Abstract
Until 2011, walrus (Odobenus rosmarus rosmarus) population inhabiting the Pechora Sea remained among the less-studied parts of the Atlantic subspecies. Composition and level of anthropogenic pollutants in these walruses have never been studied before the present work. We collected samples of skin and subcutaneous tissues from 15 adult males in the Pechora Sea and from 1 adult female from adjacent White Sea during the years 2011–2017. These samples were analyzed for polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and organochlorine pesticides. The highest concentrations were found for PCBs (∑PCB: \(\stackrel{-}{x}\) ± SE = 5700 ± 3090 ng g−1 lipid; range 40–50,400 ng g−1 lipid, n = 16), followed by pesticides oxychlordane (\(\stackrel{-}{x}\) ± SE = 1090 ± 412 ng g−1 lipid; range 31–5070 ng g−1 lipid, n = 16), and 4,4′-DDE (\(\stackrel{-}{x}\) ± SE = 460 ± 300 ng g−1 lipid; range 2.5–4920 ng g−1 lipid, n = 16). PBDEs showed the lowest concentrations (∑PBDE: \(\stackrel{-}{x}\) ± SE = 13 ± 6.5 ng g−1 lipid; range 0.7–104 ng g−1 lipid, n = 16). Compared with walruses from Svalbard, the studied animals have considerably lower levels of oxychlordane, comparable levels of PBDEs, and exceeding burden of PCBs. Concentrations found in walruses are closer to polar bears than to other pinnipeds from this region (ringed and harp seals). Although all walruses in the sample group were mature males with exception of one mature female from the same location, concentrations of pollutants varied in very broad range. Three groups of walruses were distinguished by concentrations of analyzed persistent organic pollutants (POPs): low POP concentrations; high POP concentrations; intermediate POP concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic pollution is among the most serious current threats to marine ecosystems (AMAP 2017). Persistent organic pollutants (POPs) represent major part of this environmental problem due to their global distribution, high toxicity, and bioaccumulation. POPs enter the Arctic marine ecosystems mainly through the long-range atmospheric transport which considerably exceeds contributions from local sources (Wania and Mackay 1993, 1996). Being on the top of marine food chain, marine mammals are exposed to substantial risk of POPs’ effect. Polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and organochlorine pesticides are widespread organic pollutants reaching considerable concentrations in marine mammals. In developed countries in the 1970s and 1980s, commercial production and use of almost all of these xenobiotics were banned, and numerous studies revealed substantial decrease of their presence in marine biota by present time (Simmonds 2017; Riget et al. 2018). However, recent studies have demonstrated that in some marine mammals (cetaceans), levels of PCB declined slowly from 1990 to 1998, and then remained relatively stable from 1998 to 2012 (Jepson et al. 2016). An increasing trend of several POPs is shown in recent years for polar bears in Greenland (Riget et al. 2016).
As marine mammals are generally considered as proper indicators of marine ecosystems’ status, monitoring of POPs’ composition and concentrations in their tissues is still important ongoing challenge. In 2015, the Ministry of Natural Resources and Environment of the Russian Federation approved an official List of flora and fauna species—indicators of the marine ecosystems’ sustainability in the Russian Arctic Zone. The list contains 61 species including 5 mammals, one of which is walrus (Odobenus rosmarus Linnaeus, 1758). Russian Arctic waters are inhabited by walruses belonging to three subspecies—the Atlantic walrus (O.r. rosmarus Linnaeus, 1758), the Pacific walrus (O.r. divergens Illiger, 1811), and the Laptev walrus (O.r. laptevi Tchapski, 1940) taxonomic status of which is disputable (Fay 1981; Lindqvist et al. 2009). According to the International Union for Conservation of Nature’s (IUCN) assessment, the species has category Vulnerable A3c in the Red List of Threatened Species.Footnote 1 The Atlantic walrus subspecies is classified by IUCN as Near Threatened. Besides, the Atlantic and Laptev walruses are listed in the Red Data Book of the Russian Federation. Any hunt of these two subspecies in Russian waters is prohibited since 1957 (Resolution of the Council of Ministers of the RSFSR No. 738 (e), 21.11.1956).
Lipophilic pollutants enter in walruses via food and are accumulated in adipose tissue. According to Stromberg et al. (1990), over 98% of lipophilic pollutants in pinnipeds reside in blubber. Even POPs deposited in blubber may be harmful to an animal (Tartu et al. 2017a). In periods when a walrus utilizes its lipid reserves (e.g., seasonal food shortage, molting, and breeding), pollutants from blubber are mobilized via blood stream to other organs where they may also be harmful.
