Abstract
Hematological studies concerned with the determination of normal values of blood parameters in animals have been increasing. However, studies on normal concentration of blood constituents of free-living birds still are not very common, and less than 5% of the species of birds have been analyzed, mostly in captivity. Avian hematology has been used in ornithological studies, because it provides biological data about these animals, their biology, and can be very important in the understanding of ecological and behavioral issues. The main purpose of the study was to investigate the concentrations of certain plasma biochemical parameters in nestlings of Rockhopper Penguins (Eudyptes chrysocome) at the crèche phase and the potential influence of some factors such as sex. We captured 95 nestling Rockhopper during the period 24–31 January 2017. All nestlings were randomly selected from colonies in Saunders Island (Falkland Islands). All the sampled birds were between 25 and 45 days of age, with mean weight of 1.778 ± 0.314 kg and mean bill length of 36.0 ± 2.8 mm. No differences in blood parameters or body condition between sexes were found. No parameters but total protein and urea were related to body index. Body index showed a negative significant relationship with urea levels in blood, with penguins in worse condition (those relatively lighter) showing higher levels of urea in blood than those that were relatively heavier. Same trend was observed for total proteins. Urea concentration in blood would be used as a tool in future studies, particularly in young Rockhoppers when they are in crèche phase, a period of high level of mortality mainly by predation. Plasma urea was the single variable that reflects the best body index and also has a rationale background explaining this relationship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hematological studies carried out on several aspects of the biochemistry and physiology of birds have increased, in particular those concerned with the determination of normal values of blood chemistry parameters (Nirmalan and Robinson 1971; Carpenter 1975; Gee et al. 1981). However, studies on normal concentration of blood constituents of free-living birds are not very common, and less than 5% of the species of birds have been analyzed, mostly in captivity (Ferrer 1993 and references therein). Avian blood chemistry has been used in ornithological studies, because it provides biological data about these animals, their biology, and the detection of possible pathological states. Determination of nutritional and physiological conditions can be very important in the understanding of ecological and behavioral issues.
Hematological values, including chemical components, are known to be influenced by many factors: physiological state, age, sex, nutritional condition, circadian rhythm, seasonal changes, captivity, pollutants, and plasma storing methods (Okumura and Tasaki 1969; Twiest and Smith 1970; Chilgren and DeGraw 1977; DeGraw et al. 1979; Gee et al. 1981; Rehder et al. 1982; Rehder and Bird 1983; Chaplin et al. 1984; Groscolas 1986; Ferrer et al. 1987; Garcia-Rodriguez et al. 1987a, b; Cherel and Le Maho 1988a, b; Viñuela et al. 1991; Jenni-Eiermann and Jenni 1992). So when we try to study the influence of one of these factors, we must be sure that the others are controlled (Ferrer 1990).
An adequate knowledge of blood chemistry is greatly recommended for those projects involving research and management of populations as far as they can be valuable for the assessment of the nutritional levels and health status of species (Ferrer 1993; Ferrer and Dobado-Berrios 1998; Ferrer et al. 2017). Additionally, many hypotheses in ecology rely in differences among individuals in nutritional levels (Ferrer 1993; Angelier et al. 2011). These kinds of studies performed in long-lived birds, like seabirds, are important providing basic data that are interesting in veterinary, taxonomy, and ecology (Balasch et al. 1976; Polo et al. 1992; Work 1996; Uhart et al. 2003; Groscolas et al. 2008; Bourgeon et al. 2010; Crossin et al. 2012; Ferrer et al. 2017). Populations of many penguin species have declined substantially in the past two decades (Trathan et al. 2015) due to several reasons, including increasing pollution of the oceans. Consequently, developing new monitoring tools, for example blood analyses to detect abnormal effects in blood constituents, would be necessary. To do that, we need to know which the reference values are in the species and which factors are modulating them.
