Abstract
Magnetic resonance contrast agents have demonstrated their clinical usefulness in a variety of organs for improved detection of various neoplastic, inflammatory and functional abnormalities. Gadolinium chelates are the most widely used. They are extracellular, non-specific contrast agents. Their use in many clinical indications is justified because, in conjunction with improved imaging techniques, these safe and image-enhancing contrast agents add morphologic and functional information compared with unenhanced MR images. This article describes the commercially available compounds, and summarizes their approval status on the international market regarding indications and doses. Their mechanisms of action, biodistributions, toxicities and tolerance profiles in normal and high-risk patient populations are described. Additionally, this article reviews the specific recommendations by the manufacturers for patients at risk. Finally, their main clinical applications are reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging contrast agents are diagnostic pharmaceutical compounds containing paramagnetic or superparamagnetic metal ions that affect the MR-signal properties of surrounding tissues. Paramagnetic agents are mainly positive enhancers that reduce the T1 and T2 relaxation times and increase tissue signal intensity on T1-weighted MR images. They are administered to enhance tissue contrast, to characterize lesions and to evaluate perfusion and flow-related abnormalities [1, 2, 3, 4, 5]. Presently, they are administered in 40%–50% of all MR imaging examinations [2]. The active constituent of extracellular MR imaging contrast agents currently available for clinical use is gadolinium (Gd), a paramagnetic metal in the lanthanide series. The following Gd complexes are currently available on the international market (Table 1): Magnevist (gadopentetate dimeglumine; Schering, Berlin, Germany), Dotarem (gadoterate meglumine; Guerbet, Aulnay-sous-Bois, France), Omniscan (gadodiamide; Nycomed, Oslo, Norway) and ProHance (gadoteridol; Bracco, Milan, Italy). In addition, Gadovist (gadobutrol; Schering, Berlin, Germany) has recently received approval in Germany and Switzerland, and MultiHance (gadobenate dimeglumine; Bracco, Milan, Italy) is an extracellular contrast agent but also a liver-specific product. Optimark (gadoversetamide; Mallinkrodt, St. Louis, Mo.) is available only in the USA. These Gd complexes can be classified into four main categories according to their biochemical structure, e.g. macrocyclic vs linear, and to their charge, ionic vs non-ionic (Fig. 1).
Paramagnetic extracellular contrasts agents are administered intravenously and are excreted unchanged by passive glomerular filtration. For clinical use, the recommended dose is 0.1 mmol/kg; however, gadodiamide and gadopentetate are approved for MR angiography at doses up to three times the standard, and gadoterate at a dose of twice the standard (Table 1). In addition, the doses approved for central nervous system imaging range from 0.1 to 0.3 mmol/kg except for gadobenate. After injection, they are rapidly distributed into the intravascular space and then throughout the extracellular space. Extracellular fluid contrast agents diffuse freely into and out of the extracellular space but do not enter tissues with specialized vascular barriers. These agents accumulate in tissues with abnormal vascularity and in regions where the blood-brain barrier is disrupted. As extracellular nonspecific contrast agents, most Gd complexes are equally effective, because of similar relaxivities (Table 2) and biodistributions. New contrast agents are being developed; criteria for their suitability include diagnostic efficacy, safety, stability, pharmacology and cost [3].
This review article focuses on the mechanisms of action of non-specific extracellular contrast agents. Their biodistributions, diagnostic efficacies, toxicities and tolerance profiles, and their main clinical applications are summarized.
Mechanisms of action
The actions of these paramagnetic agents are attributable to short-range dipolar interactions dominated by the high magnetic moment of their unpaired electrons. Among the metal ions having unpaired electrons (Gd3+, Dy3+, Mn3+ and Fe3+), Gd is the most powerful with seven unpaired electrons and most paramagnetic agents are based on it. At the molecular level, the strong paramagnetism of Gd disturbs the relaxation of nearby water protons, causing decreases of both T1 and T2 relaxation times; thus, the effect is indirect, being induced via changes in proton relaxation. Although both T1 and T2 are affected, the effects on T1 relaxation times are stronger with the concentrations used in clinical practice [1, 3, 4, 5]. Shortening of T1 in tissues, as observed after the intravenous administration of Gd chelates at the standard 0.1 mmol/kg dose, produces an increase of signal intensity (positive enhancement) [4, 5]. The effect on image contrast mainly depends on field strength, pulse sequence, imaging parameters, distribution of the Gd chelate and its local concentration [6]. The expected postcontrast decrease of T1 relaxation time is more pronounced on spin-echo sequences with short repetition and echo times, and gradient-echo images with short repetition times and a high flip angle. In gradient-echo imaging, the T1sensitivity can be optimized by using short TR (TR<T1) and short TE values (to minimize the loss of signal due to T2 effects). T1 relaxivity profiles are nearly identical for the currently available Gd contrast agents as the clinical imaging field range [6]. At high concentrations, e.g. as observed in the normal urinary tract, T2 effects predominate and cause a decrease of signal intensity on T1- and T2-weighted MR images.