Concentrations and composition of the POPs in walruses also can be used as an additional tool to understand feeding features of the animals. Walruses are considered rather benthic stenophagous animals feeding primarily on bivalve mollusks (Fay 1982; Gjertz and Wiig 1992; Sheffield et al. 2001). Although the fact that some individual walruses can prey upon seals is generally known (Gjertz and Wiig 1992; Seymour et al. 2014), proportion of such animals in a population and extent of their predation are not known. High concentrations of the pollutants may be related to walrus preying upon seals, while animals with low contamination feed on invertebrates (Muir et al. 1995; Wiig et al. 2000; Wolkers et al. 2006). Thus, data on composition and burden of POPs in walruses might contribute to better understanding of the role of walruses in marine ecosystems.
Despite the facts as above mentioned, until now only one attempt to study POPs’ pollution of walruses in Russian part of the subspecies range has taken place (Wiig et al. 2000) when biopsy of 28 walruses from Franz Josef Land were analyzed. A population of Atlantic walrus resides in the SE part of the Barents Sea (also known as the Pechora Sea) (Semenova et al. this volume). Recently, it was demonstrated that the group of walruses inhabiting this area shows low, but significant genetic distinction from walruses in Svalbard—Franz Josef Land and should be managed conservatively, as a separate, small population (Andersen et al. 2017). In the Pechora Sea, oil production has been rapidly developing since less than a decade. Background information on the contamination of marine mammals and other keystone species in this area is extremely important. The current study was carried out to fill this gap, and assess the composition and levels of POPs in the Pechora Sea population of walrus. Considering this finding and raising economic activity in this area, it is especially relevant to obtain actual data on main parameters, including POPs’ burden, affecting the welfare of this population group. This study focuses on PCBs, PBDEs, and organochlorine pesticides.
Material and methods
Sample collection, storage, and handling
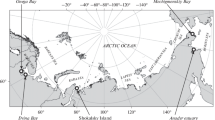
Samples of skin and subcutaneous tissues were collected from 16 Atlantic walruses in 2011–2017: 1 in the White Sea, 1 on Kolguev Island, 1 on Matveev Island, and 13 on Vaigach Island (Fig. 1, Table 1). All samples were taken in July–August except for one collected in the White Sea in May 2017.
Sampling locations (see Table 1 for list of the samples)
Fifteen of the walruses were mature males, and 1 from the White Sea—mature female. In four cases, samples (skin with subcutaneous blubber total weight about 100–150 g) were taken from stranded corpses of walruses. The rest 12 samples were taken from temporally immobilized animals. Animals were immobilized with ethorphine and reversed by naltrexone (Ølberg et al. 2017). Samples from immobilized animals were collected as described by Wolkers et al. (2006)—using hollow 150-mm-long stainless steel tube (diameter 6 mm). Three–four samples (skin with subcutaneous tissue 7–8 cm long) were taken from each animal. All samples were wrapped separately in aluminum foil and frozen (− 20 ºC).
To assess age classes of sampled walruses, the following measurements were taken: straight body length (all walruses in the sample); tusk length (measured for 9 animals); and tusk perimeter (measured for 11 animals).
All field studies of walruses, including immobilization and sampling, were approved by special permits issued by the Federal Supervisory Natural Resources Management Service (Moscow, Russia), which is responsible for control and supervision of any invasive studies of endangered species in a wild domain.
Chemical analyses
The samples were analyzed at the Research and Production Association "Typhoon" of the Federal Service for Hydrometeorology and Environmental Monitoring (Obninsk, Russia). The following persistent organic pollutants (POPs) were determined: PCBs (congeners’ IUPAC numbers: 1, 3, 4 + 10, 8, 15, 17 + 18, 22, 28 + 31, 33, 37, 44, 49, 52, 70, 74, 87, 95, 99, 101, 110, 119, 128, 138 + 158, 149, 151, 153 + 168, 171, 177, 178, 183, 187, 188, 191, 194, 199, 201, 202, 205, 206, 208, and 209), PBDEs (congeners: 7, 15, 17, 28, 47, 49, 66, 71, 85, 99, 100, 126, 138, 153, 154, 155, 166, 181, 183, 190, 203, 205, 206, 207, and 209), chlorinated pesticides (2,4′-DDE, 2,4′-DDT, 2,4′-DDD, 4,4′-DDE, 4,4′-DDT, 4,4′-DDD, cis-nonachlor, cis-chlordane, mirex, trans-nonachlor, trans-chlordane, α-HCH, β-HCH, γ-HCH, aldrin, hexachlorobenzene, heptachlor, heptachlor epoxide, oxychlordane, octachlorostyrene, alpha-endosulfan, and endrin).
