogi Y, Ono K, Murata Y, Takahashi M, Suginohara K, Uemura S (2001) Maximum stenosis of extracranial internal carotid artery: effect of luminal morphology on stenosis measurement by using CT angiography and conventional DSA. Radiology 221:802–809
Leclerc X, Godefroy O, Pruvo JP, Leys D (1995) Computed tomographic angiography for the evaluation of carotid artery stenosis. Stroke 26:1577–1581
Marcus CD, Ladam-Marcus VJ, Bigot JL, Clement C, Baehrel B, Menanteau BP (1999) Carotid arterial stenosis: evaluation at CT angiography with the volume-rendering technique. Radiology 211:775–780
Randoux B, Marro B, Koskas F, Duyme M, Sahel M, Zouaoui A, Marsault C (2001) Carotid artery stenosis: prospective comparison of CT, three-dimensional gadolinium-enhanced MR, and conventional angiography. Radiology 220:179–185
Marks MP, Napel S, Jordan JE, Enzmann DR (1993) Diagnosis of carotid artery disease: preliminary experience with maximum-intensity-projection spiral CT angiography. Am J Roentgenol 160:1267–1271
Cumming MJ, Morrow IM (1994) Carotid artery stenosis: a prospective comparison of CT angiography and conventional angiography. Am J Roentgenol 163:517–523
Moll R, Dinkel HP (2001) Value of the CT angiography in the diagnosis of common carotid artery bifurcation disease: CT angiography vs digital subtraction angiography and color-flow Doppler. Eur J Radiol 39:155–162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Berg, M.H., Ikonen, A.E.J. et al. Carotid artery stenosis: reproducibility of automated 3D CT angiography analysis method. Eur Radiol 14, 665–672 (2004). https://doi.org/10.1007/s00330-003-2130-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-2130-2