Gadolinium is chelated to a ligand to reduce the toxicity inherent in free Gd ions; however, chelation also alters the pharmacokinetic parameters of the complex [7, 8], as it decreases the T1 rate constant of the tissues that take up the contrast agent [2]. One of the major aspects of this altered pharmacokinetic profile is its excretion pathway. After chelation, the rate of renal excretion of the Gd complex is increased approximately 550-fold compared with pre-chelation values [9, 10].
Although the clinical applications of the four Gd chelates most commonly used are broadly similar, there are differences in their physicochemical properties which arise from structural design differences; these latter include the presence or absence of an overall change of the Gd chelate and the use of linear vs macrocyclic structures for the organic ligands (Fig. 1) [7]. Diethylene triamine penta-acetic acid (DTPA), the first commercially available ligand, is linear and is excreted via the kidneys. The macrocyclic chelates are more stable than the linear chelates, but this difference seems to have little consequence in practice. Despite differences among the chelating molecules, e.g. ionic charge and linearity, these MR imaging contrast agents appear to have remarkably similar diagnostic efficacies and safety profiles [7, 8, 11, 12, 13, 14, 15, 16, 17].
Biodistribution
Gadolinium complexes are called non-specific as they are hydrophilic, do not bind to proteins or receptors, are excreted unmetabolized in urine and are considered extracellular-fluid markers. Gd chelates have low molecular masses (approximately 500 Da). Because of their small size, they are rapidly cleared from the intravascular space through the capillaries into the interstitial space, and therefore their biodistribution is non-specific. They do not cross an intact blood-brain barrier. Due to their rapid equilibration in the interstitial space of both normal tissues and tumours, the use of dynamic MR imaging after bolus injection makes the best use of the narrow imaging window with a transiently increased tumour-to-normal tissue contrast. Gd chelates are excreted unchanged by passive glomerular filtration with >95% excreted by 1 day [18]; <0.1% of the injected dose is eliminated via feces. The biological elimination half-life is approximately 1.5 h [8] with renal clearance for healthy persons of 1.1–1.8 ml min–1 kg–1. There is no detectable biotransformation, decomposition or serum protein binding. Biobistribution of the four commercially available Gd chelates has been studied in mice and rats using a radiolabeling technique [7, 18]. The macrocyclic chelates, gadoterate and gadoteridol, had the lowest residual Gd in both rodents. The lowest-to-highest order of residual whole body Gd at 14 days was: gadoteridol≈gadoterate=gadopentetate<<gadodiamide [7].
Dose administration
The standard dose is 0.1 mmol/kg; however, higher or lower doses may be diagnostically more adequate [19] and Gd chelates are approved for different dose regimens (Table 1). Potential advantages of using Gd chelates at higher doses include better lesion enhancement, delineation and detectability of central nervous system (CNS) neoplasms [20]. Higher doses (0.2–0.3 mmol/kg) may also be required for MR angiography [21], and gadodiamide has been approved for a dose up to 0.3 mmol/kg for body imaging. Haustein et al. [22] showed that a gadopentetate dimeglumine dose of 0.3 mmol/kg injected at a flow rate of approximately 1 ml/s was safe and well tolerated. Yuh et al. [23] observed no significant side effects of gadoteridol for doses up to three times the standard dose. Lower doses also appear to be useful for MR urography [24] and CNS imaging [19].
Efficacy
The contrast between the tissues in MR images is mainly attributable to three parameters: the amount of protons (proton density) and their two relaxation times T1 and T2. As it is difficult to modify the amount of water or fat in vivo, the substances used to increase contrast act by modifying the relaxation times. The four most widely used Gd complexes have similar relaxivities (Table 2), thereby explaining the similar diagnostic efficacies of these agents.
