Abstract
Habitat loss and climate change are major processes affecting biodiversity, especially in the Arctic which is experiencing rapid sea ice decline. Loss of sea ice habitat for ice-dependent species such as polar bears (Ursus maritimus) has been associated with declines in body condition, reproductive output, survival, and abundance. Monitoring habitat use can therefore provide insights into population responses to sea ice loss, especially for vulnerable demographic groups such as subadults. Here, we used resource selection functions to examine habitat selection patterns of subadult male and female (n = 21) and adult female (n = 37) polar bears in the Southern Beaufort Sea population from 2007 to 2011. We found that polar bears displayed broad similarities in seasonal habitat selection by using nearshore areas in winter/spring and ranging farther offshore into the multiyear ice in summer/autumn. However, there were differences in habitat use among age, sex, and reproductive classes. Adult females with cubs-of-the-year differed the most among classes and selected landfast ice in spring, allowing them to hunt for seal birth lairs while reducing risk of intra-specific predation. Adult females with older cubs and solitary adult females used active sea ice, which allowed them to hunt adult seals, while subadult females used a mix of active and landfast ice. Subadult males had similar selection for landfast ice as females with cubs-of-the-year, potentially as a mechanism to reduce intra-specific competition and/or kleptoparasitism. The Arctic faces continued warming and understanding variation in habitat use patterns can assist in identifying which bears are most vulnerable to loss of different sea ice habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and fragmentation are key drivers of biodiversity loss (Brook et al. 2008; Mantyka-Pringle et al. 2012) and anthropogenic climate change similarly threatens global biodiversity (Scheffers et al. 2016). Climate change and habitat loss can interact synergistically to negatively affect species (Opdam and Wascher 2004; Brook et al. 2008; Mantyka-Pringle et al. 2012). As changes to habitats are predicted to continue due to climate change (Mantyka-Pringle et al. 2012; IPCC 2014), understanding habitat use and requirements can aid conservation efforts. Habitat use can vary among age classes in many species (Mattson et al. 1987; Reid et al. 1994; Whitehead et al. 2002; Kokurewicz 2004; Crawford et al. 2012). However, habitat use is often modeled with little or no consideration for age or reproductive class, which can result in incomplete assessments of habitat requirements due to variation within populations (Aebischer et al. 1993; Durner et al. 2009; McCall et al. 2016). Understanding variation in habitat use and selection among demographic groups can help identify which groups are most vulnerable to habitat change, and improve predictions about population responses to future change.
The Arctic is warming at a faster rate than the rest of the world (Wassmann et al. 2011; IPCC 2014; Parkinson 2014), resulting in sea ice extent reductions, increased open water duration (Comiso 2002; Parkinson and Cavalieri 2008; Stroeve et al. 2012; Parkinson 2014), earlier sea ice breakup, and later freeze-up (Stirling and Parkinson 2006; Stern and Laidre 2016; Stroeve and Notz 2018). Sea ice is critical habitat for many Arctic species and sea ice decline has negatively affected the population dynamics of various species including many Arctic marine mammals (Laidre et al. 2008; Post et al. 2009; Kovacs et al. 2011). For example, sea ice is essential for polar bears (Ursus maritimus) due to its use as a platform for movement and foraging on their main prey [ice-associated ringed seals (Pusa hispida) and bearded seals (Erignathus barbatus)] (Stirling and Archibald 1977; Smith 1980). Because sea ice affects energy intake and use, polar bear body condition and growth are affected by sea ice availability (Stirling et al. 1999; Rode et al. 2010; Durner et al. 2017). Climate change-induced sea ice decline thus affects the availability of polar bear critical habitat and sea ice loss has been associated with negative effects on polar bear body condition, reproduction, survival, and population abundance (Regehr et al. 2010; Rode et al. 2010; Bromaghin et al. 2015; Lunn et al. 2016). Polar bears are threatened by future sea ice declines (Wang and Overland 2009, 2012; IPCC 2014; Regehr et al. 2016) and it is therefore important to understand habitat use and requirements in the warming Arctic.
In particular, the Southern Beaufort Sea (SB) polar bear population has undergone extensive reductions in habitat availability due to declines in sea ice concentration by 9.3% decade−1 and ice-covered days by 17.5 days decade−1 (Stern and Laidre 2016), with associated declines in SB polar bear body condition, reproductive output, survival, and abundance (Regehr et al. 2010; Rode et al. 2010; Bromaghin et al. 2015). Studies on polar bear habitat use have identified selection for intermediate to high sea ice concentrations over the shallow continental shelf, which is a biologically productive region (Durner et al. 2009; Laidre et al. 2018; Lone et al. 2018b). Sea ice can be further categorized as stable landfast ice or active sea ice, which differ in their availability of prey (Stirling et al. 1993; Pilfold et al. 2016; Reimer et al. 2019). Landfast ice is lower-quality foraging habitat where ringed seal pups and adults in birth lairs are hunted by polar bears (Smith and Stirling 1975; Smith 1980; Stirling et al. 1993; Reimer et al. 2019). In contrast, active sea ice is high-quality foraging habitat along leads between the fast ice and drifting offshore ice and is the habitat where juvenile/adult ringed seals and bearded seals are available to polar bears (Stirling and Archibald 1977; Stirling et al. 1993; Amstrup et al. 2000; Pilfold et al. 2014; Reimer et al. 2019). Adult females with cubs-of-the-year (COY) select landfast ice in spring, likely to protect cubs from the threat of infanticide from adult males, whereas other age classes select active ice (Stirling et al. 1993; Freitas et al. 2012; Pilfold et al. 2014; McCall et al. 2016). Intra-specific competition may also influence distribution within a population and further segregate habitat use due to dominant individuals excluding subordinates from optimal habitat (Egbert and Stokes 1976; Mattson et al. 1987; Pilfold et al. 2014). For example, competition for food resources affects variation in grizzly bear (U. arctos) habitat use, whereby adult females and subadults avoid or are excluded from the habitats used by dominant adult males (Egbert and Stokes 1976; Mattson et al. 1987). Similarly, polar bears also differ in their competitive ability as adult males are the largest class and subadults are inexperienced hunters that adults can kleptoparasitize (Stirling et al. 1993; Pilfold et al. 2014). However, knowledge of variation in habitat use within the SB population primarily comes from bear captures and seal kill sites (Pilfold et al. 2014) and observational surveys (Stirling et al. 1993), whereas the use of telemetry to track the movements of different demographic groups of SB polar bears and examine habitat use is lacking.
