Abstract
Testicular microlithiasis (TM) is an uncommon condition characterized by calcium deposits within the seminiferous tubules. On ultrasound (US), it is seen as multiple, uniform, nonshadowing echogenic foci in the testis. Although its true prevalence in the general population is still unknown, reported prevalences range from 0.6 to 9%. The TM is often associated with germ cell tumor (GCT) or intratubular germ cell neoplasia. The incidence of GCT in patients with TM was reported as 6–46%. There are several reports demonstrating interval development of GCT in patients with TM. These may suggest a premalignant nature of TM; however, more recent studies show a lower incidence of associated GCT and no interval development of tumor in relatively longer duration follow-up. Additionally, previously reported cases of interval tumor development had predisposing factors for testicular GCT. According to the recent literature, it is suggested that both TM and testicular GCT may be caused by a common defect, such as tubular degeneration, and TM may present as a marker for such abnormalities; however, because of a high incidence of association with GCT, it is prudent to follow up patients with TM with physical examination and US at least annually and to encourage self-examination. The routine use of biochemical tumor markers, abdominal and pelvic CT, or testicular biopsy does not seem to be justified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Definition
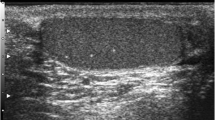
Testicular microlithiasis (TM) is an uncommon condition characterized by calcium deposits within the seminiferous tubules. The ultrasonographic appearance was first described by Doherty et al. [1]. On ultrasound, TM is seen as multiple, uniform, nonshadowing echogenic foci of 1–3 mm, scattered throughout the testicular parenchyma (Fig. 1). The TM is generally uniform and bilaterally symmetric [2].
The definition of TM varies among different authors. Some define TM as more than three echogenic foci in one testis [3] and others define as five or more foci in one testis [4]. More recently, “five or more foci on at least one US image” is the most commonly used criterion since it was first defined as classic TM by Bennett et al. [6] (see also [5, 7, 8]). As a fewer number of microliths may also be associated with an increased risk of testicular cancer, some authors define limited TM as fewer-than-five echogenic foci in contrast to classic TM (Fig. 2) [6, 7].
Histopathology
Histologically, TM refers to intratubular deposits consisting of calcified central cores surrounded by multiple concentric layers of cellular debris, glycoprotein, and collagen within the seminiferous tubules (Fig. 3) [9]. Some authors, however, suggest that the microliths are located outside the tubules and have been present since early stage of testicular development [10]. The microcalcification may be initiated by sloughing of degenerated cells into the tubule. The major defect is believed to be in the breakage of the basement membrane of the seminiferous tubule [9]. As a result of obstruction and degeneration possibly associated with an immunological process, it causes precipitation of a glycoprotein resulting in microlith formation [9]. Failure of Sertoli cells to phagocytize degenerating cells within the tubule has been suggested as the underlying cause of microcalcifications [10, 11].
Histopathological findings of testicular microlithiasis in a 49-year-old patient with seminoma. Photomicrograph (hematoxylin–eosin stain, ×125 magnification) demonstrates microcalcifcation (Ca) surrounded by cellular debris within the seminiferous tubules (arrows). (From [52])
Histopathologically, Renshaw classified intratesticular calcifications into three types, which include ossifications, hematoxylin bodies, and laminated or psammomatous calcifications [12]. True ossification is most common in teratoma. The hematoxylin bodies which consist of amorphous dystrophic calcifications are rare and only seen in germ cell tumors and burned out tumors [12]. The laminated or psammomatous calcifications, which correspond to TM, are more common and can be seen in many conditions including normal prepubertal testis, infertility, undescended testis, inguinal hernia, Klinefelter syndrome, and germ cell tumor [12]. Although histopathologically the hematoxylin bodies could be differentiated from laminated calcifications, it is impossible to differentiate these calcifications on US [2, 12].
The US findings of TM do not always correlate with histolopathological findings. In a series by Backus et al., calcification was present on pathological examination only in 10 patients of 22 who showed TM on US [5]. In another report, pathologic diagnosis of TM corresponded with the US findings in only 17 (61%) patients of 28 [3]. Problems in preparation, sampling error, and sectioning artifact may be the causes of this discrepancy [5]. On MR imaging, microliths are usally not visualized on both T1- and T2-weighted images.