Osmolality
Non-ionic complexes with low osmolality (Table 2) were developed to improve tolerance and allow the use of higher doses [18, 25]. Because of the low amounts of compounds injected for MR imaging, the ionic charge of the Gd complexes is not a crucial factor, as the increased plasma osmolality following administration of Gd chelates is very low, unlike what is observed with iodinated contrast media. Indeed, from a safety viewpoint, it is inappropriate to compare ionic and non-ionic MR imaging contrast agents with ionic and non-ionic CT contrast agents [1]. Low-osmolarity chelates could be advantageous when higher doses are required. In addition, it has been shown in a rat model that the extravasation of ionic gadopentetate was associated with higher incidences of necrosis, haemorrhage and oedema than non-ionic gadoteridol [26]. The first commercially available agents had a 0.5-M osmolarity. Manufacturers now provide 1-M solutions (Table 2) in order to increase the quality of bolus injection and to reduce the volume of injected solution.
Toxicity
Free-ion Gd3+ is acutely toxic owing to its tendency to precipitate and be deposited in liver, lymph nodes and bones which prolongs its half-life [2, 7]. It may also obstruct calcium-ion passage through muscle cells, and block the flow of calcium in bone epiphyses and nerve tissue cells, causing the arrest of neuromuscular transmission. A primary factor contributing to the toxicity of Gd complexes is the extent to which Gd can replace some endogenous metals, especially zinc. This phenomenon is called trans-metallation. Another parameter, which governs toxicity at least partially, is the stability of the complex. It must be as high as possible to avoid any in vivo decomplexation. Decomplexation is an equilibrium which is characterized by a constant called the thermodynamic stability constant (LogK therm). Macrocyclic complexes have the highest thermodynamic stability constants [1]. Moreover, at physiological pH, the study of the conditional stability constants (LogK cond) showed that non-ionic structures are the most stable (Table 2). Although some differences in stability constants do exist, they appear to be of minor clinical importance [2].
There are several ways to assess the toxicity of a drug, including determination of its 50% lethal dose (LD50) and the frequencies and types of adverse events. The LD50 is defined as the dose of a drug that, when administered to test animals, results in the acute death of half of the test population. The LD50 value is the highest for gadodiamide (34 mmol/kg), and lowest for gadopentetate (7 mmol/kg; Table 2) [1, 2, 25], representing respective excesses of approximately 300 and 60 times the typical diagnostic dose of 0.1 mmol/kg, and has minor practical impact.
Tolerance and adverse events
Gadolinium chelates are extremely well tolerated at both standard and higher doses, with no clinically relevant difference among these agents. The safety of MR contrast agents has been shown to be comparable in several studies [2, 25, 26, 27, 28, 29]. The frequency of single adverse events is approximately 1% or less of all patients [2]. A meta-analysis of data obtained for 13,439 patients enrolled in phase IIIb–IV studies who received 0.1 or 0.2 mmol/kg of Gd-DTPA showed an adverse event rate of 1.15% [28]. In patients with a known history of allergy, the incidence of adverse events was found to be 2.6%. No correlation was found between patient age and the frequency of adverse events. Fast bolus injections were tolerated without added risk. The adverse events that have been reported are comparable to those observed with iodinated contrast media. The most common adverse events are nausea, headache and vomiting. They are not severe and do not require specific treatment. Anaphylactoid reactions, involving respiratory, cardiovascular, cutaneous, gastrointestinal and/or genitourinary manifestations, have been reported but are anecdotal. Their true prevalence appears to be between 1/100,000 and 1/500,000 [2]. Most patients who experienced anaphylactoid reactions had a past history of respiratory difficulties or respiratory allergic disease. In 1991, Niendorf et al. [16] reported that the risk of adverse reactions to gadopentetate dimeglumine was 3.7 times higher in patients with a prior history of reaction to iodinated contrast media. This observation was further supported by Murphy et al. [27], who reviewed 36 adverse reactions to Gd which were observed among 21,000 patients who received Gd-based contrast media: 4 patients had previously reacted to iodinated contrast media. Premedication of these patients remains controversial. To date, the nature of these "allergy-like" reactions has not been explained. A known hypersensitivity to one of the constituents of the Gd contrast agent is considered as a contraindication by manufacturers. The management of severe adverse reactions to Gd chelates requires immediate intervention and thus radiologists must be trained and equipped to resuscitate patients experiencing such reactions. This recommendation is included in the package insert information provided by manufacturers.
