Abstract
We studied distribution and growth of Serripes groenlandicus and Macoma calcarea in the southeastern part of the Pechora Sea. The hypothesis was tested that trends in the site-to-site variability of population characteristics of these two bivalve species were driven by their feeding types (suspension-feeder and deposit-feeder, respectively). However, such a trend was found only in the abundance distribution of these species and site-to-site variability in growth rates of S. groenlandicus. M. calcarea density on silty sediments was almost twice as high as on sandy sediments, while Serripes biomass was almost 1.5 times higher on sandy sediments than on silty sediments. The slowest-growing Serripes were found at the deepest stations and in habitats with the largest content of fine fractions (silt) in sediments. Differences in the growth of S. groenlandicus could reflect variability of feeding conditions of this suspension-feeder (e.g., hydrodynamic conditions). Thus, the growth rate of S. groenlandicus was sensitive to environmental conditions, which means it can be used as an indicator of their changes. In general, S. groenlandicus in the Pechora Sea is very slow-growing compared to other areas (maximum life span and shell length are 28 years and 70 mm, respectively). Their growth rate was closest to that in Arctic-influenced locations. On the contrary, the maximum life span and shell length of M. calcarea in the Pechora Sea (15 years and 30 mm, respectively) were similar to those in other parts of the distribution area. No significant differences were found in the group growth of M. calcarea from different studied localities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Pechora Sea is an unusual Arctic basin. Atlantic, Arctic, and river waters collide there within a relatively small area. As a result, the water mass of the Pechora Sea is highly dynamic and is characterized by local temperature and salinity gradients. Therefore, a significant spatial–temporal heterogeneity in the distribution of benthic invertebrates may be expected. In recent decades, a strong anthropogenic factor has come into play in the Pechora Sea due to exploration and development of oil and gas fields though oil production has already begun only in one field on the Russian Arctic shelf. In the light of all this, the Pechora Sea seems promising for studying bioindicator properties and population organization of marine benthic invertebrates.
Growth parameters of organisms reflect ontogenetic changes of their individual growth, abiotic and biotic conditions of local biotopes, and the trends of key environmental variables. Bivalves are popular model objects for studies of the intraspecific variability of the growth rate. Growth parameters are very sensitive to a changing environment and can be fairly easily studied in bivalves using shell morphology. Biotopic diversity can often be reliably described by their growth rate variability, even within the same area. In some cases, the growth rate variability is quite comparable in different habitats of the same area and within the distribution area of the species (Jorgensen 1990; Gerasimova et al. 2003; Sukhotin et al. 2007).
Boreal-arctic bivalves Serripes groenlandicus (Mohr) and Macoma calcarea (Gmelin) are very common infaunal invertebrates. They often dominate in soft sediments of the Arctic seas (Zenkevich 1963; Pogrebov et al. 1997; Dahle et al. 1998; Denisenko et al. 2003; Britayev et al. 2010) and have a relatively long life span: up to 17 years for M. calcarea (Petersen 1978) and more than 30 years for S. groenlandicus (Kilada et al. 2007). Therefore, the population characteristics of both species may serve as good indicators in environmental monitoring. Nevertheless, the population features of M. calcarea in the Arctic seas have never been properly studied, with only fragmentary information on their abundance in some regions being available (Neyman 1963, 1969; Britayev et al. 2010).
Population studies of S. groenlandicus (the Greenland Cockle) (Menis and Oganesyan 1997; Gudimova 2002; Christian et al. 2010; Shulgina et al. 2015) were facilitated by the commercial importance of this species. Besides, these molluscs are a major component of trophic webs in the Arctic and Far Eastern seas because they are the food object of marine mammals such as walruses (Fisher and Stewart 1997; Born et al. 2003) and seals (Hjelset et al. 1999) and demersal fish such as cod and haddock (Dolgov and Yaragina 1990). However, there is little information about the distribution patterns of S. groenlandicus in different parts of its distribution area (Menis and Oganesyan 1997; Christian et al. 2010; Shulgina et al. 2015). Within the range, Serripes is known to be distributed from the minimal depth (1–5 m) to 125 m (Naumov 2006; Denisenko 2014), its preferred sediments are silty and silty-sandy (Christian et al. 2010; Shulgina et al. 2015), and the density is usually not very high, only rarely exceeding 30 ind. m−2 (Naumov 2006; Borisovets et al. 2017).
Noteworthy, the age of S. groenlandicus can be easily and reliably determined based on its external shell morphology (Maximovich and Gerasimova 2004; Ambrose et al. 2012). The growth characteristics of this species were used for assessing the spatial–temporal habitat diversity and even long-term climatic and hydrological trends (Gerasimova et al. 2003; Ambrose et al. 2006; Carroll et al. 2009, 2011). However, the spatial variability of the growth rate of S. groenlandicus has been studied only in a few locations: West Greenland (Petersen 1978), the White Sea, the Barents Sea (but not in the Pechora Sea) (Kuznetsov 1960; Gerasimova et al. 2003), and around the Svalbard Archipelago (Carroll et al. 2011). In these studies, it was shown that the growth rate of the Greenland Cockle was sensitive to environmental variability and could be a valuable proxy of ecosystem variation. The geographical differences of the growth rate of S. groenlandicus can be affected by water temperature (Kuznetsov 1960; Andrews 1972), salinity (Andrews 1972), and feeding conditions (Gerasimova et al. 2003; Ambrose et al. 2006; Carroll et al. 2011; Denisenko 2014). At the same time, the variability of the molluscan growth in different parts of the same region was mainly determined by the feeding conditions. The spatial differences in the growth rates of Serripes across sites around the Svalbard Archipelago (Carroll et al. 2011) appear to be associated with the proximity to the relatively warm, nutrient-rich Atlantic waters.
The growth characteristics of the Greenland Cockle in the Pechora Sea are almost unstudied. This is all the more unfortunate considering the diversity of its hydrological characteristics as well as the fact that its southern part has been almost entirely occupied by the community of this cockle for many years (Pogrebov et al. 1997; Dahle et al. 1998; Denisenko et al. 2003, 2005).
A large area in the southeastern part of the Pechora Sea was covered by a network of stations during two expeditions, in 2012 and 2013 (Online Resource 1). In this way, we had a chance to study ecological characteristics of mass representatives of benthic fauna in a previously unstudied area. The aim of this research was to compare the degree of heterogeneity of the abundance (density, biomass) and growth of S. groenlandicus and M. calcarea in the Pechora Sea. The hypothesis examined in this study is that the distribution and growth of these species in the southeastern part of the Pechora Sea is a reflection of the habitat conditions. The molluscs differed in feeding type: S. groenlandicus is a suspension-feeder, while M. calcarea is mainly a deposit-feeder (Naumov 2006). Accordingly, it was hypothesized that their distribution and growth would reflect sediment types in the study area.
Materials and methods
Study area and sampling
The temperature and salinity regimes of the Pechora Sea, which is located in the southeastern part of the Barents Sea (Online Resource 1), are mainly formed under the influence of meteorological conditions, the discharge of the Pechora River, and the inflow from the Barents Sea, the Kara Sea, and the White Sea (Zenkevich 1963; Matishov 1992; Pavlov and Pfirman 1995; Adrov and Denisenko 1996; Dahle et al. 1998). The currents influencing the study area are branches of the warm Kolguev-Pechora current (Atlantic water), the Litke cold current (Arctic water), and the coastal White Sea and Pechora currents (warm in summer and cold in winter). Ice also has a certain effect on the water temperature. Most of the Pechora Sea is covered with ice from October–November to the end of June-July (Adrov and Denisenko 1996; Nikiforov et al. 2005). In winter, the water temperature in the shallow-water Pechora Sea is close to freezing point, − 1.8 ÷ 0 °C. The highest water temperature (up to 6–10 °C in the southern regions) was recorded in August–September (Adrov and Denisenko 1996; Danilov et al. 2004). Seasonal variations in salinity in the Pechora Sea are due to river runoff, the influence of the White Sea waters, and formation and melting of ice. Salinity increases in winter, when the river runoff is the lowest. The typical salinity range in the open Pechora Sea is 33–35. In summer, the salinity in the open part of the Sea, usually decreases down to 26–33. The granulometric composition of the Pechora Sea sediments varies with depth. The sand fraction usually predominates down to a depth of 50 m, and pelites are mainly registered deeper than 100 m. Mixed sediments (sand, mud, clay) predominate in the middle part of the Sea (Ivanov et al. 2001; Danilov et al. 2004).