Prevalence
Owing to the use of higher-frequency US transducers (7–14 MHz) and an increased general knowledge of the association of TM with germ cell tumors (GCT), more cases of TM have recently been reported; however, the true prevalence of TM in the general population is still unknown. The reported prevalences are variable, ranging from 0.6 to 9%, depending on the study population and the criteria for TM used [13, 14]. Using a criterion of five or more microliths in one US image, the prevalence was reported as low as 0.68% [8]. Higher prevalences (2.7 [15] and 9% [14]) have been reported when a criterion of five or more microliths in one testis, rather than in one image, was used.
Whereas most previous works investigating the prevalence of TM are retrospective studies done with patients referred for testicular US (Table 1), more recently a few prospective studies have been reported. In a study with 1504 asymptomatic men of 18–35 years, Peterson et al. reported the prevalence of TM as 5.6% using a criterion of greater than five microliths in one testis [4]. In another study done with 1079 patients referred for testicular US, Middleton et al. [7] reported that 18.1% of the patients had TM: 3.7% had classic TM (i.e., five or more microliths in one US image) and 14.4% had limited TM (i.e., less than five microliths in one US image). The prevalence of classic TM in this study is similar to that of other reports.
Testicular microlithiasis can be seen at all ages but is reported to be more common in childhood [13]. Its relative prevalence has been reported in previous literature as 1:2100 for adults, 1:618 for boys, and 1:15 for boys with cryptorchidism [16]. Among different races, TM has been reported as most prevalent in black men (14.1%), followed by Hispanic (8.5%), Asian (5.6%), and white men (4.2%) [4].
Although TM typically presents with a bilateral and diffuse appearance, its number and distribution are variable (Fig. 4). In a series of 42 cases with TM, Backus et al. reported that only 20 cases showed classic diffuse and symmetric distribution [5]. The distribution was asymmetric between the two testes in 8 patients and was unilateral in 1 patient [5]. According to their study, the number of microliths in one US image was 5–19 in 57% of patients. In another study with asymptomatic men, Peterson et al. reported that US demonstrated 5–25 microliths per one testis in 70% of cases [4].
Unilateral involvement was documented in case reports in which the involved testis later developed testicular cancer [17, 18, 19]. Subsequently, in a study with 159 infertile men, Thomas et al. found that all 10 cases of TM in infertile men showed unilateral involvement [20]. In recent literature, the incidence of unilateral involvement ranges 19–33% [4, 21]. Focal TM has also been reported (Fig. 5). Backus et al. reported in their series peripheral clustering in 12 (28%) of 42 patients with TM [5]. Although TM is usually confined within the testis, there is one report in which the microcalcification was also seen in the epididymis [22].
These focal or scanty microliths should be differentiated from a solitary echogenicity or multiple focal echogenicities which are seen in various conditions. Solitary hyperechoic lesions may be seen in scar tissue, fibrosis, or burned out tumors (usually with larger calcifications). Focal echogenicities can be seen in benign processes including orchitis, scar, granulomas, sarcoidosis, chronic infarction, and following chemotherapy or radiation therapy [5, 11, 13]. Unlike the tiny, evenly sized, round echogenicities of TM, these are often irregular, larger in size, and less well defined (Fig. 6) [11, 13].
Association with germ cell tumor
Testicular microlithiasis is often associated with GCT (Fig. 7) or intratubular germ cell neoplasia (IGCN). The histological types of associated GCT include seminoma, teratoma, and mixed GCT in decreasing order of frequency [19]. In a study using mammographic technique, microcalcifications were present in 74% of testicular tumors vs 16% with benign conditions [23].
The relative risk for cancer in association with TM was reported to be anywhere between 2 [14] and 20 [8]. It has been reported that TM becomes less significant in terms of tumor risk when detected in older patients [8].
Since Salisz and Goldman reported concurrent GCT in a patient with TM, the association of the two conditions has been described in several case reports (Table 2) [24]. Patel et al. reported that 3 of 4 patients with TM in their series had GCT [25] and Backus et al. found GCT in 40% of cases with TM (Table 1) [5]. Literature reviews by several authors report the incidence of GCT in patients with TM as 30–35% (Table 1) [25, 26, 27].