Reported reactions at the injection site include pain, warmth and localized oedema. Several delayed-onset reactions, typically developing 1–4 days after administration, have been reported, and included swelling and pain at and near the injection site [2, 17]. They progressively peaked and then resolved over a few days.
At present, the use of MR imaging contrast agents in paediatric patients over 2 years old is approved for the four most common Gd complexes [30, 31, 32]. No significant adverse clinical events or clinically important trends in vital signs have been reported in association with the use of Gd chelates in this patient group [33]. In addition, the use of gadopentetate dimeglumine is approved in Europe at doses up to 0.2 mmol/kg for CNS studies and body MRI in infants (day 1 of life). Gadodiamide is also approved in Europe for babies from 6 months of age at a dose of 0.1 mmol/kg, whereas a 0.1-mmol/kg dose of gadoteridol can be injected in children of 2 years and above. Additional studies are ongoing to evaluate the use of MR imaging contrast agents in paediatric patients.
High-risk patients
Renal insufficiency
Gadolinium chelates are excreted unchanged by passive glomerular filtration and the package insert information provided by manufacturers usually indicates that "caution should be exercised in patients with severely impaired renal function" or that, as there are no studies with the contrast agent in patients with impaired renal function, its use cannot be recommended for this group of patients (Table 3); however, several studies suggest that Gd chelates are well tolerated in patients with renal insufficiency [16, 34, 35, 36]. No significant change of serum creatinine levels was observed in patients with moderate or severe renal impairment after the administration of 0.1 mmol Gd-DTPA [35] or Gd-DOTA [36]. In addition, Gadobutrol (1 mol/l) at a dose of 0.1 or 0.3 mmol/kg b.w. proved to be a safe MR agent in a study of 21 patients with impaired renal function [37, 38]. Gd chelates can be eliminated by dialysis, with more than 95% of the administered dose being removed by the third dialysis session [34]. In patients with chronic renal failure on hemodialysis, Gd chelates are efficiently cleared. The mean half-life of the plasma concentration of Gd-DTPA is 1.87±0.71 h in patients on dialysis, which is comparable to the value obtained for patients with normal renal function [16]. According to the study conducted by Yoshikawa and Davies [34], gadoteridol at a dose of 0.3 mmol/kg could be safely administered to patients with end-stage renal disease on dialysis. Tombach et al. [39] documented the safety and dialysability of 1-M gadobutrol for doses of 0.1 and 0.3 mmol/kg b.w. in a phase-III clinical trial. They showed that the mean eliminated fraction increased from 68.2±12.7 to 98.0±1.8%, respectively, after one or three 3-h haemodialysis sessions.
Sickle cell disease and anaemia
To date, there is no evidence to suggest any basis for increased clinical concern regarding the administration of the currently used Gd complexes to patients with sickle cell anaemia or other haemoglobinopathy. Moreover, there is no report of a sickle-cell crisis being triggered by injection of any paramagnetic MR contrast agents [2].
Pregnancy
Gadolinium complexes easily cross the placental barrier and appear within the fetal urinary bladder only moments after IV administration. They are then excreted into the amniotic fluid, subsequently swallowed by the fetus and pass unaltered into the fetal gastrointestinal tract, from which they pass into the circulation and are filtered by the fetal kidney and again excreted in the urine. To date, no data are available that enable evaluation of the clearance rate of Gd chelates from the amniotic fluid by the mother. Because of this lack of information, Gd chelates have not been approved for use in pregnant women (Table 3).
Lactation
Gadopentetate dimeglumine is excreted in very low concentrations in human breast milk over approximately 33 h after IV administration. Its concentration in breast milk peaks at approximately 4.75 h and decreases to less than one-fifth of this level 22 h after injection [40, 41]. For this reason, and as an extra precaution, it is recommended that nursing mothers pump their breasts before and do not breast feed for 24–48 h after Gd administration to ensure that the nursing child does not receive the drug in any appreciable quantity (Table 3). Kubik-Huch et al. [42] measured the amount of gadopentetate dimeglumine excreted into human breast milk following IV injection of a clinical dose. The cumulative amount detected during the 24 h was <0.04% of the administered dose. The amount transferred to a nursing infant would be more than 100 times less than the permitted IV dose (200 µmol/kg) for neonates. The package insert information recommendation of a 24-h suspension of breast feeding for lactating women should thus be reconsidered [42].