In this study, we examined variation in SB polar bear habitat use between adult females of different reproductive status and male and female subadults using global positioning system (GPS) satellite-linked telemetry and resource selection functions (RSFs). Habitat use was compared between demographic groups and RSFs were used to predict subadult and adult female habitat selection in each season. We hypothesized that habitat selection would vary the most among classes during primary hunting/reproduction seasons (winter and spring). Researching variation in habitat use within the SB can improve our understanding of habitat requirements for different demographic groups, with implications for foraging success, energetics, and models of population-wide habitat use.
Methods
Study area
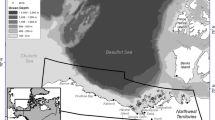
The study area was located in the southern Beaufort Sea from Pearce Point, Northwest Territories, Canada to Icy Cape, Alaska, USA, and offshore up to 80°N (Fig. 1). The clockwise Beaufort Gyre, wind, and currents affect ice drift patterns in this region (Proshutinsky et al. 2002; Bromaghin et al. 2015; Pongracz and Derocher 2017). There is a narrow continental shelf in the Beaufort Sea and primary productivity is driven by sea ice algae (Horner and Schrader 1982). The region is characterized by stable shorefast ice that forms each year, open-water leads that occur in spring at the boundary of the shorefast ice (active sea ice zones), and drifting pack ice farther offshore (Stirling et al. 1993; Pilfold et al. 2014; Pongracz and Derocher 2017). Open-water leads between the shorefast ice and the pack ice are important regions of biological productivity (Stirling et al. 1993; Bromaghin et al. 2015; Moore et al. 2018). When annual sea ice melts in summer, SB polar bears either travel north to multiyear ice in the Polar Basin or move onto land (Amstrup et al. 2000; Atwood et al. 2016; Pongracz and Derocher 2017).
Field sampling
Field work was conducted in April–May of 2007–2010 in the Canadian portion of the southern Beaufort Sea. Subadult male and female (3–4 years old) and adult female (≥ 5 years old) polar bears were immobilized using tiletamine hydrochloride and zolazepam hydrochloride (Zoletil®, Laboratoires Virbac, Carros, France) following standard procedures (Stirling et al. 1989) and fitted with GPS collars (Telonics, Mesa, AZ) linked to the Argos satellite system (CLS America Inc., Lanham, MD) that collected locations every four hours (Pongracz and Derocher 2017). The programmable releases (CR-2a, Telonics, Mesa, AZ) on the collars were set for 1 year for subadults and 2 years for adults and subadult collars had a corrodible link. Erroneous data, dropped collar data, locations on land, and locations from one adult female that traveled outside the study area to Wrangel Island, Russia (Johnson et al. 2017) were excluded from analyses. Capture and handling protocols followed the Canadian Council on Animal Care guidelines and were approved by the University of Alberta BioSciences Animal Care and Use Committee.
Habitat use
At each polar bear GPS location, eight environmental covariates were extracted: sea ice concentration (Ice; %), distance to 5% sea ice concentration contour (IceEdge; km), two types of ice thickness (FirstYear, OldIce; %), three types of ice floe size (SmallFloe, VastFloe, FastIce; %), and distance to land (DistLand; km) (Table S1 in Online Resource 1). These variables were chosen for ecological reasons relevant for polar bears: Ice is a key feature that influences polar bear habitat use, IceEdge represents the edge of the sea ice, ice thickness/floe type characterizes the seascape and prey availability (nearshore stable fast ice versus active ice) (Stirling et al. 1993; Pilfold et al. 2014), and DistLand is important because polar bears select nearshore habitats (Durner et al. 2009; Lone et al. 2018b). Sea ice concentration was obtained from satellite passive microwave data from the National Snow and Ice Data Center (daily SSM/I with a resolution of 25 km; Boulder, CO; Cavalieri et al. 1996; https://nsidc.org/data/NSIDC-0051/versions/1; accessed 09 April 2011) and sea ice thickness/floe size data were obtained from the Canadian Ice Service and extracted from a satellite remote sensor (weekly AMSR-E with a resolution of 6.5 km; Spreen et al. 2008; https://iceweb1.cis.ec.gc.ca/Archive/page1.xhtml; accessed 25 October 2011). ArcGIS was used to calculate the distance to land for each polar bear location (ArcGIS v.10.6.1, Environmental Systems Research Institute, Redlands, CA).
Use of each environmental covariate was compared among five SB polar bear classes: adult females with COY, adult females with older cubs (yearlings and two-year-old cubs), solitary adult females, subadult females, and subadult males. The environmental variables were non-normally distributed (Shapiro–Wilk test, p ≤ 0.05) and standard transformations did not improve normality; therefore, Kruskal–Wallis and Dunn’s non-parametric tests were used to examine differences in the use of environmental variables among classes in each season.