Sampling was carried out from the R/V “Dalnie Zelentsy” at 40 stations in SE Pechora Sea in July, August, and October of 2012–2013 (Online Resource 1; Table 1). The choice of the study area was determined by the plans for prospective oil field development in this area. The distance between stations varied from 5 to 30 km, on the average, about 20 km. The coordinates of the stations were determined with the help of a GPS navigator. Samples were taken with a 0.1 m2 van Veen grab (three replicates at each station). The sampling depth varied from 6 to 72 m. Samples were gently sieved through 0.7 mm mesh size screens. All animals were sorted out from the sediment, preserved in 75% alcohol and subsequently identified to the species or the lowest taxonomic level possible. All specimens of each species were counted and weighed (up to 0.001 g). The specimens of S. groenlandicus and M. calcarea were selected from the samples and used for further analysis.
The environmental data including the sediment granulometry, near-bottom temperature, salinity, oxygen concentration, and pH were determined at each station (3 replicates) during the same surveys. The granulometric analysis was performed using the standard two-stage procedure (Petelin 1967): (1) determining the proportion of coarse fractions by sieving the sediments through mesh sieves of 10 mm, 5 mm, 3 mm, 1 mm, 0.5 mm, and 0.25 mm; and (2) separating the sediments into fractions of 0.25–0.1 mm and 0.1–0.05 mm, 0.05–0.01 mm, 0.01–0.005 mm using aqueous analysis.
Analysis of spatial distribution of Serripes groenlandicus and Macoma calcarea
The similarity of the stations in respect of abiotic parameters was assessed using a cluster analysis (UPGMA algorithm) and multi-dimensional scaling (MDS) on the basis of Euclidian distance. The results of the cluster analysis and the ordination were verified by ANOSIM analysis. To assess the contribution of individual abiotic variables to the differences in station groups, the Simper analysis was used. Spearman rank correlation coefficient (at significance level α ≤ 0.05) and Mantel test (Legendre and Legendre 2012) were used to estimate the role of the environmental factors in the distribution of S. groenlandicus and M. calcarea.
Analysis of growth heterogeneity of the molluscs
Shell length (L, Online Resource 2) of bivalves was measured to the nearest 0.1 mm. The age of molluscs was determined by counting annual growth marks on the shells. It should be noted that age determination on the basis of external shell morphology is known to be inaccurate (MacDonald and Thomas 1980; Thompson et al. 1980; Murawski et al. 1982; Appeldoorn 1983; Wenne and Klusek 1985; Zolotarev 1989; Cardoso et al. 2013). In particular, determining the age of M. calcarea from western Greenland by counting external growth rings was highly problematic (Petersen 1978). Physicochemical methods relying on determination of the ratio of oxygen stable isotopes, magnesium and strontium content and radiography of shell have been successfully used in the last decades for determining the age of bivalves (Zolotarev 1989). In particular, they were used to confirm the annual character of the inner structure labels of the shell (Zolotarev 1989; Witbaard 1996; Khim 2001, 2002; Khim et al. 2003; Ambrose et al. 2006; Kilada et al. 2007; Cardoso et al. 2013). However, these methods are time- and effort-consuming, and so are not practical for processing large amounts of material. This means that the analysis of external shell morphology, however imperfect, remains the most common method of determining the ages of bivalves (Zolotarev 1989; Ambrose et al. 2012). To note, the age of S. groenlandicus can be reliably and relatively easily determined based on external shell morphology (Maximovich and Gerasimova 2012; Ambrose et al. 2012). The annual nature of external growth rings was confirmed in Serripes by isotopic analysis (Khim 2001, 2002; Khim et al. 2003; Ambrose et al. 2006).
Some criteria allowing one to distinguish additional rings among annual rings with a greater certainty have been proposed (Pannella and MacClintock 1968; Kennish and Olsson 1975; Taylor and Brand 1975; Okera 1976). To increase the objectivity of identification of annual rings on the molluscan shells (especially those of Macoma), we took into account several aspects (Online Resource 3), namely: rings associated with seasonal growth delays should be completely cyclic and equally expressed on both valves; they usually show through in the light; and there is often a stepped zone between adjacent growth rings. It should also be noted that we have many years of experience in using growth rings on molluscan shells for estimating the age of many bivalve species at the White Sea, including M. calcarea and S. groenlandicus (Maximovich and Gerasimova 2012; Gerasimova and Maximovich 2013).
Both individual and group (average) growth characteristics were used to analyze the variability of bivalve growth rate in the study area. Individual growth history of each mollusc (individual age series) was reconstructed by measuring the size of all growth rings (Online Resource 2). Group growth parameters of the bivalves (mean age series) were obtained for each station by averaging individual growth characteristics. Both individual and mean age series were used in the study of the heterogeneity of growth characteristics in the study area.
For growth pattern reconstruction the von Bertalanffy equation was used:
where Lt is the shell length (mm) at time t (year); L∞ (asymptotic or theoretically maximal length, mm), k (rate at which L∞ is approached, year−1) and t0 (theoretical time at which Lt = 0, year) are constants.
Growth age series were compared using an algorithm suggested by Maximovich (1989): a pairwise comparison of growth age series and their clustering was carried out using the analysis of residual variances with regard to growth curves. Significance of variance distinctions was estimated by Fisher’s F-statistic (F). The ratio of Fisher’s F-statistic to the critical F value at p < 0.05, F/Fcr, was used as a measure of distance between the compared age series. F/Fcr < 1 meant the absence of significant differences between the compared growth rows. Clustering was performed using the method of weighed pairgroup average. If the age series did not indicate that the growth rate slowed down with age, then the von Bertalanffy equation could not be used for their approximation (Maximovich 1989). In these cases, the age series were approximated for cluster analysis by the linear equation:
where a and b are constants.
Results
The area under consideration is located in the southeastern part of the Pechora Sea (Online Resource 1). Coordinates and environmental data for each station are presented in Table 1. As shown by the analysis of the grain size distribution, small-grained fraction (particles smaller than 0.25 mm) predominated in the sediments. This fraction is mainly represented by fine sand (on the average, 60%) and silt (30%). Hydrological-hydrochemical parameters of near-bottom waters (Table 1) in general corresponded to the literature data (Adrov and Denisenko 1996).
Neither M. calcarea nor S. groenlandicus were found at 4 stations (1, 7, 25, and 31). At the other stations at least one of the species was registered. The station similarity analysis (using the cluster and MDS analyses) in respect of abiotic parameters showed (Fig. 1) that there were two significantly different groups of stations (ANOSIM analysis, p < 0.05). The main contribution to the differences of these clusters (94%, Simper analysis) was made by fine sand and silt (Fig. 1A). Neither near-bottom water temperature nor oxygen concentration were taken into consideration in the analyses because the sampling in two surveys (2012 and 2013) was performed in different months (August, October, and July, respectively, Table 1).