Indications
This section does not deal with evidence-based medicine. It has been reported in numerous publications that Gd chelates improve sensitivity, specificity and diagnostic accuracy of MR procedures [5, 21, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60]. Indications for their use include the evaluation of tumoral, traumatic and infectious disease processes. They are now also being used for contrast-enhanced MR angiography (see below) and can provide accurate data of myocardium using echo-planar MRI [49].
Central nervous system
The most common indications for contrast-enhanced MR imaging of the CNS are: primary neoplasms (Fig. 2); brain metastases; demyelinating diseases; infectious and inflammatory processes; and vascular anomalies. In the past few years, contrast-enhanced MR has been shown to be useful for the early diagnosis of cerebral ischaemia and infarction. Controversial issues concerning contrast-agent administration include: Which dose should be administered, and what is the optimal time interval between contrast-medium injection and MR imaging? A low dose (0.05 mmol/kg) is sufficient for the evaluation of pituitary adenomas and acoustic neurinomas because of their high intrinsic contrast [43], whereas it has been shown that double or triple doses (0.2–0.3 mmol/kg) are preferable for the detection of cerebral ischaemia in functional and dynamic studies, to characterize brain tumours and to differentiate tumour recurrence from tissue necrosis [19, 20, 23]. In addition, a multicentre study [50] showed that Gd-enhanced MR images provided additional information on 96% of intradural, extra- and intramedullary tumours, and 53% of extradural tumours.
A case of infiltrative glioma located in the left parietal and temporal lobes. a Unenhanced T1-weighted image shows an ill-defined hypodense area (arrow). b A Gd injection allows delineation of a hypervascular tumour (arrows). c On the proton-density-weighted image, the glioma appears heterogeneous. d Coronal Gd-enhanced T1-weighted image clearly demonstrates the hypervascular component of the glioma
Abdomen and pelvis
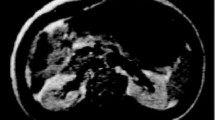
Continuous attempts are being made to improve the detection and characterization of lesions, and to determine accurately the extent of malignant tumour dissemination [51, 52]. The combined use of Gd chelates and breath-hold imaging (increased tumour-to-parenchyma contrast ratio) results in better tumour detection in most organs, as compared with unenhanced MR imaging. Most MR examinations of the abdomen and pelvis include multiphasic dynamic imaging after bolus injection of a Gd chelate. The contrast agent (0.1 mmol/kg) is administered (manually or with a power injector), and is followed within seconds by a rapid flush of 10–20 ml of saline. Dynamic gradient-echo MR imaging after bolus injection of Gd chelates allows imaging of the whole liver during the arterial, portal (Fig. 3), venous, and delayed phases. Poorly vascularized liver tumours are best detected during the portal phase and highly vascularized liver tumours during the arterial phase (Fig. 4) [5, 36, 45]. Dynamic Gd-enhanced MR also proved to be superior to helical CT for the detection of hypervascular tumours [51, 52]. In addition, Gd chelates provide useful information for characterizing liver [44, 45] and renal [53, 54] tumours, and in the pretherapeutic assessment of uterine [55], ovarian [56], bladder and prostate tumours [57, 58]. Controversies remain concerning the best contrast agent for MR imaging of the liver, either for detection or characterization: extracellular compounds; superparamagnetic particles; manganese or gadobenate dimeglumine which has a nonspecific extracellular phase followed by specific uptake by functioning hepatocytes prior to its excretion into the bile. Future developments of dynamic Gd-enhanced MR include non-invasive assessment of microcirculation and vascular permeability with potential monitoring of tumour therapy, including antiangiogenic therapy [46].