More recently, larger series investigating the association of TM and GCT have been published, with reported incidences ranging beween 6 and 46% (Table 1). Ganem et al. reported that 36% of patients with TM had testicular cancer in a series of 1100 patients who underwent testicular US [28]. Cast et al. found 7 (21%) GCT in 33 patients with TM [8], and Derogee et al. reported 29 (46%) GCT in 63 patients with TM [3]. Bach et al. reported in their series of 48 patients with TM that testicular tumor occurred in 27% of patients with TM and in 8% of those without TM [14]. Recently, in a prospective study, Middleton et al. reported a lower incidence of tumor association (8%) [7]. The authors suggested that this lower incidence is probably due to the differences in study design (cases of isolated TM could be missed in retrospective studies) and US techniques between the studies resulting in overestimation of the incidence in previous retrospective studies [7].
Testicular microlithiasis has been reported to be also associated with contralateral testicular cancer following orchiectomy for testicular cancer [29]. In a study with 156 patients with prior orchiectomy, Bach et al. reported that TM was highly associated with contralateral testicular cancer; cancer was found in 22% with TM vs 2% without TM (odds ratio=12.0, p=0.002) [29].
In terms of the relation between the severity of TM and tumor development, only a few studies have been reported [6, 7]. In a retrospective study, Bennett et al. reported that 7 patients (18%) of 39 with classic TM had GCT, whereas only 1 patient (2%) of 65 with limited TM had GCT [6]. On the contrary, Middleton et al. later reported in a prospective study that there was no significant difference in tumor incidence between classic and limited TM [7].
Several cases of interval development of GCT in patients with TM have been reported in the literature (Table 3). Average interval for tumor development was 48 months (range 6–132 months) after initial diagnosis of TM. In some reports TM was extensive [16, 18, 30], whereas others showed scanty microliths [17, 24].
Contrary to these reports of interval tumor development, there are several reports that documented no tumor development during mean follow-up periods of 28–72 months (Table 4). In the largest series, Bennett et al. reported that none of 72 patients with TM developed testicular neoplasm for an average of 45 months [6].
Intratubular germ cell neoplasia is a precancerous lesion of the testis that occurs in 0.3–0.8% of general population [31]. It occurs in up to 46% of the patients with testicular atrophy [32]. Bilateral IGCN occurs in 4.5–6.0% [32]. All GCT except spermatocytic seminoma are known to be preceded by IGCN [31], and up to 50% of patients with IGCN develop testicular cancer within 5 years [33].
Testicular microlithiasis is commonly associated with IGCN (Table 5) [34, 35]. The reported incidence of IGCN in the contralateral testis in patients with testicular cancer ranged from 4.5 to 22% [32, 36]. Song et al. reported that IGCN was detected pathologically in the same testis in 14 (67%) of 21 patients with TM and GCT [37]. In the study by Backus et al., IGCN was diagnosed in 5 patients of 42 with TM, one of whom had concurrent testicular cancer [5]. In a recent study, Bach et al. reported all 36 patients with IGCN in their series had testicular cancer [14]. Eight of these patients had TM and 28 did not.
The high association of TM with GCT or IGCN and several reports of interval development of GCT in patients with TM may raise concern that the presence of TM may significantly increase the risk of developing testicular cancer; however, several pieces of evidence mitigate against a causal relationship between TM and testicular neoplasm. Firstly, most patients with TM (more than 90%) do not show tumor at presentation and no interval development of tumor was observed in the larger series in recent literature (Table 4) [7]. Many reported cases of interval development of GCT already had underlying risk factors which predisposed to testicular GCT, including cryptorchidism, infertility, and a history of contralateral testicular tumor (Table 3). Secondly, in a recent study of asymptomatic men 18–35 years old, the prevalence among different racial groups and the geographic distribution of TM in the United States were different from those of testicular tumor [4]. For example, TM is more common (14.1%) in black than in white (4%) men, but testicular tumor is much less common in black men [4]. The TM is most common in men from the southeastern United States, but the incidence of testicular cancer is lowest in that region [4]; therefore, it is likely that both TM and testicular cancer are caused by a common defect, such as tubular degeneration [18], and TM may present as a marker of these intrinsic testicular abnormalities [8].