About 1–3 g of tissue from each sample was homogenized in a mortar with anhydrous Na2SO4. A half of this dry mixture was transferred to a glass column containing quartz wool. Further mixture of isotope-labeled surrogate standards in n-nonan was added, and finally, the rest of the sample was transferred to the column. The isotope-labeled surrogate standards mixture included PCB (13C12) congeners (28, 52, 101, 138, 153, 180, and 209), 13C12–4,4′-DDE, 13C12–4,4′-DDT, 13C6-β-HCH, 13C6-γ-HCH, 13C6-HCB, 13C10-oxychlordane, and 13C10-trans-nonachlor. The column bed was eluted with 300 ml of hexane–dichloromethane (1:1 by volume). Samples were concentrated to volume about 1 cc by rotary evaporation. Fractionation was accomplished with about 1 cc of dichloromethane and purified using column chromatography method.
The extract was purified in two stages. The first stage included removal of lipids and steroids using method of gel-filtration on Bio-Beads S-X3 column. During the second stage, impurities preventing the analysis were removed using column chromatography method: first in multilayer column with acid and alkaline silicagel and after that—in column with activated alumina. PCB, DDE isomers, mirex, and HCB were eluted from the column with alumina with the help of hexane–dichloromethane (97:3) (fraction I). Planar PCBs, polar pesticides, and PBDE were eluted with hexane–dichloromethane (40:60) (fraction II). All fractions were concentrated by a combination of rotary evaporation and nitrogen evaporation, and performance standard (PCB 155) was added before analysis.
For determination of PCBs and pesticides, each fraction was injected into a gas chromatograph (Agillent 7200Q-TOF) with mass spectral resolution R > 9000. Fractionation was accomplished in quartz capillary column DB-5MS 30 m × 0.25 mm × 0.25 mcm in temperature regime of 80–280 ºC. Chromato-mass spectrometric detection of PBDE was performed by analysis of aliquot of the fraction II on the Agilent 5977A in chemical ionization regime detecting negatively charged ions (Chemical Ionization—Negative Ion). Gas-reagent was methane. Fractionation was accomplished in quartz capillary column DB-5HT 15 m × 0.25 mm × 0.1 mcm in temperature regime of 80–320 ºC. Assessment of lipid percentage in a sample was performed gravimetrically after lipids were removed in column Bio-Beads S-X3.
For quality assurance, every 10 samples were analyzed along with blank sample consisting of all laboratory reagents and with native sample with matrix spike. Trace concentrations of PCB28, PCB101, PCB118, PCB105, and HCB were detected in some of the blanks. In such cases, their concentrations were subtracted from native sample results. To control completeness of analytes extraction, each sample was supplemented with mixture of isotope-labeled surrogate standards: 13C6-HCB, 13C6 beta-BCH, 13C6 gamma-BCH, 13C4-Heptachlor, 13C10-Oxychlordane, 13C12-Dieldrin, 13C12-p,p-DDE, 13C12-p,p-DDT, 13C10-Nonachlor, 13C8-Mirex, 13C12PCB-28, 13C12PCB-52, 13C12PCB-101, 13C12PCB-118, 13C12PCB-138, 13C12PCB-153, and 13C12PCB-180. Recovery for the 13C-standards varied from 65 to 85%. Duplicate samples were analyzed for every 5 samples. Results for duplicates varied by less than 30% for all major OC analytes. Detection accuracy was also regularly tested by SRM1945 analysis (Organic in Whole Blubber certified). SRM1945 is a certified reference material from the National Institute of Standards and Technology (NIST) made available via LGC Promochem (Teddington, Middlesex, U.K.). For PCB18, PCB209, PCB156, g-HCH, 2,4-DDT, and 4,4-DDT, 15–20% of concentrations in SRM deviated from the assigned values.
Since 2013, the laboratory regularly participates in international intercalibrate tests under programs NCP-AMAPIII-7, NCP-AMAPIII-10, and NCP-AMAPIII-11; since 2015—in AMAP intercalibration ring tests. In 2013 and 2016, it also took tests under the UNEP programs CIND/INCA.