Resource selection models
RSFs are an effective method for modeling polar bear habitat selection using GPS locations and ecologically relevant environmental covariates, such as sea ice concentration, ice type, and distance to land (Manly et al. 2002; Durner et al. 2009; Rode et al. 2010; Pilfold et al. 2014; McCall et al. 2016). We created separate RSF models for subadults (pooled) and adult females (pooled) in the SB in each season from 2007 to 2011: winter (December–February), spring (March–May), summer (June–August), and autumn (September–November). A discrete choice modeling approach was used for the RSFs, which involved modeling polar bear selection for the environmental covariates with a used versus available habitat approach (Durner et al. 2009; Laidre et al. 2015, 2018; McCall et al. 2016). The used habitat locations were the polar bear GPS locations (1 location/day selected randomly) while available habitat locations were 75 randomly generated locations within a buffer around each used location (Laidre et al. 2015, 2018; Hauser et al. 2017). The radius of the buffer was based on seasonal mean movement rates between GPS locations for each class to estimate the distance a bear could travel in 3 days (overall mean hourly movements rates: 2.50 km/h for subadults and 2.07 km/h for adults) (Laidre et al. 2015, 2018). Because it can be expected that selection will be more similar within than between bears, a random-effect term for each individual was included in a generalized linear mixed effects model approach (McCall et al. 2016). The exponential RSFs were modeled with the lme4 package in R (R Core Team 2019) using the logistic regression equation:
where w(x) is the relative probability of selection, X is the value for the environmental covariates, and β values are the coefficients from the RSF model output. Thirty-four a priori RSF models (Table 1) were created using combinations of the environmental covariates based on ecological hypotheses: Ice (included in every model because it is a key feature in polar bear habitat use), ice thickness/floe type to characterize the seascape, and DistLand (Durner et al. 2009; Laidre et al. 2018). Variables were screened for collinearity in each season and models with correlated variables (|r|> 0.6) were removed (Table S2 in Online Resource 1) (Durner et al. 2009; Pilfold et al. 2014). Ice was modeled as a quadratic term because polar bears exhibit an intermediate preference (Durner et al. 2009; Pilfold et al. 2014).
Predicted habitat selection
Model selection was conducted using Akaike’s Information Criterion (AIC) to select the top model for subadults and adults in each season. The top model for each class in each season was used to predict the relative probability of selection across the landscape in each season using the equation of the top model (Eq. 1), β coefficients from each covariate in the top model, and the environmental conditions for a representative day in each season (winter: February 4; spring: May 7; summer: August 6; autumn: November 5) (Durner et al. 2009; McCall et al. 2016). The resulting RSF predictions were then scaled to a relative probability of selection from 0 to 1 to compare predicted selection between subadults and adults (Durner et al. 2009; Laidre et al. 2015).
RSF zones
Predicted RSF values for each age class in each season were placed into 10 equal-area bins that were ranked from 1 to 10 and the percentage of used locations falling into each bin was determined (Durner et al. 2009, 2019). In addition, the percentage of used locations in the upper 20% (i.e., optimal habitat) and upper 50% of RSF zones were calculated and chi-square tests were used to assess differences in the percentage of used locations in each RSF zone between age classes (Durner et al. 2009, 2019). The level of significance was set at α ≤ 0.05 and statistical analyses were conducted in R v.3.6.0 (R Core Team 2019).
Results
The four seasonal RSF models were constructed with 1399 locations pooled from 10 subadult males and 11 subadult females, and 2996 locations from 37 adult females (Fig. 1; Table 2). Given that some adult females were tracked for > 1 year, there were six adult females with COY, 35 adult females with older cubs, and five solitary adult females.
Habitat use
In winter, there were no significant differences in the use of Ice and FastIce among classes, and no significant differences in any environmental variables between subadult females and subadult males (Dunn’s tests, p > 0.05; Fig. 2; Table S3 in Online Resource 1). Subadults were significantly closer to land [mean 36 km (n = 11)] than adult females with older cubs [mean 60 km (n = 25)]. Adult females with COY were significantly closer to IceEdge [mean 545 km (n = 6)] and used areas with significantly less VastFloe [mean 0% (n = 6)] than all other classes [mean IceEdge > 662 km, VastFloe > 26% (n = 36)]. Adult females with COY also used areas with significantly more SmallFloe [mean 21% (n = 6)] than all classes [mean 12% (n = 30)] except subadult males [mean 14% (n = 6)].
In spring, adult females with COY used areas significantly closer to land [mean 28 km (n = 6)] with significantly more FastIce [mean 51% (n = 6)] and less VastFloe [mean 30% (n = 6)] than all other classes [mean DistLand > 42 km, FastIce < 22%, VastFloe > 58% (n = 59)] (Dunn’s tests, p ≤ 0.05; Fig. 2; Table S4 in Online Resource 1). Subadult males showed some similarities to adult females with COY by using areas significantly closer to land [mean 42 km (n = 10)] with significantly more FastIce [mean 22% (n = 10)] and less VastFloe [mean 58% (n = 10)] than adult females with older cubs/subadult females [mean DistLand > 50 km, FastIce < 12%, VastFloe > 69% (n = 45)]. Subadult males also used significantly lower Ice [mean 92% (n = 10)] than all classes [mean > 94% (n = 49)] except adult females with COY [mean 96% (n = 6)]. Adult females with older cubs used areas with significantly more OldIce [mean 5% (n = 34)] than subadults [mean < 2% (n = 21)]. Solitary adult females used areas with significantly more SmallFloe [mean 5% (n = 4)] than adult females with older cubs [mean 4% (n = 34)] and subadult females [mean 3% (n = 11)].
In summer, subadult males used areas with significantly lower Ice [mean 64% (n = 10)] and were closer to IceEdge [mean 25 km (n = 10)] than all other classes [mean Ice > 74%, IceEdge > 36 km (n = 54)] (Dunn’s tests, p ≤ 0.05; Fig. 2; Table S5 in Online Resource 1). Adult females with older cubs and subadult females used significantly more VastFloe [mean > 55% (n = 44)] than other classes [mean < 42% (n = 20)]. Adult females with COY used significantly more FastIce [mean 25% (n = 6)] and were significantly closer to land [mean 69 km (n = 6)] than other classes [mean FastIce < 9%, DistLand > 97 km (n = 58)].