Testicular microlithiasis can be associated with extragonadal GCT (Fig. 8). Isolated TM has been reported in patients with GCT in the chest and/or abdomen [11, 26, 38, 39]. Most of the cases are adults, but the occurrence in childhood and association with Klinefelter syndrome have been reported [40, 41]. Although in some reports intratesticular scar that is suggestive of burned out tumor could be found, most of these reported cases do not show focal abnormalities in the testis [11, 26, 38, 39]. The clinical significance of TM in patients with extragonadal GCT still remains unclear.
Extratesticular germ cell tumor in the mediastinum associated with testicular microlithiasis in 33-year-old man. A Longitudinal testicular US demonstrates multiple microliths scattered in the testis (T). B Contrast-enhanced CT of the chest shows homogeneously enhancing mass (arrows) in anterior mediastinum. The pathologic examination confirmed the diagnosis of seminoma
Other associated conditions
Besides GCT, TM may be associated with various conditions including infertility [23, 25, 42], cryptorchidism [43], hypogonadism, and Klinefelter’s syndrome [41], Down’s syndrome, testicular or appendiceal torsion [44], postorchiopexy testis [45], male hermaphroditism [44], neurofibromatosis, and AIDS [28]. An association of microliths in pulmonary alveoli or in the central nervous system has also been reported [5, 41].
Infertility and an undescended testis are common conditions associated with TM (Fig. 9). The reported frequency of TM in patients with infertility or undescended testis ranged from 7 to 39% [19, 43]. In studies by Janzen et al. [11] and Miller et al. [26], 37 and 39% of cases of TM were associated with an undescended testis or subfertility/infertility, respectively. In another study with 159 infertile patients, microcalcifications were found in 10 (6.2%) [20]. In this report the lesions were unilateral in all patients.
The relationship between TM and infertility is unclear. Since 30–60% of seminiferous tubules are obstructed with intratesticular concretions in patients with TM, obstruction of seminiferous tubules formed by sloughing degenerative tubular epithelium has been suggested as an underlying cause of TM in this condition [43, 46]. There may be a relationship between the degree of calcification and poor sperm function [20]. The TM was reported to be more prevalent in patients with spermatogenic defects such as severe oligospermia and reduced testicular volume [47]. On US, infertile men may show abnormal echo texture including patchy inhomogeneity, hypoechoic lesions, or echogenic foci [19, 42]. As both IGCN and GCT often occur in the atrophic testis with TM, these patients should be further investigated [48].
An undescended testis often demonstrates abnormal volume or morphology including TM. In a series of 75 patients with surgically fixed testes, structural abnormalities were seen in 53% of cases, including TM in 6 patients (8%) [49]. The presence of TM in patients with undescended testis may pose an additional risk for developing testicular cancer [50].
The role of US and recommendations
As there are big unresolved controversies on the clinical significance and malignant potential of TM, the role of US and the recommendations for follow-up studies in patients with TM vary among different authors.
Since TM is commonly associated with GCT and IGCN, many authors recommend clinical and US follow-up in patients with TM [3, 8, 14, 26, 28]. Some authors recommend annual physical examination and periodic self-examination, but no regular US follow-up [7]. Since TM is not as prevalent as reported previously and there were no cases of interval tumor development in their series, Middleton et al. suggested that less aggressive surveillance could be warranted [7]. Other authors suggest that, because of the low likelihood of association with GCT, there is no need to follow men with TM with biochemical tumor markers, US, and physical examination [4]. Peterson et al. suggest that the economic burden for evaluating and following men with TM in ages 18–35 years is greater than $18 billion in the United States [4].
Because of a high prevalence of testicular cancer in infertile men, some authors recommended biopsy or follow-up US when TM is seen in an atrophic testis [48]. Additionally, at the time of orchiectomy for testicular cancer, routine testicular biopsy of the contralateral testis was recommended by other authors since IGCN is relatively common at biopsy in these patients [14].
The recommended interval and method of follow-up studies also varies in the literature. Janzen et al. [11] and Miller et al. [26] suggest close clinical follow-up with periodic US at an interval of 6–12 months. The use of tumor markers [1, 14, 21, 26], chromosomal analysis, CT of the abdomen and chest for extratesticular GCT, and testicular biopsy have been suggested for patients with TM [26].