Focal nodular hyperplasia (FNH). a Unenhanced T1-weighted image demonstrates the lesion as a heterogeneous mass (arrow), with central hypointense area. b Early Gd-enhanced T1-weighted image shows massive peripheral enhancement of the lesion. c Delayed Gd-enhanced T1-weighted image shows late enhancement of the central scar. d Comparative T2-weighted image. The lesion appears hyperintense relative to the surrounding liver parenchyma, with a central hyperintense scar
MR angiography
Magnetic resonance angiography with contrast agents is rapidly evolving and is becoming a valid alternative to other imaging modalities for the assessment of vascular anatomy and disease. Rapid high-resolution 3D data of the entire peripheral vascular tree or abdominal and renal vessels can be obtained using contrast-enhanced MR angiography [21, 59]. Clinical experience suggests that increased and homogeneous enhancement of the vasculature can be obtained with paramagnetic agents that interact weakly with proteins as compared with conventional Gd chelates. MultiHance is such a contrast agent and it provides more intense and longer-lasting vascular enhancement that the non-protein-binding Gd chelates [21].
Breast
The injection of Gd chelates and the use of fat-suppression techniques help differentiate lesions that are strongly enhanced (fibroadenomas, carcinomas and proliferative dysplasia) from those lesions that are not or only poorly enhanced (scars, cysts and non-proliferative dysplasia). Analysis of dynamic images obtained after Gd injection as a function of time, and time-intensity curves, may be used to detect multicentric malignancies, recurrent local breast cancer or benign post therapeutic fibrosis [60, 61, 62].
Musculoskeletal system
Gadolinium chelates combined with dynamic MR imaging are extensively used to detect and characterize mass lesions, inflammatory processes and evaluate the extent of disease [63]. Dynamic Gd-enhanced MR imaging can effectively differentiate between epidural fibrosis and recurrent disk herniation.
Conclusion
Gadolinium chelates are widely used because they are well tolerated, they improve the diagnostic efficacy, and these more informative images contribute to better disease monitoring and thus guidance of disease management.
References
Mathur-de Vré R, Lemort M (1995) Biophysical properties and clinical applications of magnetic resonance imaging contrast agents. Br J Radiol 68:225–247
Shellock FG, Kanal E (1999) Safety of magnetic resonance imaging contrast agents. J Magn Reson Imaging 10:477–484
Brash RB (1992) New directions in the development of MR imaging contrast media. Radiology 183:1–11
Brash RC, Weinmann HJ, Wesbey GE (1984) Contrast enhanced NMR imaging: animal study using gadolinium-DTPA complex. AJR 142:625–630
Van Beers BE, Gallez B, Pringot J (1997) Contrast-enhanced MR imaging of the liver. Radiology 203:297–306
Rinck PA, Muller RN (1999) Field strength and dose dependence of contrast enhancement by gadolinium-based MR contrast agents. Eur Radiol 9:998–1004
Tweedle MF, Wedeking P, Krishan K (1995) Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Invest Radiol 30:372–380
Oksendal A, Hals P (1993) Biodistribution and toxicity of MR imaging contrast media. J Magn Reson Imaging 3:157–165
Chang C (1993) Magnetic resonance imaging contrast agents. Designs and physiochemical properties of gadodiamide. Invest Radiol 28 (Suppl 1):521–527
Cacheris W, Quay S, Rocklaye S (1980) The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging 8:467–481
Harpur E, Worah D, Hals P et al. (1993) Preclinical safety assessment and pharmacokinetics of gadodiamide injection: a new magnetic resonance imaging contrast agent. Invest Radiol 28:528–543
Tweedle MF, Ealon S, Eckelman W et al. (1988) Comparative chemical structure and pharmacokinetics of MRI contrast agents. Invest Radiol 23 (Suppl 1):236–239
Tweedle MF (1992) Physicochemical properties of gadoteridol and other magnetic resonance contrast agents. Invest Radiol 27 (Suppl 1):52–56