In autumn, subadults used significantly lower Ice [mean 81% (n = 15)] than adults [mean > 86%) (n = 42] and were significantly closer to IceEdge [mean < 130 km (n = 15)] than adults [mean > 144 km (n = 42)] (Dunn’s tests, p ≤ 0.05; Fig. 2; Table S6 in Online Resource 1). Subadult females used areas farthest from land [mean 177 km (n = 8)], with significantly lower amounts of OldIce [mean 11% (n = 8)] and VastFloe [mean 12% (n = 8)] compared to all other classes [mean DistLand < 127 km, OldIce > 25%, VastFloe > 19% (n = 49)]. Solitary adult females used areas with significantly more OldIce [mean 56% (n = 5)] and VastFloe [mean 43% (n = 5)] than other classes [mean OldIce < 34%, VastFloe < 25% (n = 52)]. Adult females with COY were significantly closer to land [mean 57 km (n = 6)] than other classes [mean > 110 km (n = 51)].
Resource selection models
Ice and DistLand were the most common variables retained (in all of the 8 top RSF models), followed by FastIce (5 models), IceEdge (5 models), OldIce (4 models), SmallFloe (3 models), FirstYear (1 model), and VastFloe (1 model) (Table 3; Tables S7–S10 in Online Resource 1).
Ice was a significant predictor for subadults in winter and summer, and for adults in summer and autumn (Table 4). DistLand was a highly significant predictor in every season for both age classes. FastIce was significant for both classes in winter and spring. IceEdge was significant for adults in spring and both age classes in summer/autumn. OldIce was significant for subadults in spring while SmallFloe was significant for adults in spring/autumn.
Predicted habitat selection
In winter, both adults and subadults selected for nearshore regions over the continental shelf with low FastIce, with subadults selecting for lower Ice and closer to the coast (~ 30 km offshore) than adults (~ 50 km offshore) (Fig. 3; Table 4). In spring, both classes selected low FastIce/OldIce, with subadults selecting lower Ice and closer to land (~ 30 km offshore) than adults that selected closer to IceEdge, more SmallFloe, and farther offshore (~ 50 km). Both age classes selected closer to IceEdge and the farthest offshore in summer (~ 200 km), as well as closer to IceEdge and relatively far offshore (~ 100 km) in autumn.
RSF zones
A significantly larger proportion of adult locations occurred in the highest RSF zones in winter relative to subadults (Chi-square test, χ1 = 45.14, p ≤ 0.001; Fig. 4; Table 5). In spring, subadults had a larger proportion of locations in the upper 20% of RSF zones than adults (Chi-square test, χ1 = 3.95, p = 0.05), but were not significantly different in the upper 50% of RSF zones (Chi-square test, χ1 = 0.80, p = 0.37). The proportion of locations in the highest RSF zones did not differ significantly between classes in summer (Chi-square test, χ1 = 0.02, χ1 = 1.28, p > 0.05). In autumn, adults had a larger proportion of locations in the upper 50% of RSF zones (Chi-square test, χ1 = 5.28, p = 0.02), but were not significantly different in the upper 20% of RSF zones (Chi-square test, χ1 = 0.17, p = 0.68).
Discussion
Understanding variation in habitat use and requirements within populations can be beneficial for managing vulnerable populations experiencing habitat loss. Here, we found broad similarities as well as variation in habitat selection based on age, sex, and reproductive class for SB polar bears. Broadly, SB polar bears selected nearshore habitats with intermediate to high Ice over the continental shelf, similar to studies in this and other populations (Durner et al. 2009; Wilson et al. 2014; Laidre et al. 2018; Lone et al. 2018b). DistLand was the strongest predictor in all models, which is consistent with polar bear selection for shallow nearshore habitats that are more productive than deeper waters (Pongracz and Derocher 2017; Laidre et al. 2018). FastIce was significant in winter and spring (especially for adult females with COY), which is consistent with observations that the fast ice is important habitat for predation on ringed seal pups (Stirling et al. 1993; Freitas et al. 2012). IceEdge was important for adult females in spring, indicating selection for the floe edge, while the importance of SmallFloe for adults in spring indicates new ice that has formed in an active ice area such as a lead (Pilfold et al. 2014). All bears used areas closer to shore in winter and spring, while ranging farther offshore in areas near IceEdge with more OldIce in summer and autumn. Our results agree with studies that have found that SB bears select nearshore habitats in winter/spring and travel farther offshore in summer/autumn to remain with remnant multiyear pack ice (Pilfold et al. 2014; Bromaghin et al. 2015; Pongracz and Derocher 2017). The offshore multiyear pack ice is not optimal polar bear habitat because it is over deeper, unproductive areas and it is energetically expensive for bears to travel longer distances as well as risk long-distance swims as the sea ice retreats (Pilfold et al. 2017; Pongracz and Derocher 2017). If optimal polar bear sea ice habitat continues to decline as predicted (Stern and Laidre 2016; Durner et al. 2019), SB polar bears may spend increasingly longer periods in this unproductive offshore region or more time on land (Bromaghin et al. 2015; Rogers et al. 2015; Pongracz and Derocher 2017). In turn, the SB polar bears have experienced nutritional stress (Amstrup et al. 2006; Stirling et al. 2008; Cherry et al. 2009; Rode et al. 2018) and declines in body condition, survival, and abundance (Regehr et al. 2010; Rode et al. 2010; Bromaghin et al. 2015) and these challenges may be exacerbated in the future.
While SB bears displayed broadly similar habitat selection, there was variation among age, sex, and reproductive classes, especially in winter and spring. Most notably, adult females with COY displayed the largest differences compared to all other classes and selected nearshore stable landfast ice in spring. The habitat use patterns of females with COY were consistent with the predicted selection for nearshore areas along the edge of the landfast ice in winter, when adults had the highest percentage of locations in the upper 20% RSF zones. In spring, adults had fewer locations in the upper 20% zones, potentially due to the differing selection between females with COY (high FastIce) versus other adults (low FastIce). Our results are consistent with studies in the SB that found nearshore stable landfast ice is selected by females with COY where they can hunt ringed seal pups and their mothers while balancing protection of their cubs from adult males (Taylor et al. 1985; Stirling et al. 1993; Derocher and Wiig 1999; Pilfold et al. 2014), reduce the risk of hypothermia for cubs (Blix and Lentfer 1979; Lone et al. 2018a), and because young cubs limit the mobility of adult females (Amstrup et al. 2000; Durner et al. 2009). Unfortunately, we are limited in our understanding of adult males because they cannot be collared, but they are the dominant age/sex class likely influencing the distribution of subordinates and have been observed selecting active ice and floe edges (Stirling 1974; Derocher and Stirling 1990; Stirling et al. 1993; Pilfold et al. 2014).