Considering the fact that more recent evidence mitigates against the premalignant nature of TM, and the extremely high cure rate of testicular cancer, more aggressive diagnostic studies for patients with TM may be costly and unnecessary. In addition, other patient populations at high risk for testicular cancer, such as cryptorchidism, infertility, or tumor in contralateral testis, are not aggressively followed up with US, biochemical serum markers, and abdominal and pelvic CT [4].
Currently, there is no consensus regarding the necessity, interval, duration or diagnostic modality that should be used for follow-up of patients with TM. To determine this, long-term prospective studies are needed. Until these studies are performed, it seems prudent to follow up patients with TM with physical examination and US at least annually [51]. It is also important to encourage and educate about self-examination since this may result in early detection of GCT [8]. The routine use of biochemical tumor markers, abdominal and pelvic CT or testicular biopsy does not seem to be justified for patients with isolated TM. Testicular biopsy should be considered only when there are suspicious findings on physical examination or US [21]. When testicular biopsy cannot be performed, MR imaging may be used to verify the absence of concurrent tumor in the testis (Fig. 10)
A 40-year-old patient with extensive microlithiasis. A Transaxial US demonstrates suspicious hypoechoic lesions (arrows) in both testes (T). Testicular biopsy was recommended but was refused by the patient. Magnetic resonance imaging was performed. B Transaxial and C coronal fast spin-echo T2-weighted images of both testes (TR 4000 ms, effective TE 85 ms) demonstrate no mass within either testis
References
Doherty FJ, Mullins TL, Sant GR, Drinkwater MA, Ucci AA (1987) Testicular microlithiasis: a unique sonographic appearance. J Ultrasound Med 6:389–392
Otite U, Webb JAW, Oliver RTD, Badenoch DF, Nargund VH (2001) Testicular microlithiasis: Is it a benign condition with malignant potential? Eur Urol 40:538–542
Derogee M, Bevers RFM, Prins HJ, Jonges TGN, Elbers FH, Boon TA (2001) Testicular microlithiasis, a premalignant condition: prevalence, histopathologic findings, and relation to testicular tumor. Urology 57:1133–1137
Peterson AC, Bauman JM, Light DE, McMann LP, Costabile RA (2001) The prevalence of testicular microlithiasis in an asymptomatic population of men 18 to 35 years old. J Urol 166:2061–2064
Backus ML, Mack LA, Middleton WD, King BF, Winter TC III, True LD (1994) Testicular microlithiasis: imaging appearances and pathologic correlation. Radiology 192:781–785
Bennett HF, Middleton WD, Bullock AD, Teefey SA (2001) Testicular microlithiasis: US follow-up. Radiology 218:359–363
Middleton WD, Teefey SA, Santillan CS (2002) Tesicular microlithiasis: prospective analysis of prevalence and associated tumor. Radiology 224:425–428
Cast JEI, Nelson WM, Early AS et al. (2000) Testicular microlithiasis: prevalence and tumor risk in a population referred for scrotal sonography. Am J Roentgenol 175:1703–1706
Vegni-Talluri M, Bigliardi E, Vanni MG, Tota G (1980) Testicular microliths: their origin and structure. J Urol 124:105–107
Drut R, Drut RM (2002) Testicular microlithiasis: histologic and immunohistochemical findings in 11 pediatric cases. Pediatr Dev Pathol 5:544–550
Janzen DL, Mathieson JR, Marsh JI et al. (1992) Testicular microlithiasis: sonographic and clinical features. Am J Roentgenol 158:1057–1060
Renshaw AA (1998) Testicular calcifications: incidence, histology and proposed pathological criteria for testicular microlithiasis. J Urol 160:1625–1628
Hobarth K, Susani M, Szabo N, Kratzik C (1992) Incidence of testicular microlithiasis. Urology 40:464–467
Bach AM, Hann LE, Hadar O et al. (2001) Testicular microlithiasis: What is its association with testicular cancer? Radiology 220:70–75