There are several species of penguins whose biochemical parameters in blood have been described (Aptenodytes forsteri, Groscolas 1982; A. patagonicus, Cherel et al. 1988a, b, c; Pygoscelis adeliae, P. antarctica, and P. papua, Aguilera et al. 1993; Spheniscus humboldti, Wallace et al. 1995; Spheniscus mendiculus, Travis et al. 2006). As far as we know, there are some published data on hematology of Rockhopper Penguins (Eudyptes chrysocome, Dehnhard et al. 2011) and also biochemical parameters (Ghebremeskel et al. 1989), but they focused on change in values before and after molt of adult birds. There are no reference values for nestlings of this species at the end of crèche phase. Those reference values could prove to be of interest for the conservation of this species as far as nutritional conditions at the beginning of juvenile dispersal seem to affect dispersal distances in other species (Ferrer and Morandini 2017) and because during the end of crèche phase there is a high predation rates that would be related to physiological conditions (Morandini and Ferrer pers. observ.).
The main purpose of the study was to investigate the concentrations of plasma biochemical parameters mainly related to fat and protein metabolism (urea, uric acid, triglycerides, cholesterol), in nestlings of Rockhopper Penguins at the crèche phase, to provide with reference values for this species and to analyze the potential influence of some factors such as sex. Even when sexual differences in blood chemistry are not very common in birds, being more frequent among hematocytological parameters has never been studied in this penguin species. In addition, we try to determine potential correlations between biochemical blood parameters and nutritional status, using for that body index. We investigate if concentration of some intermediate metabolism substances would be used as indicator of nutritional status, particularly, fat and protein metabolism products.
Materials and methods
Study area and species
We conducted this study in Saunders Island (51.37°S 60.09°W), located at the north coast of Falkland Islands, with 12,500 ha (Fig. 1). The Falkland Islands are located in the southwest region of the South Atlantic Ocean and it is home to penguins (Aptenodytes patagonicus, Pygoscelis papua, Eudyptes chrysocome, and Spheniscus magellanicus), shags (Phalacrocorax magellanicus and P. atriceps), and Black-browed Albatrosses (Thalassarche melanophrys).
The Rockhopper is the smallest yellow-crested penguin with a weight of 2–3.5 kg. The Southern Rockhopper Penguin (Eudyptes chrysocome chrysocome) has a global population of around 1 million. The Falkland Islands currently supports one of the largest populations (Baylis et al. 2013). The Southern Rockhopper Penguin status has been classified as vulnerable by the IUCN due to a declined by about one-third in the last 30 years. Nevertheless, recent studies showed a significant recovery of the population (Baylis et al. 2013). They breed in large colonies located from sea level to cliff-tops.
Body measurements
We captured 95 nestling Rockhoppers during the period 24–31 January 2017. All nestlings were randomly selected from colonies in Saunders Island. According to flipper length (after Dehnhard et al. 2011), all the sampled birds were between 25 and 45 days of age, in the crèche phase (Poisbleau et al. 2010). We weighed and measured all the nestlings using calipers and a rule. We measured bill length (expose culmen, see Aguilera et al. 1993) and bill depth (to the nearest 0.1 mm with digital calipers), and flipper length (to the nearest mm) and with a spring balance recorded body mass (to the nearest 10 g). To reduce variability, most of the measurements (> 90%) were made by the same observer. To estimate nutritional conditions in nestlings independently of blood parameters, we conducted a regression between cube root of mass and bill length keeping residuals of this regression (body index) as proxy of body condition. We select bill length because it is the structural measure with the best linear relationship with the age of the chicks (Poisbleau et al. 2010). Caution must be taken with this approach due to high variability in body mass in penguins’ chicks, depending on the time since the last meal was provided by their parents. We did not measured chicks that have been fed during our stay at the colony but we cannot discard this possibility.
Blood collecting procedures
Blood samples were collected between 11 a.m. and 3 p.m. avoiding variations in blood parameters due to circadian rhythms (Garcia-Rodriguez et al. 1987b; Ferrer 1990; Ferrer et al. 1994). Samples, up to 2 ml, were extracted using a 23-gauge needle and a 5-ml heparinized syringe. Blood samples were stored on portable refrigerator around 6 °C. Blood samples were carried to the laboratory within 8 h after blood withdrawal and were centrifuged at 3000 rpm for 10 min. We kept plasma stored frozen at − 24°C until analysis. Handling time including bleeding time of birds caught was below 3 min. All the birds were released at the capture point.