Adult females with older cubs used areas farthest offshore with less FastIce and more VastFloe in winter/spring, and were predicted to select for areas close to IceEdge with more SmallFloe in spring, suggesting selection for active ice zones at the floe edge. These active sea ice zones are prime habitat and can provide bears with a wide variety of large prey such as bearded seals and adult ringed seals (Stirling et al. 1993; Pilfold et al. 2014; Reimer et al. 2019). Our results are similar to the observations of Stirling et al. (1993) that females with yearlings and two-year-old cubs use the floe edge/active ice and avoid landfast ice habitat. Older cubs are less at risk of hypothermia (Blix and Lentfer 1979) and hunt more independently than COY (Stirling 1974); therefore, females with older cubs are less restricted in their movements than females with young offspring. There were fewer solitary adult females tracked but they had similar habitat selection for active ice zones. A limitation of our study is our assumption of a three-year reproductive cycle after releasing an adult female and because cub survival is low (Derocher and Stirling 1996), this may have resulted in misclassifying adult females that lost cubs.
In addition to the variation in habitat use among adult females, there were differences between subadults as well. Subadult females used more active sea ice zones than subadult males (farther from shore, more VastFloe), similar to females with older cubs/solitary adult females, while still using some landfast zones. The use of both habitat types potentially facilitates hunting or scavenging in high-quality active zones at the edge of the landfast ice near biologically productive open-water leads (Pilfold et al. 2014), while still providing access to safety/refuge in stable zones from threats such as ocean storms and long-distance swimming events (Durner et al. 2009; Pilfold et al. 2017). Furthermore, subadults are inexperienced and less efficient hunters (Stirling 1974; Bromaghin et al. 2015), and as such, intra-specific competition may be influencing the habitat use of subadult females as they may sometimes avoid or be excluded from high-quality habitat used by dominant adults (Mattson et al. 1987; Pilfold et al. 2014). Our results support Stirling et al. (1993) who found that subadult females showed a slight preference for active floe edge habitat, but they also did not avoid the landfast ice as strongly as other classes.
In contrast, subadult males displayed more similar habitat use patterns to adult females with COY by using nearshore stable landfast ice zones in spring. Subadult males may have been using habitat at the edge of the landfast ice as well as hunting/scavenging in adjacent high-quality active ice habitat (resulting in their use of lower Ice in spring), which is consistent with Pilfold et al. (2014) who found that subadult males were found in high-quality habitat. Of the demographic groups in our study, subadult males likely compete most directly with adult males, the dominant age/sex class. While older adult males are more successful at mating, subadult males have some mating success (Cronin et al. 2009; Zeyl et al. 2009; Stirling et al. 2016) and may therefore compete with adult males for mating opportunities as well as prey. Adult males may also kleptoparasitize kills made by subadult males (Stirling 1974; Stirling et al. 1993), and subadults can be killed by adult males (Amstrup et al. 2006). The use of lower-quality landfast ice habitat by subadult males may therefore be a mechanism to reduce intra-specific resource competition in the primary hunting/mating season, similar to the avoidance of adult male habitats by subadult grizzly bears (Mattson et al. 1987). These spring habitat use patterns of subadult males differ from observations of SB subadult males that used high-quality active ice zones and avoided stable landfast ice in the 1970s (Stirling et al. 1993). This may be due to differences in study design or a shift in subadult male distribution toward lower-quality habitat as the sea ice has declined.
The Arctic is projected to undergo continued warming and the SB is expected to experience further declines in sea ice habitat (Wang and Overland 2009, 2012; IPCC 2014; Stroeve and Notz 2018). Although an increasing proportion of SB polar bears have been noted to remain on land in the ice-free season (Rogers et al. 2015; Atwood et al. 2016), we found that many traveled north to the less productive multiyear sea ice in summer/autumn, which increases energetic expenditure and the risk of long-distance swims as sea ice declines (Bromaghin et al. 2015; Pilfold et al. 2017; Pongracz and Derocher 2017). In addition, sea ice drift rates have increased, which influences polar bear movements and may have detrimental effects on energy balances (Mauritzen et al. 2003; Durner et al. 2017). Landfast ice and active pack ice areas experience different drift patterns and have different associated energetic costs (Mauritzen et al. 2003; Durner et al. 2017; Blanchet et al. 2020), and the observed differential habitat use among SB demographic groups may therefore result in different energetic impacts in the population. Furthermore, we found that adult females with COY and subadult males used the lowest-quality habitat in the primary foraging season and they may therefore be most at risk to further declines in habitat. SB subadults have low survival rates (Bromaghin et al. 2015), high fasting rates (Rode et al. 2014), their condition is related to sea ice habitat availability (Rode et al. 2010), and they are more susceptible to unfavorable conditions (Molnár et al. 2010; Pongracz and Derocher 2017), which suggests that subadult males in lower-quality habitat will likely be especially vulnerable to future stressors. As Reimer et al. (2019) noted, bears in sub-optimal habitat may alter their habitat use and make riskier decisions as sea ice continues to decline, and these demographic groups are therefore important to monitor. There is a time lag between the loss of habitat and the ability to detect effects within a population, and the use of lower-quality habitat that has been observed in the SB and other polar bear populations can be an indicator of future demographic change (Laidre et al. 2018; Durner et al. 2019). Long-term research on habitat use of sea ice-dependent species and changes over time can therefore be a useful monitoring tool for vulnerable species experiencing habitat loss. Future studies would benefit from better information on habitat quality as well as larger sample sizes of all demographic classes to improve our understanding of observed patterns.