Miller F, Rosairo S, Clarke J, Sidhu P (2000) Testicular calcification: appearances, anatomical distribution and association with primary intra-testicular malignancy in 2924 patients. Radiology 217(P):366
McEniff N, Doherty F, Katz J, Schrager CA, Klauber G (1995) Yolk sac tumor of the testis discovered on a routine annual sonogram in a boy with testicular microlithiasis. Am J Roentgenol 164:971–972
Winter TC III, Zunkel DE, Mack LA (1996) Testicular carcinoma in a patient with previously demonstrated testicular microlithiasis. J Urol 155:648
Frush DP, Kliewer MA, Madden JF (1996) Testicular microlithiasis and subsequent development of metastatic germ cell tumor. Am J Roentgenol 167:889–890
Vrachliotis TG, Neal DE (1997) Unilateral testicular microlithiasis associated with a seminoma. J Clin Ultrasound 25:505–507
Thomas K, Wood SJ, Thompson AJM, Pilling D, Lewis-Jones DI (2000) The incidence and significance of testicular microlithiasis in a subfertile population. Br J Radiol 73:494–497
Furness PD III, Husmann DA, Brock JW III et al. (1998) Multi-institutional study of testicular microlithiasis in childhood: a benign or premalignant condition? J Urol 160:1151–1154
Kutcher R, Rosenblatt R, Kremer S (1992) Testicular microlithiasis (letter). Am J Roentgenol 159:1129
Ikinger U, Wurster K, Terwey B, Mohring K (1982) Microcalcifications in testicular malignancy: diagnostic tool in occult tumor? Urology 19:525–528
Salisz JA, Goldman KA (1990) Testicular calcifications and neoplasia in patient treated for subfertility. Urology 36:557–560
Patel MD, Olcott EW, Kerschmann RL, Callen PW, Gooding GAW (1993) Sonographically detected testicular microlithiasis and testicular carcinoma. J Clin Ultrasound 21:447–452
Miller RL, Wissman R, White S, Ragosin R (1996) Testicular microlithiasis: a benign condition with a malignant association. J Clin Ultrasound 24:197–202
Berger A, Brabrand K (1998) Testicular microlithiasis: a possibly premalignant condition. Report of five cases and a review of the literature. Acta Radiol 39:583–586
Ganem JP, Workman KR, Shaban SF (1999) Testicular microlithiasis is associated with testicular pathology. Urology 53:209–213
Bach AM, Hann LE, Shi W, Giess CS, Yoo HH, Sheinfeld J, Thaler HT (2003) Is there increased incidence of contralateral testicular cancer in patients with intratesticular microlithiasis? Am J Roentgenol 180:497–500
Gooding GA (1997) Detection of testicular microlithiasis by sonography. Am J Roentgenol 168:281–282
Giwercman A, Muller J, Skakkebaek NE (1991) Prevalence of carcinoma in situ and other histopathological abnormalities in testes from 399 men who died suddenly and unexpectedly. J Urol 145:77–80
Loy V, Dieckmann KP (1993) Prevalence of contralateral testicular intraepithelial neoplasia (carcinoma in situ) in patients with testicular germ cell tumour. Results of the German multicentre study. Eur Urol 23:120–122
Giwercman A, Maase H von der, Skakkebaek NE (1993) Epidemiological and clinical aspects of carcinoma in situ of the testis. Eur Urol 23:104–110
Kaveggia FF, Strassman MJ, Apfelbach L, Hatch JL, Wirtanen GW (1996) Diffuse testicular microlithiasis associated with intratubular germ cell neoplasia and seminoma. Urology 48:794–796
Parra BL, Venable DD, Gonzalez E, Eastham JA (1996) Testicular microlithiasis as a predictor of intratubular germ cell neoplasia. Urology 48:797–799
Berthelsen JG, Skakkebaek NE (1981) Value of testicular biopsy in diagnosing carcinoma in situ testis. Scand J Urol Nephrol 15:165–168
Song FL, Middleton WD, Winter TC, Swanson PE, Mack LA (1993) Association between intratubular germ cell neoplasia and testicular microlithiasis (abstr). Radiology 189(P):156
Emberton P, Moody AR (1994) Testicular microlithiasis (letter). Am J Roentgenol 162:1002–1003
Quane LK, Kidney DD (2000) Testicular microlithiasis in a patient with a mediastinal germ cell tumor. Clin Radiol 55:642–644