Statistical analyses
The values below limit of detection (LOD) were replaced by zero for statistical purposes. LOD = 0.1 ng g−1 (lipid) for PCB congeners, hexachlorobenzene, aldrin, alpha-endosulfan, endrin, and octachlorostyrene; LOD = 0.05 ng g−1 lipid for trans-nonachlor and cis-nonachlor LOD = 0.01 ng g−1 lipid, for α-HCH, β-HCH, γ-HCH, heptachlor, and oxychlordane; LOD = 0.03 ng g−1 lipid for hexachlorobenzene, trans-chlordane, cis-chlordane, 2,4′-DDE, 4,4′-DDE, 2,4′-DDD, 4,4′-DDD, 2,4′-DDT, 4,4′-DDT, and mirex; LOD = 0.003 ng g−1 lipid for PBDE congeners 85, 99, 100, 126, 138, 153, 154, 155, 166, 181, 183, 190, 203, 205, 206, 207, and 209; LOD = 0.001 ng g−1 lipid for PBDE congeners 7, 15, 17, 28, 47, 49, 66, and 71. Minimum values represent minimum detected concentrations of analytes.
Geometric and arithmetic means, and standard errors were calculated for concentrations of individual contaminants and percentage of lipid in samples. Arithmetic means are given along with standard error (\(\stackrel{-}{x}\) ± SE). Cluster analysis (Ward's method) was applied to find if there was grouping of studied walruses on contamination levels of ∑PCB, oxychlordane, 4,4′-DDE, and ∑PBDE. Correlations between concentrations of PCB153 and ∑PCB were estimated using Spearman's rank-order correlation with statistical significance at p = 0.05. The analyses were performed using PAleontological STatistics (PAST) version 3.19. Detection frequency for each analyte was estimated as a percentage of samples in which the analyte was detected.
Results
According to measurements (body length 311–390 cm, tusk length, and perimeter) and exterior of all sampled males (nodules around neck and shoulder), they were adults. Concentrations of POPs in collected samples are presented in Table 2. Average (arithmetic mean) lipid percentage of samples was \(\stackrel{-}{x}\) ±SE = 22.8 ± 4.9%, n = 16.
Organochlorine pesticides
Among 22 organochlorine pesticides, 5 analytes (endrin, alpha-endosulfan, heptachlor epoxide, heptachlor, and aldrin) were not detected in any of the samples. Trans-nonachlor, oxychlordane, and 4,4′-DDE were found in all samples (100% encounter rate). Encounter rate of the other 14 measured pesticides varied from 6 to 94% (mean 54%). Oxychlordane reached the highest concentrations (\(\stackrel{-}{x}\) ± SE = 1090 ng g−1 lipid, range 31.3–5070 ng g−1 lipid, n = 16; Table 2, Figs. 2, 3). Concentration of 4,4′-DDE follows oxychlordane’s varying from 2.5 to 4920 ng g−1 lipid (\(\stackrel{-}{x}\) ± SE = 464 ± 304 ng g−1 lipid, n = 16) (Table 2, Figs. 2, 3).
Polychlorinated biphenyls (PCB)
Four PCB congeners (PCB-1, PCB-3, PCB-4/PCB-10, and PCB-188) of 41 analyzed did not show detectable concentrations. Four congeners (PCB-74, PCB-99, PCB-101, and PCB-153 + PCB-168) were found in all samples, encounter rate of the rest 33 congeners varied from 6 to 94% (mean 48%). Pair PCB-153/168 showed the highest concentrations in 15 of the 16 samples (Table 2, Figs. 4 and 5). Concentration of pair PCB-153/168 strongly correlates with ∑PCBs (r = 0.997).
Polybrominated diphenyl ethers (PBDE)
Among 25 PBDEs analyzed, 15 congeners were not detected; the rest 10 congeners showed encounter rate from 6 to 75%. The highest concentration is found for BDE-47 (\(\stackrel{-}{x}\) ± SE = 8.94 ± 5.38 ng g−1 lipid, range 0.09–86.7, n = 16; Table 2, Figs. 5 and 6).