References
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325. https://doi.org/10.2307/1940062
Amstrup SC, Durner GM, Stirling I et al (2000) Movements and distribution of polar bears in the Beaufort Sea. Can J Zool 78:948–966. https://doi.org/10.1139/z00-016
Amstrup SC, Stirling I, Smith TS et al (2006) Recent observations of intraspecific predation and cannibalism among polar bears in the southern Beaufort Sea. Polar Biol 29:997–1002. https://doi.org/10.1007/s00300-006-0142-5
Atwood TC, Peacock E, McKinney MA et al (2016) Rapid environmental change drives increased land use by an Arctic marine predator. PLoS ONE 11:1–18. https://doi.org/10.1371/journal.pone.0155932
Blanchet M, Aars J, Andersen M, Routti H (2020) Space-use strategy affects energy requirements in Barents Sea polar bears. Mar Ecol Prog Ser 639:1–19. https://doi.org/10.3354/meps13290
Blix AS, Lentfer JW (1979) Modes of thermal protection in polar bear cubs—at birth and on emergence from the den. Am J Physiol 236:R67–R74. https://doi.org/10.1152/ajpregu.1979.236.1.r67
Bromaghin JF, Mcdonald TL, Stirling I et al (2015) Polar bear population dynamics in the southern Beaufort Sea during a period of sea ice decline. Ecol Appl 25:634–651. https://doi.org/10.1890/14-1129.1
Brook BW, Sodhi NS, Bradshaw CJA (2008) Synergies among extinction drivers under global change. Trends Ecol Evol 23:453–460. https://doi.org/10.1016/j.tree.2008.03.011
Cavalieri DJ, Parkinson CL, Gloersen P, Zwally HJ (1996) updated yearly. Sea Ice Concentrations from Nimbus-7 SMMR and DMSP SSM/I-SSMIS Passive Microwave Data, Version 1 [1979–2018]. NASA National Snow and Ice Data Center Distributed Active Archive Center, Boulder
Cherry SG, Derocher AE, Stirling I, Richardson ES (2009) Fasting physiology of polar bears in relation to environmental change and breeding behavior in the Beaufort Sea. Polar Biol 32:383–391. https://doi.org/10.1007/s00300-008-0530-0
Comiso JC (2002) A rapidly declining perennial sea ice cover in the Arctic. Geophys Res Lett 29:1956–1959. https://doi.org/10.1029/2002gl015650
Crawford JA, Frost KJ, Quakenbush LT, Whiting A (2012) Different habitat use strategies by subadult and adult ringed seals (Phoca hispida) in the Bering and Chukchi seas. Polar Biol 35:241–255. https://doi.org/10.1007/s00300-011-1067-1
Cronin MA, Amstrup SC, Talbot SL et al (2009) Genetic variation, relatedness, and effective population size of polar bears (Ursus maritimus) in the southern Beaufort Sea, Alaska. J Hered 100:681–690. https://doi.org/10.1093/jhered/esp061
Derocher AE, Stirling I (1990) Distribution of polar bears (Ursus maritimus) during the ice-free period in western Hudson Bay. Can J Zool 68:1395–1403. https://doi.org/10.1139/z90-208
Derocher AE, Stirling I (1996) Aspects of survival in juvenile polar bears. Can J Zool 74:1246–1252. https://doi.org/10.1139/z96-138
Derocher AE, Wiig Ø (1999) Infanticide and cannibalism of juvenile polar bears (Ursus maritimus) in Svalbard. Arctic 52:307–310. https://doi.org/10.14430/arctic936
Durner GM, Douglas DC, Albeke SE et al (2017) Increased Arctic sea ice drift alters adult female polar bear movements and energetics. Glob Chang Biol 23:3460–3473. https://doi.org/10.1111/gcb.13746
Durner GM, Douglas DC, Atwood TC (2019) Are polar bear habitat resource selection functions developed from 1985–1995 data still useful? Ecol Evol 9:8625–8638. https://doi.org/10.1002/ece3.5401
Durner GM, Douglas DC, Nielson RM et al (2009) Predicting 21st-century polar bear habitat distribution from global climate models. Ecol Monogr 79:25–58. https://doi.org/10.1890/07-2089.1
Egbert AL, Stokes AW (1976) The social behaviour of brown bears on an Alaskan salmon stream. Bears Their Biol Manag 40:41–56. https://doi.org/10.2307/3798913
Freitas C, Kovacs KM, Andersen M et al (2012) Importance of fast ice and glacier fronts for female polar bears and their cubs during spring in Svalbard, Norway. Mar Ecol Prog Ser 447:289–304. https://doi.org/10.3354/meps09516
Hauser DDW, Laidre KL, Stern HL et al (2017) Habitat selection by two beluga whale populations in the Chukchi and Beaufort seas. PLoS ONE 12:e0172755. https://doi.org/10.1371/journal.pone.0172755
Horner R, Schrader GC (1982) Relative contributions of ice algae, phytoplankton, and benthic microalgae to primary production in nearshore regions of the Beaufort Sea. Arctic 35:485–503. https://doi.org/10.14430/arctic2356
IPCC (2014) Climate Change 2014: Synthesis Report. Core Writing Team, Pachauri RK, Meyer LA (eds) Contribution of working groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva
Johnson AC, Pongracz JD, Derocher AE (2017) Long-distance movement of a female polar bear from Canada to Russia. Arctic 70:121–128. https://doi.org/10.14430/arctic4641
Kokurewicz T (2004) Sex and age related habitat selection and mass dynamics of Daubenton’s Bats Myotis daubentonii (Kuhl, 1817) hibernating in natural conditions. Acta Chiropterol 6:121–144. https://doi.org/10.3161/001.006.0110
Kovacs KM, Lydersen C, Overland JE, Moore SE (2011) Impacts of changing sea-ice conditions on Arctic marine mammals. Mar Biodivers 41:181–194. https://doi.org/10.1007/s12526-010-0061-0
Laidre KL, Born EW, Heagerty P et al (2015) Shifts in female polar bear (Ursus maritimus) habitat use in East Greenland. Polar Biol 38:879–893. https://doi.org/10.1007/s00300-015-1648-5