Tested parameters and sex determination
We performed biochemical analyses on a computer process-controlled multichannel autoanalyzer (Cobas Integra 400, Roche Diagnostic). We made 11 determinations in nestling birds (Table 1). Sample reproductability was > 97.3% for all parameters and was calculated measuring a sample 20 times for each parameter. Protein metabolism or catabolism affect levels of urea and uric acid, being cholesterol, triglycerides, and β-hydroxybutyrate a reflection of fat metabolism (Garcia-Rodriguez et al. 1987a; Cherel et al. 1988a, 1993; Alonso-Alvarez et al. 2003). Alkaline phosphatase and calcium levels vary according to ossification process (Viñuela et al. 1991; Dobado-Berrios and Ferrer 1997) and creatine kinase is related to muscular activity (Alonso-Alvarez et al. 2003). LDH is useful in diagnosing tissue damage. The analyses of plasma parameters were carried out in the Laboratory of Ecophysiology of the Doñana Biological Station (CSIC), Seville, Spain.
Sex was determined by means of PCR amplification of sections from CHD1-Z and CHD1-W genes that are located on the avian sex chromosomes (Griffiths et al. 1998). Using this technique, we identified 22 females and 31 males. The analyses were carried out in the laboratory of ecophysiology of the Doñana Biological Station (CSIC), Seville, Spain. Due to small samples, not all the analyses were conducted in all the birds.
Statistical analyses
All data are expressed as mean ± standard deviations (SD). Normality in distribution of variables was tested. We used a MANOVA analyses to look for the effect of sex on blood biochemistry parameters. Regression analyses were used to study relationship between body condition index and nutritional indicators (urea levels and total proteins). Statistica 8.0 software statistical package was used to perform statistical procedures, and we used an alpha value of 0.05 to assess significance of results.
Results
Mean weight of penguins was 1.778 ± 0.314 kg (range 1.000–2.600 kg) and mean bill length was 36.0 ± 2.8 mm (range 29.6–42.0). We identified 52 males and 41 females in our sample (2 were not determined due to small sample). Body measurements by sexes are presented in Table 2. As expected in penguins, females are smaller and lighter than males. However, no differences in blood parameters or body condition between sexes were found (Table 2). Mean, range, and SD of blood parameters and body condition index are presented in Table 3.
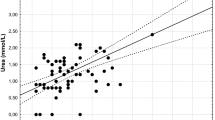
There was a significant positive relationship between cube root of body weight and bill length (r = 0.433, n = 95, p < 0.0001). The residuals of this regression are what we used as a proxy of body index. A multiple regression analysis trying to find significant relationship between body index and blood parameters was conducted (Table 4). No parameters but total protein and urea were related to body index. Body index showed a negative significant relationship with urea levels in blood (r = − 0.309, n = 83, p = 0.004; Fig. 2) and total proteins (r = − 0.244, n = 83, p = 0.030; Fig. 3), with penguins in worse condition (those relatively lighter) showing higher levels of urea and total proteins in blood than those that were relatively heavier.
Cholesterol, triglycerides, and β-hydroxybutyrate showed a non-significant negative trend, decreasing when body index increases. Uric acid concentration, contrarily, seems to show a positive relationship but again non-significant. We did not find any significant correlation among blood parameters.
Discussion
The main objective of this study was to establish reference values at the population level for several blood chemistry parameters in Rockhopper nestlings, and to determine their means and variability using free-living birds. Anyway, caution must be taken with these results. It is known that reference values in wild populations can vary among nestlings inhabiting different geographical areas (Ferrer and Dobado-Berrios 1998). Those dissimilarities are probably accounting for quantitative and qualitative differences in their respective diets. More information from other colonies of Rockhoppers in different areas is needed to stablish definitive reference values for this species. In addition to reference values, we analyzed the potential effect of a factor such as sex in these values. We used relatively high sample sizes of wild bird species to good purpose. No differences between sexes were found for any of the studied constituents. Both our present results and other previous results support that sex-related differences are exhibited mainly in hematocytological parameters (red cell number, hematocrit, hemoglobin, etc.) rather than in plasma variables (Mulley 1979; Puerta et al. 1989; Tell and Citino 1992; Ferrer and Dobado-Berrios 1998; Ferrer et al. 2017). Nevertheless, in some species of raptors, sexual differences in plasma chemistry parameters have been found, related probably with large sexual dimorphism in size (Balbontín and Ferrer 2005).