Discussion
In the studied group of walruses, PCBs reached the highest concentrations: arithmetic mean of ∑PCBs is about 3 times higher than in ∑organochlorine pesticides, and 457 times higher than ∑PBDE. In our walrus samples, oxychlordane prevails among analyzed organochlorine pesticides (Fig. 2), PCB-153 is the major PCB congener (Fig. 4), and BDE-47 has shown the highest level among PBDE congeners (Fig. 6). This corresponds to domination or at least considerable concentrations of these specific compounds in Atlantic walruses from other parts of the subspecies range (Muir et al. 1995; Wolkers et al. 2006; Scotter et al. 2018), as well as in polar bears (Ursus maritimus Phipps) (Henriksen et al. 2001; Lie et al. 2003; Tartu et al. 2017b), beluga whales (Delphinapterus leucas Pallas) (Muir et al. 1996), ringed (Pusa hispida Schreber), and harp (Pagophilus groenlandicus Erxleben) seals (Muir et al. 2003; Savinov et al. 2011).
Concentrations of pollutants in the studied group of 15 adult males and one adult female walruses have considerable individual variations. It concurs with findings of Wolkers et al. (2006) and Scotter et al. (2018) for adult male walruses in Svalbard area. Earlier Muir et al. (1995) reported about the same phenomenon in walruses in the Eastern Hudson Bay (Canada). To compare variations of pollutants’ concentrations in our samples with published data on walruses from Svalbard and on other mammals (polar bear; ringed and harp seals) inhabiting areas neighboring to the Pechora Sea, we assess ratio of maximum concentration of dominating pollutants to detected minimum one (max/min, Tables 3 and 4). In our sample, this ratio of oxychlordane concentrations is close to Norwegian one and considerably exceeds the same parameter found in subcutaneous fat of adult polar bear females from Svalbard and adipose tissue of adult harp seal females and adult ringed seal males from the White Sea (5, 11, and 30 times correspondingly). Variation of BDE-47 concentrations in our sample is almost 16 times higher than in walruses from Svalbard, 33 times higher than in adult polar bear females from Svalbard, and 438 times higher than in adult male ringed seals from the White Sea. In our sample, PCB-153 has shown the broadest spread of values, with the max/min ratio exceeding the same index in Svalbard walruses 146 times. Max/min ratio for Σ14PCBs in our sample is close to the same ratio in Σ18PCBs reported for walruses in Svalbard area, this is 29 times higher than this index for Σ16PCBs in polar bears, 198 times higher than variation index of Σ10PCBs in harp seals, and 328 times higher than this index for Σ10PCBs in ringed seals.
Thus, broad variation of POPs’ levels revealed in the studied 16 adult walruses surpass dispersion of the same POPs’ concentrations not only in other Arctic pinnipeds and polar bears but also in similar group of adult Atlantic walrus males from Svalbard.
Although basically walruses are benthos eaters, some individuals may prey upon seals (Fay 1982; Born et al. 1995). Wolkers et al. (2006) and Muir et al. (1995) for walruses in Svalbard and Canada correspondently demonstrated that the more seals contribute to the walrus diet the higher POPs’ burden the walruses have. It was also shown that there was clear separation of high and low contaminated walruses into two different clusters in Principal Component Analysis (PCA) of fatty acids from their blubber suggesting differences in diet between these groups that was likely due to a varying proportion of seals in the diet of individual animals (Wolkers et al. 2006). At the same time, recent studies of relationships between diet composition and POPs’ bioaccumulation in walruses from Svalbard area (Scotter et al. 2018) using stable isotope ratios of carbon and nitrogen did not reveal any correlation of trophic level and POPs’ concentrations.
By absolute levels of pollutants, walruses in our sample had considerably lower levels of oxychlordane, similar levels of PBDEs, and exceeding burden of PCBs compared to specimens from Svalbard area (Wolkers et al. 2006) (Tables 3 and 4). Walruses of the Pechora Sea similar to the Svalbard conspecifics showed an order of magnitude higher levels of the pollutants than ringed and harp seals. At the same time comparing to polar bears walruses have lower levels of PBDEs and PCBs, and similar and even higher burden of oxychlordane.
Comparing levels of POPs in the above-mentioned species it is necessary to note that tissue burdens at these higher trophic levels also depend on the species-specific ability to absorb, metabolize and/or eliminate these compounds (O’Hara and Becker 2002). Specifically, polar bears have capacity to metabolize chlordane what leads to comparable concentrations of the compound and its metabolites in prey and in the bear (Letcher et al. 1996).