Distribution of Macoma calcarea and Serripes groenlandicus
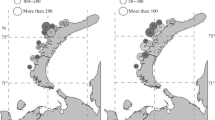
Macoma calcarea were found at 30 stations (out of 40) at depths from 8 to 52.5 m. Temperature of the near-bottom water varied at these stations between − 1.7 (station 36, July 2013) and 10.7 °C (station 21, October 2012), salinity varied from 24 (station 32) to 33.5 (stations 2, 8). Sixty-three percent of the stations with Macoma had silty-sand sediments, while 37% had sandy-silt sediments. The density and biomass of the molluscs varied from 3 to 193 ind. m−2 and from 0.003 to 57.5 g m−2, respectively, with the average values of 40 ± 8 ind. m−2 (n = 30) and 12 ± 3 g m−2 (n = 30). The greatest Macoma abundance was mainly recorded in the eastern part of the study area (Fig. 2) and up to depths of 20 m. There were almost no statistically significant correlations between the abundance of M. calcarea and the abiotic parameters except for a weak correlation with the silt proportion in the sediments (Spearman rank correlation coefficient 0.39). The maximum Macoma density and biomass (193 ind. m−2 and 56 g m−2) were found at a depth of 12 m in silt sand sediments (St. 33) where M. calcarea and S. groenlandicus were prevalent. In general, M. calcarea dominated by biomass at 6 stations, accounting for 17–66% of the total biomass of the zoobenthos.
Serripes groenlandicus were found at 29 stations at depths from 6 to 52.5 m. Temperature of the near-bottom water varied at these stations from -1.7 (station 36, July 2013) to 10.7 °C (station 21, October 2012) and salinity from 24 (station 32) to 33.5 (stations 2, 8). At 72% of these stations fine sand predominated in the sediments (the proportion of fine sand was 76% on the average), while 28% of the stations had a higher proportion of silt (72% on the average). Serripes density and biomass varied from 3 to 37 ind. m−2 and from 1 to 317 g m−2, respectively (Fig. 3), with the average values of about 12 ± 2 ind. m−2 (n = 29) and 98 ± 16 g m−2 (n = 29), respectively. The highest values of biomass were mainly recorded at depths of 15–25 m, though the absolute maximum (317 g m−2) was recorded at a depth of 35 m (St. 10). Station 10 was practically a sandy biotope, with sediments containing 90% of fine sand. S. groenlandicus often dominated in benthic communities (26 stations), where it accounted for 24 to 90% of the total macrobenthos biomass. Biomass of more than 100 g m−2 was registered at 10 stations, 8 of them had sediments with a high proportion (80% in average) of fine sand. The molluscan density at the stations was not high, with no more than 1–2 large specimens (longer than 40–50 mm) being encountered in most samples. No statistically significant correlations were registered between the abundance of S. groenlandicus and environmental parameters.
To analyze the correlation in the distribution of environmental parameters and the bivalves, the Mantel test was used. The comparison matrix of the stations according to abiotic parameters included Euclidian distance values, while the comparison matrix of the stations according to molluscan density or biomass included Bray–Curtis similarity indices. The Mantel correlation coefficient was statistically significant only in case of the comparison of the clustering results of stations by abiotic parameters and biomass of M. calcarea and S. groenlandicus. The coefficient was positive, but very small (0.13, permutation probability 0.046). The same parameter calculated using density values of M. calcarea and S. groenlandicus was 0.05 (permutation probability 0.15). All the sampled stations were divided into two clusters on the basis of the similarity of abiotic characteristics (Fig. 1). The analysis of the molluscan abundance in these clusters showed that the density of Macoma on silty sediments was almost twice as high as on sandy sediments (58 vs. 32 ind. m−2), while Serripes biomass was almost 1.5 times higher on sandy sediments than on silty sediments (106 vs. 77 g m−2). However, due to the large variation of the molluscan abundance in each cluster, these differences were not statistically significant.
Analysis of growth heterogeneity of Serripes groenlandicus and Macoma calcarea
Macoma calcarea
Individual growth
As mentioned above, age determination of M. calcarea using external shell morphology is problematic, primarily because of the strong erosion of the upper part of the shell. As a result, it was very difficult to estimate the size of the first growth rings and to determine the correct number of growth rings. However, in July 2013, a significant number of young molluscs not older than 1–2 years (sizes less than 3 mm) with well-discernible first rings were collected. Based on this material, we were able to estimate the variability range of the first and the second growth rings of M. calcarea in the study area. This allowed a fairly reliable reconstruction of the individual growth history of older individuals. A similar situation was noted by Petersen (1978), who could distinguish growth rings only in small Macoma.
In total, growth rings were measured in 158 individuals. The maximum shell length and age of M. calcarea was about 30 mm and 15 years, respectively. However, it was possible to determine the age of large individuals (more than 25 mm) only in few cases. In such molluscs the growth rings, as a rule, were difficult to distinguish. Therefore, older individuals could be among them as well. To study the individual growth variability, molluscs with well-discernible growth rings were selected. In most collected specimens only the first five (or four) growth rings were well-discernible while later rings were detected with much less certainty. In total, 23 specimens of M. calcarea were selected for this analysis. Differences in the individual growth rate were substantial: the size of the fifth growth ring varied from 4.7 to 8.1 mm. For approximation of individual age series, only linear equation could be used because the growth rate did not slow down with age. Using cluster analysis, the complex of age series was divided into two clusters combining specimens with a similar growth rate (Online Resource 4). However, the differences in the growth rate between the groups were not large (Fig. 4). The majority of individuals belonged to the group of relatively slow-growing (2) molluscs (5.5 mm in 5 years) (Fig. 4, Online Resource 4). Only five specimens formed a group of relatively fast-growing (1) clams (7 mm in 5 years). Interestingly, the distinctions between individual and group growth models were manifested as early as at the time formation of the second growth ring (Fig. 4) and persisted afterwards. Cases of compensatory growth (an increase of the growth rate after a period of slow growth) were rare. There was a positive correlation between the size of the second and that of the third growth ring (Spearman's rank correlation, rs = 0.62, n = 23, p = 0.002).
Group growth
Mean age series could be constructed only for eight stations (2, 14, 16, 27, 30, 36, 38, 39), which were used for the assessment of M. calcarea group growth variation in the study area. At the other stations too few Macoma were collected and (or) their growth rings were poorly discernible. Each age series included the mean size of the molluscs at the stage of the first 5–6 growth rings (Fig. 5a). Group growth variation was lower than the individual growth variation. The average size of the 5th growth ring varied between stations less than 1.5 times, from 5.5 to 7.5 mm. There were no significant differences in the group growth of M. calcarea (Online Resource 5). Therefore, a joint model of Macoma growth in the study area was constructed by averaging all individual size of growth rings at each age (Fig. 5b). The molluscs had a very low growth rate in the first 3–4 years of life (less than 2 mm per year) but maintained a relatively high growth rate (an average of about 3 mm per year) during the next 5–11 years. The clams older than 12 years had the lowest growth rate, less than 1–1.5 mm per year. In relative terms, the annual shell increments were maximum in one-year-old specimens (about 90% per year), changed little at an age of 5–11 years (an average of about 24% per year), and were minimum in individuals older than 12 years (less than 5% per year).
Serripes groenlandicus
Individual growth
As already noted, the number of Serripes at the study area was not high, with only 1–2 large specimens being found in most samples. Their shells were often badly damaged, and so the total shell length was not always possible to determine. In total, growth rings were measured in 55 individuals. The maximum shell length and age of S. groenlandicus was about 60–70 mm and 24–28 years, respectively. To study the individual growth variability, 33 individuals were selected, in which the size of the first 5–7 growth rings was determined starting from the second one (Fig. 6). The first ring, as a rule, was difficult to distinguish because of the strong erosion of the upper part of the shell. Differences in the individual growth of the molluscs were substantial: the size of the fifth growth ring varied from 12 to 21 mm. Only linear equation could be used to approximate the age series (Fig. 6). Using cluster analysis, the complex of age series was divided into three clusters uniting specimens with no significant differences of growth characteristics (Online Resource 6). Each cluster comprised specimens from different stations.
As in the case of M. calcarea, the distinctions between individual growth models were manifested as early as at the second year (Fig. 6) and often persisted afterwards. Cases of compensatory growth were rare. The size of the second growth ring correlated positively with that of the third growth ring (Spearman's rank correlation, rs = 0.47, n = 31, p = 0.007).