Blood values in young Rockhopper Penguins were similar to those described for other penguin species (Aguilera et al. 1993) or adults of the same species (Ghebremeskel et al. 1989, see Tables 5, 6), with the expected differences between adults and juveniles in some parameters as has been described in several species (Casado et al. 2002; Ferrer et al. 2017 and references therein). Metabolic status is different between adult and nestlings. In our case, young penguins exhibited lower values of uric acid and higher values for urea, cholesterol, or triglycerides than adults (Ghebremeskel et al. 1989) or adults of other penguin species (Aguilera et al. 1993). Contrary, levels of ALP are higher in our study because plasma ALP activity is higher in immature birds than in adults (Puerta et al. 1989; Viñuela et al. 1991; Dobado-Berrios and Ferrer 1997; Ferrer et al. 2017). Those differences are due to the different rate of osteoblastic activity and bone growth in young and adults birds (Viñuela et al. 1991; Dobado-Berrios and Ferrer 1997). The mean concentration of urea in the blood of the young Rockhoppers was greater than those for uric acid, even though they are considered to be uricotelic (Gee et al. 1981). This is interesting, because adult Pygoscelis penguins usually exhibit blood urea concentration lower than those for uric acid (Aguilera et al. 1993). Mean levels of urea and uric acid and also creatinine decrease with age in other species (Rosskopf et al. 1982; Casado et al. 2002) suggesting that adults are in better condition than young. In other seabird species, however, these seem to be the opposite, with young birds in better nutritional status than adults (Ferrer et al. 2017).
An additional aim of this study was to find a proxy of body condition using intermediate metabolite in blood. This parameter would be used as a tool in future studies, particularly in young Rockhoppers when they are in crèche phase, a period of high level of mortality mainly by predation. Plasma urea was the single variable that reflects the best body index and also has a rationale background explaining this relationship. The increase in plasma urea level could be explained by the use of own proteins as energy source during fasting period (Garcia-Rodriguez et al. 1987a; Castellini and Rea 1992). Urea levels in blood have been used as index of body condition in raptors (e.g., Ferrer 1992, 1993, 1994). Urea levels increase as a response to starvation and decrease after refeeding because proteins are actively mobilized as energy source, increasing the nitrogenous excretion components released into the blood (Garcia-Rodriguez et al. 1987a; Alonso-Alvarez et al. 2002, 2003). Urea is not sensitive to recent ingest (in contrast to glucose concentration for example), and increase and decrease in blood concentration are slow (Garcia-Rodriguez et al. 1987a; Ferrer 1990). Consequently, this could be considered a good indicator not only of acute fasting, but also of mid-term nutritional condition. Thanks to these characteristics, urea level in blood has been used as indicators of nutritional condition in several species in different ecological contexts (Ferrer et al. 1987; Ferrer 1992, 1994; Alonso-Alvarez and Ferrer 2001; Casado et al. 2002; Balbontín and Ferrer 2005).
It is well known that uric acid concentration increases during fasting periods (Garcia-Rodriguez et al. 1987a; Cherel et al. 1988a, b; Robin et al. 1988; Alonso-Alvarez et al. 2003) because it is the major compound for nitrogen excretion in birds. In contrast, changes in urea concentrations are usually thought to be of minor importance. However, the increase of plasma urea concentration found in several species of large size penguins (Groscolas 1982; Cherel et al. 1988a; Cherel and Le Maho 1988a, b; Cherel et al. 1993) as well as smaller ones (Cherel et al. 1993; Alonso-Alvarez et al. 2003) is in line with evidence showing that prolonged starvation increases the capacity of bird liver slices to generate urea (Lemonde 1959). Plasma uric acid concentration has been used as an index of protein catabolism, because birds excreted mostly uric acid as an end product of metabolized nitrogen (Griminger and Scanes 1986). Urea is a minor pathway for protein degradation in birds but the activity of liver arginase (the enzyme on which urea production in birds depends) has increased after a prolonged fast, and also the rise of urea during protein catabolism may be explained by a greater arginine availability. The time a bird keeps in using up its fat reserves and the moment it starts using its own muscle tissues as an energy source basically varies according to the individual’s initial condition and the species’ capacity for storing fat reserves. It has been shown in Emperor Penguins, Aptenodytes forsteri (Groscolas 1982, 1986; Robin et al. 1988), that there are still lipid stores when β-hydroxybutyrate levels in blood begin to decrease. A critical level in the lipid composition of adipose tissue or its distribution in the body may exist to keep these aquatic birds in a good thermal isolation condition that still allows them to dive in cold water. Perhaps small penguins like Rockhoppers would be more prone to start protein catabolism, showing a more rapid increase in urea level after fasting in a short time due to severe limitation on extra fat stores.