Cluster analysis revealed that basing on concentrations of ∑PCBs, oxychlordane, 4,4′-DDE and ∑PBDEs studied individuals comprised three distinct groups (Fig. 7), and one walrus (ID 1814) from Vaigach Island shows extraordinary high level of PCBs: ∑PCBs = 50,400 ng g−1 lipid with main contribution of CB-153/CB-168 (29,100 ng g−1 lipid) and CB-138/CB-158 (14,800 ng g−1 lipid). This is almost 4 times higher than maximum ∑PCB (12,702 ng g−1 lipid) in 17 adult male walruses from Svalbard area (Wolkers et al. 2006). The same walrus (ID 1814) has the highest levels of oxychlordane, 4,4′-DDE and ∑PBDEs among all 16 animals.
Female walrus from the White Sea (ID 1779) is one of the three animals comprising the group III (Fig. 7). Although this sea is out of normal range of the species, walruses occasionally enter there in spring and stay by the whelping gatherings of harp seals (Svetochev and Svetocheva 2007, 2008). The main probable reason for walruses’ seasonal visits to the White Sea is preying upon harp seal newborn pups (whitecoats) what is indirectly supported by the results of this toxicological study. In their turn, whitecoats receive POPs with mother’s milk during lactation (Espeland et al. 1997; Frouin et al. 2012) and can have levels comparable to those found in walruses. According to Muir et al. (2003) in 20 harp seal pups in the White Sea, ∑PCB varied from 405 to 1870 ng g−1 lipid, oxychlordane − 48–212 ng g−1 lipid.
Very high levels of pollutants in some walruses may be related to seal predation by these individuals. In our sample, such heavily polluted animals comprise about quarter of studied walruses. This may suggest that walruses are not that stenophagous as they are commonly considered, and such feeding flexibility allows them to inhabit areas with various benthos productivity.
Conclusion
Presented data on organochlorine pesticides, PCBs, and PBDEs in walruses of the Pechora Sea are the first information on POPs’ pollution of the species in this part of its range. The study has revealed a broad individual variation of concentrations of examined pollutants, and especially in PCBs. The scale of the variation considerably exceeds ranges known not only for ringed and harp seals and for polar bears, but also for similar group of walruses from Svalbard area. In analogy with similar findings of wide range of POPs’ burden in walruses from other regions, we propose that this phenomenon can be explained to a significant extent by notable individual difference in feeding patterns of walruses in the Pechora Sea ranging from primarily benthos eaters to individuals actively preying on seals.
Compared with walruses from Svalbard, the studied animals have considerably lower levels of oxychlordane (dominating organochlorine pesticides in the sample), comparable levels of PBDEs, and exceeding burden of PCBs.
Notes
A taxon is Vulnerable when the best available evidence indicates that it meets any of the following criteria (A to E), and it is therefore considered to be facing a high risk of extinction in the wild.
References
AMAP (2017) Chemicals of emerging arctic concern. Summary for Policy-makers. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway
Andersen LW, Jacobsen MW, Lydersen C, Semenova V, Boltunov A, Born EW, Wiig Ø, Kovacs KM (2017) Walruses (Odobenus rosmarus rosmarus) in the Pechora Sea in the context of contemporary population structure of Northeast Atlantic walruses. Biol J Linn Soc 122:897–915. https://doi.org/10.1093/BIOLINNEAN/BLX093
Born EW, Gjertz I, Reeves RR (1995) Population assessment of Atlantic walrus, vol 138. Norsk Polarinstitutt, Meddelelser
Espeland O, Kleivane L, Haugen S, Skaare JU (1997) Organochlorines in mother and pup pairs in two Arctic seal species: Harp seal (Phoca groenlandica) and hooded seal (Cystophora cristata). Mar Environ Res 44:315–330. https://doi.org/10.1016/S0141-1136(97)00010-X
Fay FH (1981) Walrus Odobenus rosmarus (Linnaeus, 1758). In Ridgeway SH, Harrison RJ (eds) Handbook of marine mammals vol 1: the Walrus, Sea Lions, Fur Seals and Sea Otter. London: Academic Press