Howard RG, Roebuck DJ, Metreweli C (1998) The association of mediastinal germ cell tumour and testicular microlithiasis. Pediatr Radiol 28:998
Aizenstein RI, Hibbeln JF, Sagireddy B, Wilbur AC, O’Neil HK (1997) Klinefelter’s syndrome associated with testicular microlithiasis and mediastinal germ-cell neoplasm. J Clin Ultrasound 25:508–510
Pierik FH, Dohle GR, van Muiswinkel JM, Vreeburg JT, Weber RF (1999) Is routine scrotal ultrasound advantageous in infertile men? J Urol 162:1618–1620
Nistal M, Paniagua R, Diez-Pardo JA (1979) Testicular microlithiasis in 2 children with bilateral cryptorchidism. J Urol 121:535–537
Jaramillo D, Perez-Atayde A, Teele RL (1989) Sonography of testicular microlithiasis. Urol Radiol 11:55–57
Kragel PJ, Delvecchio D, Orlando R, Garvin DF (1991) Ultrasonographic findings of testicular microlithiasis associated with intratubular germ cell neoplasia. Urology 37:66–68
Kessaris DN, Mellinger BC (1994) Incidence and implication of testicular microlithiasis detected by scrotal duplex sonography in a select group of infertile men. J Urol 152:1560–1561
Lenz S, Thomsen JK, Giwercman A, Hertel NT, Hertz J, Skakkebaek NE (1994) Ultrasonic texture and volume of testicles in infertile men. Hum Reprod 9:878–881
Eckardstein S von, Tsakmakidis G, Kamischke A, Rolf C, Nieschlag E (2001) Sonographic testicular microlithiasis as an indicator of premalignant conditions in normal and infertile men. J Androl 22:818–824
Riebel T, Herrmann C, Wit J, Sellin S (2000) Ultrasonographic late results after surgically treated cryptorchidism. Pediatr Radiol 30:151–155
Khan MA, Beyzade B, Potluri BS (2000) Testicular seminoma in a man with bilateral microlithiasis and a history of cryptorchidism. Scand J Urol Nephrol 34:377–379
Skyrme RJ, Fenn NJ, Jones AR, Bowsher WG (2000) Testicular microlithiasis in a UK population: its incidence, associations and follow-up. Br J Urol Int 86:482–485
Propeck PA, Desouky SS, Warner TF, Pozniak M (1993) Ultrasound of the day. Radiographics 13:693–695
Hobarth K, Szabo N, Klingler HC, Kratzik C (1993) Sonographic appearance of testicular microlithiasis. Eur Urol 24:251–255
Leenen AS, Riebel TW (2002) Testicular microlithiasis in children: sonographic features and clinical implications. Pediatr Radiol 32:575–579
Roberts IS, Loughran CF (1993) Case report: the ultrasound appearances of testicular microlithiasis (‘snow storm’ testis): a case complicated by testicular seminoma. Clin Radiol 47:65–67
Alsikafi NF, Gerber GS (1998) Bilateral metachronous testicular seminoma associated with microlithiasis. J Urol 159:1643–1644
Abu-Yousef M (2000) Case 2: abdominal. Testicular microlithiasis complicated by testicular carcinoma. J Ultrasound Med 19:354
Golash A, Parker J, Ennis O, Jenkins BJ (2000) The interval of development of testicular carcinoma in a patient with previously demonstrated testicular microlithiasis. J Urol 163:239
Cornford PA, Baird AD, Woolfenden KA (2001) Testicular microlithiasis needs long-term surveillance. Scand J Urol Nephrol 35:243–244
Whitman GJ, Hall DA, McCarthy KA, Hulka CA, Simeone JF (1994) Testicular microlithiasis: sonographic features and significance (abstr). Radiology 193 (P):335
Klauber GT, Sant GR, Long JP, McEniff NJ, Doherty FJ, Ucci AA (1996) Testicular microlithiasis in boys: preliminary results and implications. J Urol (part 2) 155:392A, abstract 326
Tuzel E, Yorukoglu K, Gumus B, Kirkali Z (1997) Testicular microlithiasis associated with teratocarcinoma and intratubular germ cell neoplasia: a case report. Int J Urol 4:530–532
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, B., Winter, T.C. & Ryu, Ja. Testicular microlithiasis: clinical significance and review of the literature. Eur Radiol 13, 2567–2576 (2003). https://doi.org/10.1007/s00330-003-2014-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-2014-5