References
Aguilera E, Moreno J, Ferrer M (1993) Blood chemistry values in three Pygoscelis penguins. Comp Biochem Phys A 105:471–473
Alonso-Alvarez C, Ferrer M (2001) A biochemical study of fasting, subfeeding, and recovery processes in yellow-legged gulls. Physiol Biochem Zool 74:703–713
Alonso-Alvarez C, Ferrer M, Velando A (2002) The plasmatic index of body condition in Yellow- legged Gulls Larus cachinnans: a food-controlled experiment. Ibis 144:147–149
Alonso-Alvarez C, Ferrer M, Vinuela J, Amat JA (2003) Plasma chemistry of the chinstrap penguin Pygoscelis antarctica during fasting periods: a case of poor adaptation to food deprivation? Polar Biol 26:14–19
Angelier F, Weimerskirch H, Chastel O (2011) Capture and blood sampling do not affect foraging behaviour, breeding success and return rate of a large seabird: the black-browed albatross. Polar Biol 34:353–361
Balasch J, Musquera S, Palacios L, Jimenez M, Palomeque J (1976) Comparative hematology of some falconiforms. Condor 78:258–273
Balbontín J, Ferrer M (2005) Condition of large brood in Bonelli’s Eagle Hieraaetus fasciatus. Bird Study 52:37–41
Baylis AMM, Wolfaardt AC, Crofts S, Pistorius PA, Ratcliffe N (2013) Increasing trend in the number of southern rockhopper penguins (Eudyptes c. chrysocome) breeding at the Falkland Islands. Polar Biol 36:1007–1018
Bourgeon S, Kauffmann M, Geiger S, Raclot T, Robin JP (2010) Relationships between metabolic status, corticosterone secretion and maintenance of innate and adaptive humoral immunities in fasted re-fed mallards. J Exp Biol 213:3810–3818
Carpenter FL (1975) Bird hematocrits: effects of high altitude and strength of flight. Comp Biochem Phys A 50:415–417
Casado E, Balbontin J, Ferrer M (2002) Plasma chemistry in booted eagle (Hieraaetus pennatus) during breeding season. Comp Biochem Phys A 131:233–241
Castellini MA, Rea LD (1992) The biochemistry of natural fasting at its limits. Experientia 48:575–582
Chaplin SB, Diesel DA, Kasparie JA (1984) Body temperature regulation in red-tailed hawks and great horned owls: responses to air temperature and food deprivation. Condor 86:175–181
Cherel Y, Le Maho Y (1988a) Five months of fasting in king penguin chicks: body mass loss and fuel metabolism. Am J Physiol 249:387–392
Cherel Y, Le Maho Y (1988b) Changes in body mass and plasma metabolites during short-term fasting in the king penguin. Condor 90:257–258
Cherel Y, Robin JP, Le Maho Y (1988a) Physiology and biochemistry of long-term fasting in birds. Can J Zool 66:159–166
Cherel Y, Robin JP, Le Maho Y (1988b) Fasting in king penguin. I. Hormonal and metabolic changes during breeding. Am J Physiol 254:170–177
Cherel Y, Robin JP, Le Maho Y (1988c) Fasting in king penguin. II. Hormonal and metabolic changes during molt. Am J Physiol 254:178–184
Cherel Y, Freby F, Gilles J, Robin J-P (1993) Comparative fuel metabolism in gentoo and king penguins: adaptation to brief versus prolonged. Polar Biol 13:263–269
Chilgren JD, DeGraw WA (1977) Some blood characteristics of white-crowned sparrows during molt. Auk 94:169–171
Crossin GT, Trathan PN, Phillips RA, Gorman KB, Dawson A, Sakamoto KQ, Williams TD (2012) Corticosterone predicts foraging behavior and parental care in macaroni penguins. Am Nat 180:E31–E41
DeGraw JI, Brown VH, Kisliuk RL, Gaumont Y, Sirotnak FM (1979) Chemistry and biology of Pteridines. Elsvier, Amsterdam
Dehnhard N, Poisbleau M, Demongin L, Quillfeldt P (2011) Do leucocyte profiles reflect temporal and sexual variation in body condition over the breeding cycle in Southern Rockhopper Penguins? J Ornithol 152:759–768
Dobado-Berrios PM, Ferrer M (1997) Age-related changes of plasma alkaline phosphatase and inorganic phosphorus, and late ossification of the cranial roof in the Spanish imperial eagle (Aquila adalberti CL Brehm, 1861). Physiol Zool 70:421–427