Group growth
Group (average) age series could be constructed only for 8 stations (10, 12, 13, 18, 19, 20, 27, 39), which were used for the assessment of S. groenlandicus group growth variability in the study area. As in the case of M. calcarea, at the other stations too few individuals were collected and (or) their growth rings were poorly discernible. Each age series included the mean sizes of the molluscs at the stage of the first 8 growth rings. Group growth variation was slightly lower than that of the individual growth. The average size of the 5th growth ring varied between stations from 14 to 19 mm. Two groups of age series were identified based on the statistical comparison of the age series (Online Resource 7). The Serripes group growth reconstruction in the clusters is shown in Fig. 7. Interestingly, the slowest growth of Serripes (an average of 26 mm in the first 8 years of life) was observed both at stations with the greatest content of silt (about 70%) in sediments and at the deepest stations (35 m) (Table 1). The molluscs grew faster (an average of 36 mm in the first 8 years of life) at stations where the proportion of silt did not exceed 16%, and fine sand fraction prevailed (an average of 74%). The average growth rate of slow-growing clams (Cluster 1) was relatively stable for all 8 years of their life, averaging 3.6 mm per year. Fast-growing molluscs (Cluster 2) demonstrated a relatively high growth rate at an age of 4–8 years (an average of about 6 mm per year).
Reconstruction of Serripes mean growth in the clusters (Online Resource 7). Each curve is a reconstruction of mollusc growth with no significant differences of group growth characteristics from different stations. Dots—average shell lengths during winter growth delay, vertical lines indicate 95% confidence intervals
Discussion
Our research demonstrated a substantial site-to-site variability in the density, biomass, and growth rates of both bivalves in the study area of the Pechora Sea. At the same time, the growth characteristics of S. groenlandicus were more sensitive to environmental conditions than those of M. calcarea.
Distribution patterns of Serripes groenlandicus and Macoma calcarea in SE Pechora Sea
The density and biomass (both mean and maximum), the ranges of vertical distribution (depth), the preferred abiotic conditions of S. groenlandicus and M. calcarea (temperature, salinity ranges, sediment types) in the SE Pechora sea were similar to those from other areas of distribution of these species (Neyman 1963; Menis and Oganesyan 1997; Kamenev et al. 2004; Naumov 2006; Shulgina et al. 2015; Borisovets et al. 2017). Noteworthy, the average abundance and the biomass of the molluscs practically coincided with those recorded in this area almost two decades ago (1991–1995) (Denisenko et al. 2003).
At this stage of the study, we were only able to relate the distribution of M. calcarea and S. groenlandicus in the SE Pechora Sea to the characteristics of the bottom sediments. As expected, the species showed opposite preferences in accordance with their feeding type: the abundance of M. calcarea (deposit-feeder) on the average was higher in sandy silts, while the biomass of S. groenlandicus (suspension-feeder) tended to increase in silty sand.
However, the species abundance did not always correlate with the sediment type. There were stations with silty sediments, where Macoma were missing or only a few individuals were found (e.g., stations 13, 29). On the contrary, a high abundance of this species was found in some sandy biotopes (e.g., stations 21, 27, 38). Similar observations are also true for Serripes. Thus, both species showed an extreme heterogeneity in the density and biomass within the preferred sediment type. It should be noted that M. calcarea is not an obligate deposit-feeder. It can also switch to suspension-feeding (Rasmussen 1973), which could affect the choice of biotope. Besides, due to a relatively low density of these species, three replicates of grab samples can hardly be considered as reliable for correct estimation of abundance. The Agassiz trawl is preferable for sampling in such cases (Gerasimova and Maximovich 2013; Shulgina et al. 2015).
The density and biomass of these two bivalve species recorded in our study could be inconsistent with their ecological properties. It is known that the structure of M. calcarea and S. groenlandicus beds in northern seas is unstable (Petersen 1978; Gerasimova and Maximovich 2013). Inter-annual changes in the quantitative characteristics of bed are typical of both species. This complicates the analysis of their distribution on the basis of a single sampling without taking into account the long-term dynamics of the bed structure.
The growth heterogeneity of Serripes groenlandicus and Macoma calcarea in the Pechora Sea
In general, the maximum shell length of M. calcarea and S. groenlandicus (30 and 70 mm accordingly) and their maximum longevity (15 and 28 years accordingly) recorded in the SE Pechora Sea fit well into the known range of similar characteristics of the same species from different geographic locations. Within the range, the life span of S. groenlandicus varied from 5 (in the Kandalaksha Bay, the White Sea) to 39 years (in eastern Canada), while the maximum length ranged from 28 mm (in the Kandalaksha Bay, the White Sea) to 110 mm (in the Sea of Japan) (Ockelmann 1958; Kuznetsov 1960; Petersen 1978; Zolotarev 1989; Kilada et al. 2007; Denisenko 2014; Shulgina et al. 2015). The corresponding values for M. calcarea were 6 years (in the Kandalaksha Bay, the White Sea) – 17 years (in West Greenland) and 18–38 mm (in the White Sea), accordingly (Petersen 1978; Antipova 1979; Naumov 2006; Lisitsyna et al. 2017).
The average annual growth rate of M. calcarea throughout the life cycle in the Pechora Sea, about 2 mm per year, was very close to that revealed by Petersen (1978) in the coastal waters of West Greenland from a wide range of depths (3–107 m). It was also close to that revealed recently in the Kara Sea from depth 20–53 m (Lisitsyna and Gerasimova 2019). However, M. calcarea in the Pechora Sea as well as in the Kara Sea had an extremely slow growth rate in the first years of life (Fig. 8). Their average shell length at an age of 1 year (July 2013) in the Pechora Sea was only 1.2 mm (the size of the growth ring was about 0.8 mm), and the length of 2-year-old molluscs (the same sample) was about 2.7 mm. Perhaps the reasons for such a low growth rate during the first years of life are related to severe temperature conditions of the Pechora Sea, which is covered with ice from October–November to the end of June-July (Adrov and Denisenko 1996; Nikiforov et al. 2005). Unlike the Pechora Sea, the White Sea, where M. calcarea grew much faster in the first years of life (Fig. 8), is free of ice by the end of May (Babkov and Golikov 1984). However, as noted earlier, the method used for age determination of bivalves on the basis of external shell morphology may potentially lead to biased growth rate estimations. Therefore, we plan to continue studying the growth character and life span of M. calcarea in the Arctic seas in the future.
Reconstruction of shell length growth of Macoma calcarea from different geographic locations. 1–3—White Sea (Lisitsyna et al. 2017): 1—depth 10–15 m, 2—depth 10–18 m, 3—depth 35–40 m; 4–6—Western Greenland (Petersen 1978): 4—depth 3–13 m, 5—depth 3–13 m, 6—depth 3–107 m; 7—Kara Sea, depth 20–53 m (Lisitsyna and Gerasimova 2019); 8—Pechora Sea, depth 8–52 m (our data)
Interestingly our results showed that there were no significant differences in Macoma group growth within the study area, despite the differences of environmental conditions (depths, sediments, etc.). The variability of M. calcarea individual growth was also relatively weak. Similar information is available for this species from the White Sea (Lisitsyna et al. 2017), from the Kara Sea (Lisitsyna and Gerasimova 2019) and from the West Greenland (Petersen 1978).
A poor variability of group growth of M. calcarea in the Pechora Sea can be considered from the point of view of the optimality of the conditions. A comparative analysis of M. calcarea growth was carried out for the stations, with a relatively high density of this species. In accordance with the Optimum Principle, the densest beds of species are formed under the most favorable conditions. The characteristics of the habitats under consideration might be optimal for M. calcarea. Bivalves in such habitats are known to have, on the average, the highest growth rate. Thus, the absence of differences in the growth patterns of M. calcarea at the stations could reflect the uniformity of habitats in terms of condition optimality. It should be noted the average abundance of M. calcarea at the analyzed stations was rather similar, 33–103 ind. m−2.