Laidre KL, Stern H, Born EW et al (2018) Changes in winter and spring resource selection by polar bears Ursus maritimus in Baffin Bay over two decades of sea-ice loss. Endanger Species Res 36:1–14. https://doi.org/10.3354/esr00886
Laidre KL, Stirling I, Lowry LF et al (2008) Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol Appl 18:S97–S125. https://doi.org/10.1890/06-0546.1
Lone K, Kovacs KM, Lydersen C et al (2018a) Aquatic behaviour of polar bears (Ursus maritimus) in an increasingly ice-free Arctic. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-27947-4
Lone K, Merkel B, Lydersen C et al (2018b) Sea ice resource selection models for polar bears in the Barents Sea subpopulation. Ecography 41:567–578. https://doi.org/10.1111/ecog.03020
Lunn NJ, Servanty S, Regehr EV et al (2016) Demography of an apex predator at the edge of its range: impacts of changing sea ice on polar bears in Hudson Bay. Ecol Appl 26:1302–1320. https://doi.org/10.1890/15-1256
Manly BFJ, McDonald LL, Thomas DL et al (2002) Resource selection by animals: statistical design and analysis for field studies. Kluwer, Dordrecht. https://doi.org/10.2307/5247
Mantyka-Pringle CS, Martin TG, Rhodes JR (2012) Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Glob Chang Biol 18:1239–1252. https://doi.org/10.1111/j.1365-2486.2011.02593.x
Mattson DJ, Knight RR, Blanchard BM (1987) The effects of developments and primary roads on grizzly bear habitat use in Yellowstone National Park, Wyoming. Bears Their Biol Manag 7:259–273
Mauritzen M, Derocher AE, Pavlova O, Wiig Ø (2003) Female polar bears, Ursus maritimus, on the Barents Sea drift ice: walking the treadmill. Anim Behav 66:107–113. https://doi.org/10.1006/anbe.2003.2171
McCall AG, Pilfold NW, Derocher AE, Lunn NJ (2016) Seasonal habitat selection by adult female polar bears in western Hudson Bay. Popul Ecol 58:407–419. https://doi.org/10.1007/s10144-016-0549-y
Molnár PK, Derocher AE, Thiemann GW, Lewis MA (2010) Predicting survival, reproduction and abundance of polar bears under climate change. Biol Conserv 143:1612–1622. https://doi.org/10.1016/j.biocon.2010.04.004
Moore SE, Stabeno PJ, Grebmeier JM, Okkonen SR (2018) The Arctic Marine Pulses Model: linking annual oceanographic processes to contiguous ecological domains in the Pacific Arctic. Deep Res Part II Top Stud Oceanogr 152:8–21. https://doi.org/10.1016/j.dsr2.2016.10.011
Opdam P, Wascher D (2004) Climate change meets habitat fragmentation: linking landscape and biogeographical scale levels in research and conservation. Biol Conserv 117:285–297. https://doi.org/10.1016/j.biocon.2003.12.008
Parkinson CL (2014) Spatially mapped reductions in the length of the Arctic sea ice season. Geophys Res Lett 41:4316–4322. https://doi.org/10.1002/2014GL060434
Parkinson CL, Cavalieri DJ (2008) Arctic sea ice variability and trends, 1979–2006. J Geophys Res Ocean 113:C07003. https://doi.org/10.1029/2007JC004558
Pilfold NW, Derocher AE, Richardson E (2014) Influence of intraspecific competition on the distribution of a wide-ranging, non-territorial carnivore. Glob Ecol Biogeogr 23:425–435. https://doi.org/10.1111/geb.12112
Pilfold NW, Hedman D, Stirling I et al (2016) Mass loss rates of fasting polar bears. Physiol Biochem Zool 89:377–388. https://doi.org/10.1086/687988
Pilfold NW, McCall A, Derocher AE et al (2017) Migratory response of polar bears to sea ice loss: to swim or not to swim. Ecography 40:189–199. https://doi.org/10.1111/ecog.02109
Pongracz JD, Derocher AE (2017) Summer refugia of polar bears (Ursus maritimus) in the southern Beaufort Sea. Polar Biol 40:753–763. https://doi.org/10.1007/s00300-016-1997-8
Post E, Forchhammer MC, Bret-Harte MS et al (2009) Ecological dynamics across the Arctic associated with recent climate change. Science 325:1355–1358. https://doi.org/10.1126/science.1173113
Proshutinsky A, Bourke RH, McLaughlin FA (2002) The role of the Beaufort Gyre in Arctic climate variability: seasonal to decadal climate scales. Geophys Res Lett 29:2100. https://doi.org/10.1029/2002gl015847
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Regehr EV, Hunter CM, Caswell H et al (2010) Survival and breeding of polar bears in the southern Beaufort Sea in relation to sea ice. J Anim Ecol 79:117–127. https://doi.org/10.1111/j.1365-2656.2009.01603.x
Regehr EV, Laidre KL, Resit Akcakaya H et al (2016) Conservation status of polar bears (Ursus maritimus) in relation to projected sea-ice declines. Biol Lett 12:20160556. https://doi.org/10.1098/rsbl.2016.0556
Reid DG, Code TE, Reid ACH, Herrero SM (1994) Spacing, movements, and habitat selection of the river otter in boreal Alberta. Can J Zool 72:1314–1324. https://doi.org/10.1139/z94-175
Reimer JR, Mangel M, Derocher AE, Lewis MA (2019) Modeling optimal responses and fitness consequences in a changing Arctic. Glob Change Biol 25:3450–3461. https://doi.org/10.1111/gcb.14681
Rode KD, Amstrup SC, Regehr EV (2010) Reduced body size and cub recruitment in polar bears associated with sea ice decline. Ecol Appl 20:768–782. https://doi.org/10.1890/08-1036.1
Rode KD, Regehr EV, Douglas DC et al (2014) Variation in the response of an Arctic top predator experiencing habitat loss: feeding and reproductive ecology of two polar bear populations. Glob Chang Biol 20:76–88. https://doi.org/10.1111/gcb.12339