Ferrer M (1990) Hematological studies in birds. Condor 92:1085–1086
Ferrer M (1992) Regulation of the period of postfledging dependence in the Spanish imperial Eagle Aquila adalberti. Ibis 134:128–133
Ferrer M (1993) Blood chemistry studies in birds: some applications to ecological problems. Trends Comp Biochem Physiol 1:1031–1044
Ferrer M (1994) Nutritional condition of Spanish imperial Eagle nestlings Aquila adalberti. Bird Study 41:120–123
Ferrer M, Dobado-Berrios P (1998) Factors affecting plasma chemistry values of the Spanish Imperial Eagle, Aquila adalberti. Comp Biochem Phys A 120:209–217
Ferrer M, Morandini V (2017) Better nutritional condition changes the distribution of juvenile dispersal distances: an experiment with Spanish imperial eagles. J Avian Biol 48:1342–1347
Ferrer M, Garcia-Rodriguez T, Carrillo JC, Castroviejo J (1987) Hematocrit and blood chemistry values in captive raptors (Gyps fulvus, Buteo buteo, Milvus migrans, Aquila heliaca). Comp Biochem Phys A 87:1123–1127
Ferrer M, Amat JA, Viñuela J (1994) Daily variations of blood chemistry values in the chinstrap penguin (Pygoscelis antarctica) during the Antarctic summer. Comp Biochem Phys A 107:81–84
Ferrer M, Morandini V, Perry L, Bechard M (2017) Factors affecting plasma chemistry values of the black-browed albatross Thalassarche melanophrys. Polar Biol 40:1537–1544
Garcia-Rodriguez T, Ferrer M, Carrillo JC, Castroviejo J (1987a) Metabolic responses of Buteo buteo to long-term fasting and refeeding. Comp Biochem Phys A 87:381–386
Garcia-Rodriguez T, Ferrer M, Recio F, Castroviejo J (1987b) Circadian rhythms of determined blood chemistry values in Buzzards and Eagle Owls. Comp Biochem Phys A 88:663–669
Gee GF, Carpenter JW, Hensler GL (1981) Species differences in hematological values of captive cranes, geese, raptors, and quail. J Wildl Manag 45:463–483
Ghebremeskel K, Williams G, Keymer IF, Horsley D, Gardner DA (1989) Plasma chemistry of rockhopper (Eudyptes crestatus), Magellanic (Spheniscus magellanicus) and gentoo (Pygoscelis papua) wild penguins in relation to moult. Comp Biochem Phys A 92:43–47
Griffiths R, Double MC, Orr K, Dawson RJ (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Griminger P, Scanes CG (1986) In: Sturkie PD (ed) Avian physiology. Springer-Verlag, New York, pp 326–344
Groscolas R (1982) changes in plasma lipids during breeding, molting, and starvation in male and female emperor penguins (Aptenodytes forsteri). Physiol Zool 55:45–55
Groscolas R (1986) Changes in body mass, body temperature and plasma fuel levels during the natural breeding fast in male and female emperor penguins Aptenodytes forsteri. J Comp Phys B 156:521–527
Groscolas R, Lacroix A, Robin JP (2008) Spontaneous egg or chick abandonment in energy-depleted king penguins: a role for corticosterone and prolactin? Horm Behav 53:51–60
Jenni-Eiermann S, Jenni L (1992) High plasma triglyceride levels in small birds during migratory flight: a new pathway for fuel supply during endurance locomotion at very high mass-specific metabolic rates? Physiol Zool 65:112–123
Lemonde A (1959) Urea production in chick liver slices. Can J Biochem Physiol 37:1187–1190
Mulley RC (1979) Haematology and blood chemistry of the black duck (Anas superciliosa). J Wildl Dis 15:437–441
Nirmalan GP, Robinson GA (1971) Haematology of the Japanese quail (Coturnix coturnix japonica). Br Poult Sci 12:475–481
Okumura JI, Tasaki I (1969) Effect of fasting, refeeding and dietary protein level on uric acid and ammonia content of blood, liver and kidney in chickens. J Nutr 97:316–320