Unlike M. calcarea, S. groenlandicus in the Pechora Sea was characterized by a very slow growth (Fig. 9). Similar results for this species in the Pechora Sea (Fig. 9, curves 22–23) were obtained in an earlier study (Denisenko 2014). The group growth rate of the Greenland Cockle in the study area was much lower than that in the areas of the Barents Sea and the Svalbard Archipelago dominated by Atlantic waters (Fig. 9, curves 10, 19–21), being close to that in the Arctic-influenced areas around the Svalbard Archipelago (Fig. 9, curves 15–17). The slow growth of Serripes revealed in our study may be associated with severe temperature conditions and a relatively low salinity (because of the river Pechora runoff) in the Pechora Sea. As noted above, the Pechora Sea is covered with ice for 7–8 months a year, and the salinity of the water in its off-shore part decreases in the summer, reaching 26–33 (Adrov and Denisenko 1996; Nikiforov et al. 2005). However, it is unlikely that the salinity conditions influence considerably the growth rate of Serripes. A much faster growth of S. groenlandicus was found in the White Sea (Fig. 9, curves 12–14), where the surface salinity was even lower (24–26) than in our study (Babkov and Golikov 1984). The temperature appears to influence the growth rate of Serripes only indirectly, by affecting the feeding conditions (Carroll et al. 2009, 2011; Ambrose et al. 2012). The Greenland Cockle, being a suspension-feeder, feeds mainly on phytoplankton or on phytodetritus depending on the habitat depth (Denisenko 2014). Accordingly, the duration of the phytoplankton vegetation period, which is determined to a large extent by the temperature regime, can have a profound effect on the growth rates of these molluscs. The analysis of the seasonal changes in the growth rate of the Barents Sea S. groenlandicus showed that their growth resumed in spring at very low temperatures (Carroll et al. 2009; Ambrose et al. 2012). Formation of annual rings began in late summer or early autumn, when the water temperature was rather high, 4–6 °C. The researchers explained this situation by a seasonal deterioration in the feeding conditions of molluscs such as reduced daylight hours and lower rate of primary production (Carroll et al. 2009; Ambrose et al. 2012). The waters of Atlantic origin, which are known to be both warmer and more productive than Arctic waters (Wassmann et al. 2006; Reigstad et al. 2011; Makarevich 2012), have little effect on the analyzed area of the Pechora Sea (Nikiforov et al. 2005). The currents in this area are mainly represented by the coastal White Sea and Pechora currents, which are warm in summer and cold in winter (Nikiforov et al. 2005). In the Pechora Sea, a high phytoplankton productivity coincides with the ice melting in June–September (Makarevich 2012). According to the long-term observations, the primary production in the study area is less than 100 gC m −2 year −1 (Makarevich 2012), which is very close to that in the Arctic water areas of the Barents Sea (Wassmann et al. 2006; Makarevich 2012). This probably explains the similarity of S. groenlandicus growth rate in the Pechora Sea and in the Arctic-influenced areas around the Svalbard Archipelago (Carroll et al. 2011). South-western (non-freezing) areas of the Barents Sea are, as a rule, almost twice as productive (Wassmann et al. 2006; Makarevich 2012).
Reconstruction of shell length growth of Serripes groenlandicus from different geographic locations. 1—Eastern Canada (Kilada et al. 2007); 2—Eastern Canada (Andrews 1972); 3–6—Western Greenland: 3—depth 3–13 m, 4—depth 3–18 m, 5—depth 25–42 m, 6—depth 80–100 m (Petersen 1978); 7–8—Bering Sea (Khim 2001); 9–11—Barents Sea: 9—Svalbard, depth 24–50 m, 10—central area, depth 88–90 m, 11—East Murman, depth 25 m (Kuznetsov 1960); 12–14—White Sea: 12—depth 4–6 m, 13–14—depth 1–3 m (Gerasimova et al. 2003); 15–21—Pan-Svalbard (Carroll et al. 2011); 22–23—Pechora Sea: 22—depth 5–6 m, 23—depth 23 m (Denisenko 2014); 24–25—Pechora Sea: 24—depth 14–25 m, 25—depth 17–35 m (our data)
Feeding conditions can also determine the variability of molluscan growth in different parts of the same region. The fastest growth of S. groenlandicus in some regions was recorded in shallow habitats (Petersen 1978; Gerasimova et al. 2003; Denisenko 2014), where more living and dead phytoplankton reaches the bottom. Intensive wave mixing ensures a constant nutrient inflow, which in turn contributes to an increased productivity of the plankton community (Denisenko 2014). The observed differences in S. groenlandicus growth in the Pechora Sea may be determined by the spatial variation of the feeding conditions. Hydrodynamic activity, an indirect indicator of feeding conditions of suspension-feeders, can be inferred from sediment characteristics such as the size distribution of sediment particles. In our study, the lowest growth rate of the cockles was registered in the deepest habitats as well as in the sediments with the largest proportion of fine-grained fractions, while the highest growth rate was recorded at stations with the lowest proportion of silt.
It is noteworthy the differences in group (mean) growth of both species in the study area were smaller than the variation of individual growth rate. Individual growth can be an indicator of internal heterogeneity of local aggregations of bivalves. Specimens with significantly different growth rates can also differ in survival, mortality rate, respiration rate, and life span (Sukhotin 1992; Sukhotin and Kulakowski 1992; Gerasimova et al. 2017).
The highest heterogeneity of individual growth in the SE Pechora Sea was observed in S. groenlandicus. As noted above, practically nothing is known about the variability of individual growth rate of M. calcarea and S. groenlandicus. However, there are more data on other bivalve species, e.g., Mytilus edulis (Kulakovskii and Kunin 1982; Sukhotin 1992; Sukhotin and Kulakowski 1992; Gerasimova et al. 2014), Mytilus trossulus (Gagaev et al. 1994); Macoma balthica (Cloern and Nichols 1978); Macoma incongrua (Maximovich and Lysenko 1986); and Mya arenaria (Gerasimova et al. 2016, 2017). The differentiation of individual growth of S. groenlandicus and Macoma calcarea apparently occurred early in the life cycle and persisted afterwards. Cases of compensatory growth were infrequent.
Mechanisms ensuring different growth patterns are understudied. The annual shell increment of Mytilus edulis mostly correlates with the shell size attained by the start of the growing season (Savilov 1953; Seed 1969; Maximovich et al. 1993; Sukhotin and Maximovich 1994; Sukhotin et al. 2002). The differences in molluscan growth at the early stages can be associated with the conditions of spat formation. The correlation between the size by the start of the second growth season and later growth rate (during the entire life cycle) was shown for several bivalve species such as Mytilus edulis (Maximovich et al. 1993), Mytilus trossulus (Gagaev et al. 1994), Macoma balthica (Cloern and Nichols 1978) Macoma incongrua (Maximovich and Lysenko 1986) and Mya arenaria (Gerasimova et al. 2016, 2017). In S. groenlandicus and M. calcarea the differences in size by the start of the second growth season could be caused by the difference in the time of juvenile settling due to prolonged (up to 2–4 months) reproduction period in the northern seas (Ockelmann 1958; Petersen 1978; Günther and Fedyakov 2000; Garcia et al. 2003). In Mya arenaria (Gerasimova et al. 2017) and Macoma balthica (Bachelet 1980) the size of the first growth ring (and correspondingly the size by the start of second growth season) varied considerably (5–6 times) due to a prolonged spawning period.
For several bivalve species at the White Sea, an almost linear dependence of the growth rate on the size of the first growth ring is shown (Gerasimova et al. 2003, 2014, 2016, 2017). Accordingly, it can be assumed that individuals that have reached a larger size before their first winter will grow faster afterwards. We could not assess the dependence of the molluscan growth rate on the size of the first growth ring, but significant linear correlation between the growth rate during the third year of life and the size of the second growth ring was found for both species (Spearman rank correlation coefficient in different habitats 0.5–0.6).