Runge VM, Parker JR (1997) Worldwide clinical safety assessment of gadoteridol injection: an update. Eur Radiol 7 (Suppl 5):243–245
Nelson KI, Gifford LM, Lauber-Huber C, Gross CA, Lasser TA (1995) Clinical safety of gadopentetate dimeglumine. Radiology 2:349–443
Niendorf HP, Haustein J, Cornelius I, Alhassan A, Clauss W (1991) Safety of gadolinium-DTPA: extended clinical experience. Magn Reson Med 22:222–228
Kanal E, Applegate G, Gillen C (1990) Review of adverse reactions, including anaphylaxis in 5260 cases receiving gadolinium-DTPA by bolus injection. Radiology 177:159
Tweedle MF (1997) The proHance story: the making of a novel MRI contrast agent. Eur Radiol 7 (Suppl 5):S225–S230
Yuh WTC, Parker JR, Carvlin MJ (1997) Indication-related dosing for resonance contrast media. Eur Radiol 7 (Suppl 5):S269–S275
Yuh WTC, Nguyen HD, Tali ET et al. (1994) Delineation of gliomas with various doses of MR contrast material. Am J Neuroradiol 15:983–989
Prince MR (1998) Contrast-enhanced MR angiography: theory and optimization. Magn Reson Imaging Clin North Am 6:257–267
Haustein J, Laniado M, Niendorf HP et al. (1993) Triple-dose versus standard-dose gadopentetate dimeglumine: a randomized study in 199 patients. Radiology 186:855–860
Yuh WTC, Fisher DJ, Engelken JD et al. (1991) MR evaluation of CNS tumors: dose comparison study with gadopentetate dimeglumine and gadoteridol. Radiology 180:485–491
Szopinski K, Szopinska M, Borowka A, Jakubovski W (2000) Magnetic resonance urography: initial experience of a low dose Gd-DTPA-enhanced technique. Eur Radiol 10:1158–1164
Olukotun AY, Parker JR, Meeks MJ, Lucas MA, Fowler DR, Lucas TR (1995) Safety of gadoteridol injection: US clinical trial experience. J Magn Reson Imaging 5:17–25
Cohan RH, Leder RA, Herzberg AJ et al. (1991) Extravascular toxicity of two magnetic resonance contrast agents: preliminary experience in the rat. Invest Radiol 26:224–226
Murphy KJ, Brunberg JA, Cohan RH (1996) Adverse reaction to gadolinium constrast media: a review of 36 cases. AJR 167:847–849
Niendorf HP, Alhassan A, Haustein J, Clauss W, Cornelius I (1993) Safety and risk of gadolinium-DTPA: extended clinical experience after more than 5,000,000 applications. Adv MRI Contrast 2:12–19
Runge VM (2000) Safety of approved MR contrast media for intravenous injection. J Magn Reson Imaging 12:205–213
Eldevik OP, Brunberg JA (1994) Gadopentetate dimeglumine-enhanced MR of brain: clinical utility and safety in patients younger than two years of age. AJNR 15:1001–1008
Hanquiret S, Christope C, Greef DD, Gordon P, Perlemuller N (1996) Clinical evaluation of gadodiamide injection in pediatric MR imaging. Pediatric Radiol 26:806–810
Niess AC, Le Mignon MM, Vitry A, Caille JM (1991) Efficacité et tolérance du DOTA-Gd lors d'une enquête multicentrique européenne. Rev Im Med 3:383–387
Ball WJ, Nadel S, Zimmerman R et al. (1993) Phase III multicenter clinical investigation to determine the safety and efficacy of gadoteridol in children suspected of having neurologic disease. Radiology 186:769–774
Yoshikawa K, Davies A (1997) Safety of ProHance in special population. Eur Radiol 7 (Suppl 5):246–250
Haustein J, Niendorf H, Krestin G et al. (1992) Renal tolerance of gadolinium-DTPA dimeglumine in patients with chronic renal failure. Invest Radiol 27:153–156
Bellin MF, Deray G, Assogba U et al. (1992) Gd-DOTA: evaluation of its renal tolerance in patients with chronic renal failure. Magn Reson Imaging 10:115–118
Tombach B, Bremer C, Reimer P et al. (2001) Renal tolerance of a neutral gadolinium chelate (gadobutrol) in patients with chronic renal failure: results of a randomized study. Radiology 218:651–657
Tombach B, Heindel W (2002) Value of 1.0 M gadolinium chelates: review of preclinical and clinical data on gadobutrol. Eur Radiol 12:1550–1556
Tombach B, Bremer C, Reimer P et al. (2002) Using highly concentrated gadobutrol as an MR contrast agent in patients also requiring hemodialysis: safety and dialysability. AJR 178:105–109
Schmiedl U, Maravilla K, Gerlach R, Dowling C (1990) Excretion of gadopentetate dimeglumine in human breast milk. AJR 154:1305–1306