Poisbleau M, Demongin L, Noordwijk HJV, Strange IJ, Quillfeldt P (2010) Sexual dimorphism and use of morphological measurements to sex adults, immatures and chicks of Rockhopper penguins. Ardea 98:217–224
Polo FJ, Celdran JF, Peinado VI, Viscor G, Palomeque J (1992) Hematological values for four species of birds of prey. Condor 94:1007–1013
Puerta ML, Munõz RP, Huecas V, Abelenda M (1989) Hematology and blood chemistry of chicks of white and black storks (Ciconia ciconia and Ciconia nigra). Comp Biochem Phys A 94:201–204
Rehder NB, Bird DM (1983) Annual profiles of blood packed cell volumes of captive American kestrels. Can J Zool 61:2550–2555
Rehder NB, Bird DM, Lague PC, Mackay C (1982) Variation in selected hematological parameters of captive red-tailed hawks. J Wildl Dis 18:105–109
Robin JP, Frain M, Sardet C, Groscolas R, Le Maho Y (1988) Protein and lipid utilization during long-term fasting in emperor penguins. Am J Physiol 254:61–68
Rosskopf Jr WJ, Woerpel RW, Rosskopf G, Water D (1982) Hematologic and blood chemistry values for common pet avian species. VM/SAC, Veterinary Medicine and Small Animal Clinician, USA
Tell LA, Citino SB (1992) Hematologic and serum chemistry reference intervals for cuban Amazon parrots (Amazona leucocephala leucocephala). J Zoo Wildl Med 23:62–64
Trathan PN, García-Borboroglu P, Boersma D, Bost CA, Crawford RJ, Crossin GT, Cuthbert RJ, Dann P, Davis LS, De La Puente S, Ellenberg U, Lynch HJ, Mattern T, Putz K, Seddon PJ, Trivelpiece W, Wienecke B (2015) Pollution, habitat loss, fishing, and climate change as critical threats to penguins. Conserv Biol 29:31–41
Travis EK, Vargas FH, Merkel J, Gottdenker N, Miller RE, Parker PG (2006) Hematology, serum chemistry, and serology of Galapagos penguins (Spheniscus mendiculus) in the Galapagos Islands, Ecuador. J Wildl Dis 42:625–632
Twiest G, Smith CJ (1970) Circadian rhythm in blood glucose level of chickens. Comp Biochem Phys 32:371–375
Uhart MM, Quintana F, Karesh WB, Braselton WE (2003) Hematology, plasma biochemistry, and sero survey for selected infectious agents in southern giant petrels from Patagonia, Argentina. J Wildl Dis 39:359–365
Viñuela J, Ferrer M, Recio F (1991) Age-related variations in plasma levels of alkaline phosphatase, calcium and inorganic phosphorus in chicks of two species of raptors. Comp Biochem Phys A 99:49–54
Wallace RS, Teare JA, Diebold E, Michaels M, Willis MJ (1995) Hematology and plasma chemistry values in free-ranging Humboldt penguins (Spheniscus humboldti) in Chile. Zoo Biol 14:311–316
Work TM (1996) Weights, hematology, and serum chemistry of seven species of free-ranging tropical pelagic seabirds. J Wildl Dis 32:643–657
Acknowledgements
We thank the Falkland Island government and Fundacion Migres that supported the present study. We are especially grateful to David and Suzan Pole-Evans for their support of this project. Two anonymous referees and especially J. P. Robin greatly improved the first version of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest in this study.
Ethical approval
Procedures used in this study comply with the current laws for working on the Falklands Islands, as well as with institutional guidelines for the care and use of animals (Spanish Council (CSIC) Ethical Committee). Permits to work in the study area and to take blood samples of Rockhoppers Penguins were granted by Falkland Government (number of license: R12/2014), as well as by the owners’ of the Saunders Island.
Rights and permissions
About this article
Cite this article
Morandini, V., Ferrer, M., Perry, L. et al. Blood chemistry values in nestlings of Rockhopper Penguins (Eudyptes chrysocome): the effect of sex and body condition. Polar Biol 41, 2533–2541 (2018). https://doi.org/10.1007/s00300-018-2389-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2389-z