Rode KD, Wilson RR, Douglas DC et al (2018) Spring fasting behavior in a marine apex predator provides an index of ecosystem productivity. Glob Chang Biol 24:410–423. https://doi.org/10.1111/gcb.13933
Rogers MC, Peacock E, Simac K et al (2015) Diet of female polar bears in the southern Beaufort Sea of Alaska: evidence for an emerging alternative foraging strategy in response to environmental change. Polar Biol 38:1035–1047. https://doi.org/10.1007/s00300-015-1665-4
Scheffers BR, De Meester L, Bridge TCL et al (2016) The broad footprint of climate change from genes to biomes to people. Science 354:aaf7671. https://doi.org/10.1126/science.aaf7671
Smith TG (1980) Polar bear predation of ringed and bearded seals in the land-fast sea ice habitat. Can J Zool 58:2201–2209. https://doi.org/10.1139/z80-302
Smith TG, Stirling I (1975) The breeding habitat of the ringed seal (Phoca hispida). The birth lair and associated structures. Can J Zool 53:1297–1305. https://doi.org/10.1139/z75-155
Spreen G, Kaleschke L, Heygster G (2008) Sea ice remote sensing using AMSR-E 89-GHz channels. J Geophys Res 113:C02S03
Stern HL, Laidre KL (2016) Sea-ice indicators of polar bear habitat. Cryosphere 10:1–15. https://doi.org/10.5194/tc-10-1-2016
Stirling I (1974) Midsummer observations on the behavior of wild polar bears (Ursus maritimus). Can J Zool 52:1191–1198. https://doi.org/10.1139/z74-157
Stirling I, Andriashek D, Calvert W (1993) Habitat preferences of polar bears in the western Canadian Arctic in late winter and spring. Polar Rec (Gr Brit) 29:13–24. https://doi.org/10.1017/S0032247400023172
Stirling I, Archibald WR (1977) Aspects of predation of seals by polar bears. J Fish Res Board Can 34:1126–1129. https://doi.org/10.1139/f77-169
Stirling I, Lunn NJ, Iacozza J (1999) Long-term trends in the population ecology of polar bears in western Hudson Bay in relation to climatic change. Arctic 52:294–306. https://doi.org/10.14430/arctic935
Stirling I, Parkinson CL (2006) Possible effects of climate warming on selected populations of polar bears (Ursus maritimus) in the Canadian Arctic. Arctic 59:261–275
Stirling I, Richardson E, Thiemann GW, Derocher AE (2008) Unusual predation attempts of polar bears on ringed seals in the southern Beaufort Sea: possible significance of changing spring ice conditions. Arctic 61:14–22. https://doi.org/10.14430/arctic3
Stirling I, Spencer C, Andriashek D (1989) Immobilization of polar bears (Ursus maritimus) with Telazol® in the Canadian Arctic. J Wildl Dis 25:159–168. https://doi.org/10.7589/0090-3558-25.2.159
Stirling I, Spencer C, Andriashek D (2016) Behavior and activity budgets of wild breeding polar bears (Ursus maritimus). Mar Mammal Sci 32:13–37. https://doi.org/10.1111/mms.12291
Stroeve J, Notz D (2018) Changing state of Arctic sea ice across all seasons. Environ Res Lett 13:103001. https://doi.org/10.1088/1748-9326/aade56
Stroeve JC, Serreze MC, Holland MM et al (2012) The Arctic’s rapidly shrinking sea ice cover: a research synthesis. Clim Change 110:1005–1027. https://doi.org/10.1007/s10584-011-0101-1
Taylor M, Larsen T, Schweinsburg RE (1985) Observations of intraspecific aggression and cannibalism in polar bears (Ursus maritimus). Arctic 38:303–309. https://doi.org/10.14430/arctic2149
Wang M, Overland JE (2009) A sea ice free summer Arctic within 30 years? Geophys Res Lett 36:L07502. https://doi.org/10.1029/2009GL037820
Wang M, Overland JE (2012) A sea ice free summer Arctic within 30 years: an update from CMIP5 models. Geophys Res Lett 39:L18501. https://doi.org/10.1029/2012GL052868
Wassmann P, Duarte CM, Agustí S, Sejr MK (2011) Footprints of climate change in the Arctic marine ecosystem. Glob Chang Biol 17:1235–1249. https://doi.org/10.1111/j.1365-2486.2010.02311.x
Whitehead AL, David BO, Closs GP (2002) Ontogenetic shift in nocturnal microhabitat selection by giant kokopu in a New Zealand stream. J Fish Biol 61:1373–1385. https://doi.org/10.1006/jfbi.2002.2147
Wilson RR, Horne JS, Rode KD et al (2014) Identifying polar bear resource selection patterns to inform offshore development in a dynamic and changing Arctic. Ecosphere 5:136. https://doi.org/10.1890/ES14-00193.1
Zeyl E, Aars J, Ehrich D et al (2009) The mating system of polar bears: a genetic approach. Can J Zool 87:1195–1209. https://doi.org/10.1139/Z09-107
Acknowledgements
We would like to thank Jodie Pongracz and Charlene Nielsen for assistance with data extraction and Nicholas Pilfold for constructive comments on modeling. We also thank Marie Auger-Méthé, Oliver Barker, Seth Cherry, Stephen Hamilton, Alysa McCall, Nicholas Pilfold, Jodie Pongracz, Vicki Sahanatien, and Mike Woodcock for their assistance in the field. We would like to acknowledge financial support from the Canadian Association of Zoos and Aquariums, Canadian Wildlife Federation, Environment and Climate Change Canada, Hauser Bears, Natural Sciences and Engineering Research Council of Canada, Polar Bears International, Polar Continental Shelf Project, Quark Expeditions, United States Department of the Interior (Bureau of Ocean Energy Management), and World Wildlife Fund Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Johnson, A.C., Derocher, A.E. Variation in habitat use of Beaufort Sea polar bears. Polar Biol 43, 1247–1260 (2020). https://doi.org/10.1007/s00300-020-02705-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02705-3