Rojski N, Weinreb J, Lih A (1993) Quantitative analysis of gadopentetate dimeglumine excreted in breast milk. J Magn Reson Imaging 3:131–132
Kubik-Huch RA, Gottstein NM, Frenzel T et al. (2000) Gadopentetate dimeglumine excretion into human breast milk during lactation. Radiology 216:555–558
Colosimo C, Manfredi R, Tartaglione T (1997) Contrast-enhanced issues in the MR evaluation of the central nervous system. Eur Radiol 7 (Suppl 5):231–237
Hamm B, Thoeni RF, Gould RG et al. (1994) Focal liver lesions: characterization with nonenhanced and dynamic contrast-material-enhanced MR imaging. Radiology 190:417–423
Bartolozzi C, Lencioni R, Donati F, Cioni D (1999) Abdominal MR: liver and pancreas. Eur Radiol 9:1496–1512
Delorme S, Knopp MV (1998) Non-invasive vascular imaging: assessing tumour vascularity. Eur Radiol 8:517–527
Ho KY, Leiner T, de Haan MW, van Engelshoven JM (1999) Peripheral MR angiography. Eur Radiol 9:1765–1774
Mahfouz AE, Hamm B, Taupitz M (1997) Contrast agents for MR imaging of the liver: a clinical overview. Eur Radiol 7:507–513
Saeed M, Higgings CB, Geschwind JF, Wendland MF (2000) T1-relaxation kinetics of extracellular, intracellular and intravascular MR agents in normal and acutely reperfused infarcted myocardium using echo-planar MR imaging. Eur Radiol 10:310–318
Sze G, Stimac GK, Barlett C et al. (1990) Multicenter study of gadopentetate dimeglumine as an MR contrast agent: evaluation in patients with spinal tumors. AJNR 11:967–974
Yamashita Y, Mitsuzaki K, Yi T et al. (1996) Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of the whole liver. Radiology 200:79–84
Oi H, Mukarami T, Kim T et al. (1996) Dynamic MR imaging and early-phase helical CT for detecting small intrahepatic metastases of hepatocellular carcinoma. AJR 166:369–374
Scialpi M, Maggio A di, Midiri M, Loperfido A, Angelelli G, Rotondo A (2000) Small renal masses: assessment of lesion characterization and vascularity on dynamic contrast-enhanced MR imaging with fat suppression. AJR 175:751–757
Balci NC, Semelka RC, Patt RH et al. (1999) Complex renal cysts: findings on MR imaging. AJR 172:1495–1500
Saez F, Urresola A, Larena JA et al. (2000) Endometrial carcinoma: assessment of myometrial invasion with plain and gadolinium-enhanced MR imaging. J Magn Reson Imaging 12:460–466
Kinoshita T, Ishii K, Naganuma H, Higashiiwai H (2000) MR findings of ovarian tumours with cystic components. Br J Radiol 73:333–339
Barentsz JO, Engelbrecht M, Jager GJ et al. (1999) Fast dynamic gadolinium-enhanced MR imaging of urinary bladder and prostate cancer. J Magn Reson Imaging 10:295–304
Hayashi N, Tochigi H, Shiraishi T, Takeda K, Kawamura J (2000) A new staging criterion for bladder carcinoma using gadolinium-enhanced magnetic resonance imaging with an endorectal surface coil: a comparison with ultrasonography. Br J Urol 85:32–36
Glockner JF (2001) Three-dimensional gadolinium-enhanced MR angiography: applications for abdominal imaging. RadioGraphics 21:357–370
White-Nunes L, Schnall MD, Orel SG et al. (1999) Correlation of lesion appearance and histologic findings for the nodes of a breast MR imaging interpretation model. RadioGraphics 19:79–92
Fisher U, Kopka L, Grabbe E (1999) Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic apprach. Radiol 213:881–888
Hulka CA, Edmister WB, Smith BL et al. (1997) Dynamic echo-planar imaging of the breast: experience in diagnosing breast carcinoma and correlation with tumor angiogenesis. Radiology 205:837–842
van der Woude HJ, Verstraete KL, Hogendoorn PC, Taminiau AH, Hermans J, Bloem JL (1998) Musculoskeletal tumors: Does fast dynamic contrast-enhanced subtraction MR imaging contribute to the characterization? Radiology 208:821–828
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellin, M.F., Vasile, M. & Morel-Precetti, S. Currently used non-specific extracellular MR contrast media. Eur Radiol 13, 2688–2698 (2003). https://doi.org/10.1007/s00330-003-1912-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-1912-x