Fay FH (1982) Ecology and biology of the Pacific walrus, Odobenus rosmarus divergens Illiger. North American Fauna 74. U.S. Department of the Interior, Fish and Wildlife Service
Frouin H, Lebeuf M, Hammill M, Fournier M (2012) Transfer of PBDEs and chlorinated POPs from mother to pup during lactation in harp seals Phoca groenlandica. Sci Total Environ 417–418:98–107. https://doi.org/10.1016/j.scitotenv.2011.11.084
Gjertz I, Wiig Ø (1992) Feeding of walrus Odobenus rosmarus in Svalbard. Polar Rec 28:57–59. https://doi.org/10.1017/S0032247400020283
Henriksen EO, Wiig Ø, Skaare JU, Gabrielsena GW, Derocher AE (2001) Monitoring PCBs in polar bears: lessons learned from Svalbard. J Environ Monit 3:493–498. https://doi.org/10.1039/B102683F
Jepson PD, Deaville R, Barber JL, Aguilar À, Borrell A, Murphy S et al (2016) PCB pollution continues to impact populations of orcas and other dolphins in European waters. Sci Rep. https://doi.org/10.1038/srep18573
Letcher RJ, Norstrom RJ, Lin S, Ramsay MA, Bandiera SM (1996) Immunoquantitation and microsomal monoxygenase activities of hepatic cytochromes P450 1A and P450 2B and chlorinated hydrocarbon contaminant levels in polar bear. Toxicol Appl Pharmacol 137:127–140. https://doi.org/10.1006/taap.1996.0065
Lie E, Bernhoft A, Rigetb F, Belikov SE, Boltunov AN, Derocher AE, Garner GW, Wiig Ø, Skaare JU (2003) Geographical distribution of organochlorine pesticides (OCPs) in polar bears (Ursus maritimus) in the Norwegian and Russian Arctic. Sci Total Environ 306:159–170. https://doi.org/10.1016/S0048-9697(02)00490-4
Lindqvist C, Bachmann L, Andersen LW, Born EW, Arnason U, Kovacs KM, Lydersen C, Abramov AV, Wiig Ø (2009) The Laptev Sea walrus Odobenus rosmarus laptevi: an enigma revisited. Zool Scr 38:113–127. https://doi.org/10.1111/j.1463-6409.2008.00364.x
Muir DCG, Segstro MD, Hobson KA, Ford CA, Stewart REA, Olpinski S (1995) Can seal eating explain elevated levels of PCBs and organochlorine pesticides in walrus blubber from eastern Hudson Bay (Canada)? Environ Pollut 90:335–348. https://doi.org/10.1016/0269-7491(95)00019-N
Muir DCG, Ford CA, Rosenberg B, Norstrom RJ, Simon M, Béland P (1996) Persistent organochlorines in beluga whales (Delphinapterus leucas) from the St Lawrence River estuary-I. Concentrations and patterns of specific PCBs, chlorinated pesticides and polychlorinated dibenzo-p-dioxins and dibenzofurans. Environ Pollut 93:219–234. https://doi.org/10.1016/0269-7491(96)00006-1
Muir DCG, Savinova T, Savinov V, Alexeeva L, Potelov V, Svetochev V (2003) Bioaccumulation of PCBs and chlorinated pesticides in seals, fishes and invertebrates from the White Sea, Russia. Sci Total Environ 306:111–131. https://doi.org/10.1016/S0048-9697(02)00488-6
O’Hara TM, Becker PR (2002) Persistent organic contaminants in Arctic marine mammals. In: Vos JG, Bossart GD, Fournier M, O’Shea T (eds) New perspectives: toxicology and the environment, vol 3—systems. Toxicology of marine mammals. Taylor & Francis Group. London, pp 168–205
Ølberg R-A, Kovacs KM, Bertelsen MF, Semenova V, Lydersen C (2017) Short duration immobilization of Atlantic walrus (Odobenus rosmarus rosmarus) with etorphine, and reversal with naltrexone. J Zoo Wildl Med 48:972–978. https://doi.org/10.1638/2016-0232R.1
Riget F, Vorkamp K, Bossi R, Sonne C, Letcher RJ, Dietz R (2016) Twenty years of monitoring of persistent organic pollutants in Greenland biota. A review. Environ Pollut 217:114–123. https://doi.org/10.1016/j.envpol.2015.11.006
Riget F, Bignert A, Braune B, Dam M, Dietz R, Evans M, Green N, Gunnlaugsdóttir H, Hoydal KS, Kucklick J, Letcher R, Muir D, Schuur S, Sonne C, Stern G, Tomy G, Vorkamp K, Wilson S (2018) Temporal trends of persistent organic pollutants in Arctic marine and freshwater biota. Sci Total Environ 649:99–110. https://doi.org/10.1016/j.scitotenv.2018.08.268
Savinov V, Muir DCG, Svetochev V, Svetocheva O, Belikov S, Boltunov A, Alekseeva L, Reiersen LO, Savinova T (2011) Persistent organic pollutants in ringed seals from the Russian Arctic. Sci Total Environ 409:2734–2745. https://doi.org/10.1016/j.scitotenv.2011.02.039