Differences in the settlement time may also be associated with genetic differences. Mytilus edulis juveniles settled at different times do differ genetically (Gosling and Wilkins 1985). However, there are no data on the genetic composition of S. groenlandicus and M. calcarea populations in the Barents Sea.
Variability of individual growth rate may be associated with inter-annual changes of the growth conditions. This was shown for several bivalve species: Mytilus edulis (Dare 1976; Sirenko and Saranchova 1985; Zotin and Ozernyuk 2006), S. groenlandicus (Ambrose et al. 2006; Carroll et al. 2009, 2011; Denisenko 2014), Arctica islandica (Josefson et al. 1995; Witbaard 1996), and Macoma balthica (Beukema and Cadee 1991). Inter-annual variations in the annual shell increments of the S. groenlandicus and A. islandica have even been used for analyzing long-term climatic and hydrological trends (Witbaard 1996; Ambrose et al. 2006; Carroll et al. 2009, 2011; Denisenko 2014). Due to shell damage in many specimens of Serripes in the present study it was impossible to determine their age. The study of inter-annual changes of the growth could be the subject of future research. However, if the growth rate is mostly determined by the characteristics of the initial period of molluscan growth, the use of shell sclerochronology for the retrospective analyses of long-term climatic and hydrological trends might prove problematic.
Conclusion
Our study showed that in the southeastern part of the Pechora Sea the average density and biomass of S. groenlandicus and M. calcarea were rather stable over the past 20 years and quite comparable with those in other areas. The molluscan stock was mostly concentrated at depths < 20–25 m. We found only a correlation between the distribution of M. calcarea and S. groenlandicus in the SE Pechora Sea and the characteristics of the bottom sediments. Both species showed opposite preferences, which were explained by their feeding characteristics. M. calcarea density on silty sediments was on the average almost twice as high as on sandy sediments, while Serripes biomass was almost 1.5 times higher on sandy sediments than on silty sediments.
The growth rate of S. groenlandicus (but not of M. calcarea) was sensitive to environmental conditions, which means it can be used as an indicator of their changes. S. groenlandicus in the Pechora Sea was much more slow-growing than in other areas. In general, the growth rate of these cockles was closest to that in Arctic-influenced areas around the Svalbard Archipelago. At the same time, a significant variability of both group and individual growth of S. groenlandicus in the study area was revealed. The variation of S. groenlandicus group growth probably reflects the differences in feeding conditions of this suspension-feeder, which could arise from the features of local biotopes (e.g., hydrodynamic conditions). The slowest-growing Serripes were found at the deepest stations and in habitats with the largest content of fine-grained fractions (silt) in the sediments.
Unlike S. groenlandicus, the characteristics of the group growth of M. calcarea were very similar both in different habitats of southeastern Pechora Sea and in different parts of their distribution. The variability of individual growth was also relatively poor. The maximum life span and shell length of M. calcarea in the Pechora Sea were 15 years and 30 mm, respectively.
Noteworthy, the variation of group growth for both species was less pronounced than the variation of individual growth. The latter was to a great extent determined by the characteristics of the initial period of molluscan growth.
References
Adrov NM, Denisenko SG (1996) Oceanographic characteristics of the Pechora Sea. In: Matishov GG, Tarasov GA, Denisenko SG, Denisov VV, Galaktionov KV (eds) Biogeocenoses of glacial shelf of the western Arctic seas. Kola Centre RAN, Apatity, pp 164–179 (in Russian)
Ambrose WG, Carroll ML, Greenacre M, Thorrold SR, McMahon KW (2006) Variation in Serripes groenlandicus (Bivalvia) growth in a Norwegian high-Arctic fjord: evidence for local- and large-scale climatic forcing. Glob Change Biol 12:1595–1607
Ambrose WG et al (2012) Growth line deposition and variability in growth of two circumpolar bivalves (Serripes groenlandicus, and Clinocardium ciliatum). Polar Biol 35:345–354
Andrews JT (1972) Recent and fossil growth rates of marine bivalves, Canadian Arctic, and Late-Quaternary Arctic marine environments. Palaeogeogr Palaeoclimatol Palaeoecol 11:157–176
Antipova TV (1979) Distribution, ecology, growth and production of bivalve molluscs of the Barents and Kara Seas. PhD thesis (in Russian)
Appeldoorn RS (1983) Variation in the growth rate of Mya arenaria and its relationship to the environment as analyzed through principal component analysis and the ω parameter of von Bertalanffy equation. Fish Bull 81:75–85
Babkov AI, Golikov AN (1984) Hydrobiocomplexes of the White Sea. Publishing House of Zoological Institute, Leningrad (in Russian)
Bachelet G (1980) Growth and recruitment of the tellinid bivalve Macoma balthica at the southern limit of its geographical distribution, the Gironde estuary (SW France). Mar Biol 59:105–117
Beukema JJ, Cadee GC (1991) Growth rates of the Macoma balthica in the Wadden Sea during a period of eutrophication: relationships with concentrations of pelagic diatoms and flagellates. Mar Ecol Prog Ser 68:249–256
Borisovets EE, Sokolenko DA, Yavnov SV (2017) Distribution of greenland smoothcockle Serripes groenlandicus (Bivalvia, Cardiidae) in Peter the Great Bay (Japan Sea). Izvestiya TINRO 189:88–102 (in Russian)
Born EW, Rysgaard S, Ehlme G, Sejr M, Acquarone M, Levermann N (2003) Underwater observations of foraging free-living Atlantic walruses (Odobenus rosmarus rosmarus) and estimates of their food consumption. Polar Biol 26:348–357
Britayev TA, Udalov AA, Rzhavsky AV (2010) Structure and long-term dynamic of the soft-bottom communities of the Barents Sea bays. Uspekhi sovremennoy biologii 130:50–62 (in Russian)
Cardoso JFMF, Santos S, Witte JIJ, Witbaard R, van der Veer HW, Machado JP (2013) Validation of the seasonality in growth lines in the shell of Macoma balthica using stable isotopes and trace elements. J Sea Res 82:93–102
Carroll ML, Johnson BJ, Henkes GA, McMahon KW, Voronkov A, Ambrose WG Jr, Denisenko SG (2009) Bivalves as indicators of environmental variation and potential anthropogenic impacts in the southern Barents Sea. Mar Pollut Bull 59:193–206
Carroll ML, Ambrose WG Jr, Levin BS, Locke VWL, Henkes GA, Hop H, Renaud PE (2011) Pan-Svalbard growth rate variability and environmental regulation in the Arctic bivalve Serripes groenlandicus. J Mar Syst 88:239–251
Christian JR, Grant CGJ, Meade JD, Noble LD (2010) Habitat requirements and life history characteristics of selected marine invertebrate species occurring in the Newfoundland and Labrador Region. Can Manuscr Rep Fish Aquat Sci 2925:vi–207
Cloern JE, Nichols FH (1978) A von Bertalanffy growth model with a seasonally varying coefficient. J Fish Res Board Can 35:1479–1482
Dahle S, Denisenko SG, Denisenko NV, Cochrane SJ (1998) Benthic fauna in the Pechora Sea. Sarsia 83:183–210
Danilov AI, Mironov YU, Spichkin VA (eds) (2004) Variability of natural conditions in the shelf zone of the Barents and Kara Seas. AANII, SPb (in Russian)
Dare PJ (1976) Settlement, growth and production of the mussel, Mytilus edulis L., in Morecambe Bay, England. Fishery Investigations. Ser. II, vol 28(1). Her Majesty’s Stationery Office, London
Denisenko SG, Denisenko NV, Dahle S, Cochrane SJ (2005) The zoobenthos of the Pechora Sea revisited: a comparative study. In: Bauch HA, Pavlidis YA, Polyakova YI, Matishov GG, Koc N (eds) Pechora Sea environments: past, present, and future. Berichte zur Polar- und Meeresforschung, vol 501. pp 55–74
Denisenko SG (2014) Climate effect on growth of bivalve mollusc Serripes groenlandicus Bruguiere, 1789 in southeast part of the Barents Sea. J Siberian Fed Univ Ser Biol 7:57–72 (in Russian)
Denisenko SG, Denisenko NV, Lehtonen KK, Andersin AB, Laine AO (2003) Macrozoobenthos of the Pechora Sea (SE Barents Sea): community structure and spatial distribution in relation to environmental conditions. Mar Ecol Prog Ser 258:109–123
Dolgov AV, Yaragina NA (1990) Daily feeding rhythms and food intake of the Barents Sea cod and haddock in the summer of 1989. ICES Council Meeting (Collected Papers), vol 6, Copenhagen, Denmark
Fisher KI, Stewart REA (1997) Summer foods of Atlantic walrus, Odobenus rosmarus rosmarus, in northern Foxe Basin, Northwest Territories. Can J Zool 75:1166–1175
Gagaev SY, Golikov AN, Sirenko BI, Maximovich NV (1994) Ecology and distribution of the mussel Mytilus trossulus septentrionalis Clessin, 1889 in the Chaun inlet of the East Siberian Sea. In: Ecosystems, flora and fauna of Chaun inlet of the East Siberian Sea. Part I. Exploration of fauna of the seas, vol 44 (55). Russian Akademy of Sciences, St-Petersburg, pp 254–258 (in Russian)
Garcia EG, Thorarinsdottir GG, Ragnarsson SA (2003) Settlement of bivalve spat on artificial collectors in Eyjafjordur, North Iceland. Hydrobiologia 503:131–141
Gerasimova AV, Maximovich NV, Saminskaya AA (2003) The length growth of Serripes groenlandicus Briguiere in the Chupa Inlet (The Kandalaksha Bay, the White Sea). Vestnik Sankt-Peterburgskogo universiteta Seriya 3 Biologiya 4:28–36 (in Russian)
Gerasimova A, Maximovich N (2013) Age-size structure of common bivalve mollusc populations in the White Sea: the causes of instability. Hydrobiologia 706:119–137
Gerasimova AV, Ivonina NY, Maximovich NV (2014) The length growth rate variability of Mytilus edulis L. (Mollusca, Bivalvia) in the waters of the Keret Archipelago (Kandalaksha Bay, the White Sea). Vestnik Sankt-Peterburgskogo universiteta Seriya 3 Biologiya 4:22–38 (in Russian)
Gerasimova AV, Martynov FM, Filippova NA, Maximovich NV (2016) Growth of Mya arenaria L. at the northern edge of the range: heterogeneity of soft-shell clam growth characteristics in the White Sea. Helgol Mar Res 70:1–14
Gerasimova AV, Ushanova EV, Filippov AA, Filippova NA, Stogov IA, Maximovich NV (2017) Long-term changes in cohort structure of the soft-shell clam Mya arenaria in the White Sea: growth rate affects lifespan and mortality. Mar Biol Res 14(1):51–64
Gosling EM, Wilkins NP (1985) Genetics of settling cohorts of Mytilus edulis (L.): preliminary observations. Aquaculture 44:115–123
Gudimova EN (2002) Bottom invertebrates of the Barents Sea: resources, perspectives of use, ecology. In: Kalinnikov VT (ed) “The Kola Scientific Center of the Russian Academy of Sciences—70 years”: Environmental management in the Euro-Arctic region: the experience of the 20th century and prospects. Izd-vo Kol'skogo nauchnogo tsentra RAN, Apatity, pp 1–8 (in Russian)
Günther C-P, Fedyakov V (2000) Seasonal changes in the bivalve larval plankton of the White Sea. Mar Biodivers 30:141–151
Hjelset AM, Andersen M, Gjertz I, Lydersen C, Gulliksen B (1999) Feeding habits of bearded seals (Erignathus barbatus) from the Svalbard area, Norway. Polar Biol 21:186–193
Ivanov GI, Krylov AA, Ponomarenko TV (2001) Fractional structure of bottom sediments of the Pechora Sea. In: Sedimentation processes and evolution of marine ecosystems in marine periglacial conditions, vol 1. MMBI of the KSC, Apatity, pp 80–95 (in Russian)
Jorgensen CB (1990) Bivalve filter feeding: hydrodynamics, bioenergetics, physiology and ecology. Olsen and Olsen, Fredensborg
Josefson A, Jensen J, Nielsen T, Rasmussen B (1995) Growth parameters of a benthic suspension feeder along a depth gradient across the pycnocline in the southern Kattegat, Denmark. Mar Ecol Prog Ser 125:107–115
Kamenev GM, Kavun VY, Tarasov VG, Fadeev VI (2004) Distribution of bivalve mollusks Macoma golikovi Scarlato and Kafanov, 1988 and Macoma calcarea (Gmelin, 1791) in the shallow-water hydrothermal ecosystem of Kraternaya Bight (Yankich Island, Kuril Islands): connection with feeding type and hydrothermal activity of Ushishir Volcano. Cont Shelf Res 24:75–95
Kennish MJ, Olsson RK (1975) Effects of thermal discharges on the microstructural growth of Mercenaria mercenaria. Environ Geol 1:41–64
Khim B-K (2001) Stable isotope profiles of Serripes groenlandicus shells. II. Occurrence in Alaskan coastal water in south St. Lawrence Island, northern Bering Sea. J Shellfish Res 20:275–281
Khim B-K (2002) Stable isotope profiles of Serripes groenlandicus shells. I. Seasonal and interannual variations of Alaskan Coastal Water in the Bering and Chukchi Seas. Geosci J 6:257–267
Khim B-K, Krantz DE, Cooper LW, Grebmeier JM (2003) Seasonal discharge of estuarine freshwater to the western Chukchi Sea shelf identified in stable isotope profiles of mollusk shells. J Geophys Res 108:1–10
Kilada RW, Roddick D, Mombourquette K (2007) Age determination, validation, growth and minimum size of sexual maturity of the Greenland smoothcockle (Serripes groenlandicus, Bruguiere, 1789) in eastern Canada. J Shellfish Res 26:443–450
Kulakovskii EE, Kunin BL (1982) Preliminary results for growing mussels on artificial substrates in the White Sea. In: Ecological studies of mariculture perspective objects in the fauna of the White Sea. Exploration of fauna of the seas, vol 27(35). Zoological Institute of Russian Academy of Sciences, Leningrad, pp 17–24 (in Russian)
Kuznetsov VV (1960) The White Sea and biological features of its flora and fauna. Russian Academy of Sciences, Leningrad (in Russian)
Legendre P, Legendre L (2012) Numerical ecology. developments in environmental modelling, vol 24, 3rd English edn. Elsevier, Amsterdam
Lisitsyna KN, Gerasimova AV, Maximovich NV (2017) Demecological investigations of Macoma calcarea (Gmelin) in the White Sea. In: Pugachev ON (ed) Exploration, sustainable use and protection of natural resources of the White Sea. XIII conference (collection papers), St. Petersburg, 17–20 October 2017, St. Petersburg, pp 123–126 (in Russian)
Lisitsyna KN, Gerasimova AV (2019) Growth and distribution of bivalve molluscs Macoma calcarea (Gmelin) in the Kara Sea. In: Proceedings of the VII International conference “Marine Research and Education”, Moscow, 19–22 November 2018. OOO “PoliPRESS”, Tver, pp 18–21 (in Russian)
MacDonald BA, Thomas MLH (1980) Age determination of the soft-shell clam Mya arenaria using shell internal growth lines. Mar Biol 58:105–109