Scotter SE, Tryland M, Nymo IH, Hanssen L, Harju M, Lydersen C, Kovacs KM, Klein J, Fisk AT, Routti H (2018) Contaminants in Atlantic walruses in Svalbard part 1: relationships between exposure, diet and pathogen prevalence. Environ Pollut 244:9–18. https://doi.org/10.1016/j.envpol.2018.10.001
Seymour J, Horstmann-Dehn L, Wooller MJ (2014) Proportion of higher trophic-level prey in the diet of Pacific walruses (Odobenus rosmarus divergens). Polar Biol 37:941–952. https://doi.org/10.1007/s00300-014-1492-z
Sheffield G, Fay FH, Feder H, Kelly BP (2001) Laboratory digestion of prey and interpretation of walrus stomach contents. Mar Mamm Sci 17:310–330. https://doi.org/10.1111/j.1748-7692.2001.tb01273.x
Simmonds MP (2017) Of poisons and plastics: an overview of the latest pollution issues affecting marine mammals. In: Butterworth A (eds) Marine mammal welfare. Animal welfare, vol 17. Springer, Cham, pp 27–37. https://doi.org/10.1007/978-3-319-46994-2_3
Stromberg JO, Andersen LG, Bjork G (1990) State of the marine environment in Antarctica. UNEP Regional Seas Report and Studies 129. UN Environ Prog, Nairobi, Kenya
Svetochev VN, Svetocheva ON (2007) Spring distribution of Atlantic walruses (Odobenus rosmarus rosmarus L.) in the White Sea (according to vessel-based observations, 2005-2006). In Problems of study, rational use and conservation of natural resources of the White Sea. Materials of the 10th international conference. Arkhangelsk, pp 213–217 (In Russian)
Svetochev VN, Svetocheva ON (2008) Distribution of Atlantic walruses (Odobenus rosmarus rosmarus L.) in the White, Barents and Kara seas in 2004-2007. In: Marine mammals of the Holarctic, collection of scientific papers, pp 543–544 (In Russian)
Tartu S, Bourgeon S, Aars J, Andersen M, Polder A, Thiemann GW, Welker JM, Routti H (2017a) Sea ice-associated decline in body condition leads to increased concentrations of lipophilic pollutants in polar bears (Ursus maritimus) from Svalbard, Norway. Sci Total Environ 576:409–419. https://doi.org/10.1016/j.scitotenv.2016.10.132
Tartu S, Lille-Langøy R, Størseth TR, Bourgeon S, Brunsvik A, Aars J et al (2017b) Multiple-stressor effects in an apex predator: combined influence of pollutants and sea ice decline on lipid metabolism in polar bears. Sci Rep. https://doi.org/10.1038/s41598-017-16820-5
Wania F, Mackay D (1993) Global fractionation and cold condensation of low volatility organochlorine compounds in polar regions. Ambio 22:10–18
Wania F, Mackay D (1996) Tracking the distribution of persistent organic pollutants. Environ Sci Technol 30:390A–396A. https://doi.org/10.1021/es962399q
Wiig Ø, Berg V, Gjertz I, Seagars DJ, Skaare JU (2000) Use of skin biopsies for assessing levels of organochlorines in walruses (Odobenus rosmarus). Polar Biol 23:272–278. https://doi.org/10.1007/s003000050444
Wolkers H, van Bavel B, Ericson I, Skoglund E, Kovacs KM, Lydersen C (2006) Congener-specific accumulation and patterns of chlorinated and brominated contaminants in adult male walruses from Svalbard, Norway: indications for individual-specific prey selection. Sci Total Environ 370:70–79. https://doi.org/10.1016/j.scitotenv.2006.06.005
Acknowledgements
Different components of studies during the last 3 years were supported by a number of organizations and funds including WWF Russia, Norwegian Polar Institute, Lukoil foundation. The Norwegian–Russian Environmental Commission facilitated the collection of the samples that made this study possible. The authors acknowledge all colleagues who helped to collect samples, especially Vladislav Svetochev and veterinarian Mikhail Alshinetsky. Critical remarks and valuable advices of reviewers are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
This article belongs to the special issue on the "Ecology of the Pechora Sea", coordinated by Alexey A. Sukhotin.
Rights and permissions
About this article
Cite this article
Boltunov, A., Semenova, V., Samsonov, D. et al. Persistent organic pollutants in the Pechora Sea walruses. Polar Biol 42, 1775–1785 (2019). https://doi.org/10.1007/s00300-019-02457-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02457-9