Makarevich PR (2012) Primary products of the Barents Sea. Vestnik Murmanskogo Gosudarstvennogo Tekhnicheskogo Universiteta 15:786–793 (in Russian)
Matishov (1992) An international ecological expedition in the Pechora Sea, Novaya Zemlya, Vaygach, Kolguyev and Dolgiy Islands, July 1992. MMBI Report. Russian Academy of Sciences, Apatity (in Russian)
Maximovich NV, Lysenko VI (1986) Growth and production of the bivalve Macoma incongrua in Zostera beds in the Knight Bay, Sea of Japan. Biologiya Morya 1:25–30 (in Russian)
Maximovich NV (1989) Statistical comparison of growth curves. Vestnik Leningradskogo universiteta Seriya 3 Biologiya 4:18–25 (in Russian)
Maximovich NV, Minichev YS, Kulakovskii EE, Sukhotin AA, Chemodanov AV (1993) Dynamics of structural and functional characteristics of the White Sea mussel beds in the suspended aquaculture. In: Research Mariculture White Sea Mussels (eds) Proceedings of Zoological Institute of Russian Academy of Sciences, vol 253. Zoological Institute of Russian Akademy of Sciences, St. Petersburg, pp 61–82 (in Russian)
Maximovich NV, Gerasimova AV (2004) Age determination of the White Sea bivalves by the shell morphology. In: Proceedings of the V Scientific Session of the Marine Biological Station of St. Petersburg State University, St. Petersburg, Russia, 6 February 2004, pp 29–30 (in Russian)
Menis DT, Oganesyan SA (1997) Some features of the biology of the greenlandic Serripes groenlandicus in the southeast of the Barents Sea. In: Investigations of commercial invertebrates in the Barents Sea. Izd-vo PINRO, Murmansk, pp 122–129 (in Russian)
Murawski SA, Ropes JW, Serchuk FM (1982) Growth of the ocean quahog, Arctica islandica, in the Middle Atlantic Bight. Fish Bull 80:21–34
Naumov AD (2006) Bivalve molluscs of the White Sea. Experience of ecological and faunistic analysis, Zoological Institute of RAS, St. Petersburg (in Russian)
Neyman AA (1963) Quantitative distribution of benthos on the shelf and upper horizons of the slope of the eastern part of the Bering Sea. Trudy VNIRO 48:145–205 (in Russian)
Neyman AA (1969) Bentos of the West Kamchatka shelf. Trudy VNIRO 65:223–232 (in Russian)
Nikiforov SL, Dunaev NN, Politova NV (2005) Modern environmental conditions of the Pechora Sea (climate, currents, waves, ice regime, tides, river runoff, and geological structure). In: Bauch HA, Pavlidis YA, Polyakova YI, Matishov GG, Koc N (eds) Pechora Sea environments: past, present, and future, vol 501. Berichte zur Polar- und Meeresforschung, pp 7–38
Ockelmann WK (1958) The zoology of East Greenland. Marine Lamellibranchia. Medd om Gronland 122:1–256
Okera W (1976) Observations on some population parameters of exploited stocks of Senilia senilis (=Arca senilis) in Sierra Leone. Mar Biol 38:217–229
Pannella G, MacClintock C (1968) Biological and environmental rhythms reflected in Molluscan shell growth. Memoir 2:64–80
Pavlov VK, Pfirman SL (1995) Hydrographic structure and variability of the Kara Sea: implications for pollutant distribution. Deep Sea Res Part II 42:1369–1390
Petelin VP (1967) Analysis of particle size distribution of marine sediments. Nauka, Moscow (in Russian)
Petersen GH (1978) Life cycles and population dynamics of marine benthic bivalves from the Disko Bugt area of West Greenland. Ophelia 17:95–120
Pogrebov VB, Nekrasova NN, Ivanov GI (1997) Macrobenthic communities of the Pechora Sea: the past and the present on the threshold of the Prirazlomnoye oil-field exploitation. Mar Pollut Bull 35:287–295
Rasmussen E (1973) Systematics and ecology of the Ice-Fjord Marine Fauna (Denmark). Ophelia 11:1–507
Reigstad M, Carroll J, Slagstad D, Ellingsen I, Wassmann P (2011) Intra-regional comparison of productivity, carbon flux and ecosystem composition within the northern Barents Sea. Prog Oceanogr 90:33–46
Savilov AI (1953) Growth and its variability in the White Sea invertebrates Mytilus edulis, Mya arenaria and Balanus balanoides. Part I. Mytilus edulis in the White Sea. Proc Inst Oceanol USSR Acad Sci 7:198–258 (in Russian)
Seed R (1969) The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores. II. Growth and mortality. Oecologia 3:317–350
Shulgina LV, Sokolenko DA, Davletshina TA, Zagorodnaya GI, Borisovets EE, Yakush EV (2015) Characteristics of bivalve mollusc Serripes groenlandicus in connection with its rational use. Izvestiya TINRO 181:263–272 (in Russian)
Sirenko BI, Saranchova OL (1985) Two-year observations of the growth of the mussels Mytilus edulis L. in cages in the Chupa Inlet (the White Sea). In: Ecological studies of perspective objects of mariculture in the White Sea. Zoological Institute of Russian Akademy of Sciences, Leningrad, pp 23–28 (in Russian)
Sukhotin AA (1992) Respiration and energetics in mussels (Mytilus edulis L.) cultured in the White Sea. Aquaculture 101:41–57
Sukhotin AA, Kulakowski EE (1992) Growth and population dynamics in mussels (Mytilus edulis L.) cultured in the White Sea. Aquaculture 101:59–73
Sukhotin AA, Maximovich NV (1994) Variability of growth rate in Mytilus edulis L. from the Chupa Inlet (the White Sea). J Exp Mar Biol Ecol 176:15–26
Sukhotin AA, Abele D, Pörtner H-O (2002) Growth, metabolism and lipid peroxidation in Mytilus edulis: age and size effects. Mar Ecol Prog Ser 226:223–234
Sukhotin AA, Strelkov PP, Maximovich NV, Hummel H (2007) Growth and longevity of Mytilus edulis (L.) from northeast Europe. Mar Biol Res 3:155–167
Taylor AC, Brand AR (1975) A comparative study of the respiratory responses of the bivalves Arctica islandica (L.) and Mytilus edulis L. to declining oxygen tension. Proc R Soc Lond Ser B Biol Sci 190:443–456
Thompson I, Jones DS, Dreibelbis D (1980) Annual internal growth banding and life history of the ocean quahog Arctica islandica (Mollusca: Bivalvia). Mar Biol 57:25–34
Wassmann P et al (2006) Food webs and carbon flux in the Barents Sea. Prog Oceanogr 71:232–287
Wenne R, Klusek Z (1985) Longevity, growth and parasites of Macoma balthica (L.) in the Gdansk Bay (South Baltic). Pol Arch Hydrobiol 32:31–45
Witbaard R (1996) Growth variations in Arctica islandica L. (Mollusca): a reflection of hydrography-related food supply. ICES J Mar Sci 53:981–987
Zenkevich LA (1963) Biology of the Seas of the USSR. George Allen & Unwin, London
Zolotarev VN (1989) Sclerochronology of marine bivalve molluscs. Izd-vo “Naukova Dumka”, Kiev (in Russian)
Zotin AA, Ozernyuk ND (2006) Retrospective analysis of environmental impact on growth parameters in White Sea mussel Mytilus edulis. Biol Bull 33:623–626
Acknowledgements
The authors are grateful to all participants of the expeditions to the Barents Sea in 2012–2013 for their invaluable assistance in material collection.
Funding
This work was supported by the RFBI Grant 16–34-00216 and RFBI Grant 18–05-60157.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the special issue on the “Ecology of the Pechora Sea”, coordinated by Alexey A. Sukhotin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gerasimova, A.V., Filippova, N.A., Lisitsyna, K.N. et al. Distribution and growth of bivalve molluscs Serripes groenlandicus (Mohr) and Macoma calcarea (Gmelin) in the Pechora Sea. Polar Biol 42, 1685–1702 (2019). https://doi.org/10.1007/s00300-019-02550-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02550-z