Abstract
Although benthic fauna in the Pechora Sea (SE Barents Sea) is generally well-studied, information on the bottom communities in the shallows near islands and the mainland is still sparse. Shallow marine areas in the Pechora Sea serve as important feeding grounds for numerous benthophagous fish, waterfowl and marine mammals, including the Atlantic walrus (Odobenus rosmarus rosmarus). To study the patterns of macrozoobenthic distribution in the shallows and evaluate the ecological state of the zoobenthic populations close to walrus haul-outs, sampling was performed in 2014 and 2016 around an archipelago in the Pechora Sea. In 2014, the average biomass, the Shannon's Diversity Index and the predominance of filter feeders in benthic communities were, in general, similar to the respective characteristics in neighboring deeper areas studied in the 1990s. In 2016, significant differences in species number and in biomass were recorded compared to 2014. An increase was observed in Atlantic boreo-Arctic species. Analysis of the trophic structure showed a slight decrease in the proportion of filter feeders and a significant increase in the proportion of subsurface deposit feeders. However, the Shannon's Diversity and Ecological Stress Indices indicated that the macrozoobenthos in the study area was in a state of equilibrium. Changes in the zoobenthos may result from several factors, such as an increase in water temperature, sediment re-deposition under wind-induced waves and the plowing of bottom sediments by walruses during their foraging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Pechora Sea is a special region of the Barents Sea owing not only to the peculiarities of its oceanographic characteristics—such as shallowness, the influence of massive freshwater discharge and a long period of sea-ice cover (Terziev et al. 1992; Adrov and Denisenko 1996)—but also due to the absence of any anthropogenic impact before the mid-1990s (Denisenko 2013). This was confirmed by a chemical analysis of the waters and bottom sediments in the Pechora Sea carried out in 2000–2001 (Skibinskiy et al. 2003). Unlike other areas of the Barents Sea, no large-scale commercial fishing ever occurred in the Pechora Sea, thus its marine biota remained undisturbed until the end of the 1990s (Denisenko 2013). Due to its environmental features and habitats—where waterfowl stop during their seasonal migrations (Krasnov et al. 2002), the southern boundary of the Atlantic walrus distribution is found and the edible cockles Serripes groenlandicus and other bivalve molluscs reach high biomasses—the Pechora Sea has been regarded as one of the most important areas of the Barents Sea (Larsen et al. 2004). Until recently, the bottom macrofauna of the Pechora Sea has been considered well-studied due to abundant information collected at networks of stations during benthic surveys that have been conducted since the 1920s (Brotskaya and Zenkevich 1939; Dahle et al. 1998; Denisenko et al. 2003; Menis 2003; Denisenko 2006a; 2013, Anisimova et al. 2010). Despite these intensive studies prior to the 2000s, it appeared that shallow areas with depths of less than 10 m were still under-explored. For example, in 2006, a large sublittoral population of blue mussels (Mytilus edulis) was discovered in the Pechora Sea near Dolgiy Island (Sukhotin et al. 2008).
A serious risk factor that can affect the benthos is commercial oil production like that which began at several oil fields in the Pechora Sea in the early 2000s, and is imminent in Pechora Bay’s Korovonskaya Inlet in the near future. Despite the absence of large oil spills to date, the accumulation of hydrocarbons in bottom sediments near the oilfields has caused a negative impact on benthic organisms at the local scale (Mitskevich and Telitsina 2002; Samokhina 2009). A significant negative correlation was reported between the concentration of aromatic hydrocarbons in sediments and the abundance and biomass of zoobenthos in some areas of the Pechora Sea (Samokhina 2009).
Besides anthropogenic impacts, natural stressors can also affect the benthos in the Pechora Sea. It has been found that natural fluctuations of the total biomass of macrozoobenthos in the area are associated with water temperature variations (Denisenko 2006a, 2013). Another influencing factor is the mass consumption of macrozoobenthos by walruses. The number of walruses in the Pechora Sea has been increasing since 1991 (Haug and Nilssen 1995; Boltunov et al. 2010; Lydersen et al. 2012; Anufriev et al. 2017). They have permanent haul-outs on Kolguev, Vaygach, Dolgiy and Matveev islands (Anufriev et al. 2017; Semenova et al. in press). The latter two belong to the Nenetskiy Nature State Reserve, where any anthropogenic activity is prohibited. Nevertheless, both islands are located relatively close to oil exploitation areas such as the Prirazlomnoye oil field. Walruses have massive haul-outs on these coasts, spending almost the entire ice-free period there—from June to October (Semenova et al. in press). According to observations in other areas in the Arctic, during the open-water period walruses spend about 75% of their time in the water feeding near haul-outs (Levermann et al. 2003). In 2011, the number of walruses registered on Matveev and Dolgiy islands exceeded 500 individuals (Anufriev et al. 2017); by 2017, the number of walruses had increased up to 2000 specimens (Bogomolova et al. 2017). During the ice-free time only males were present on the mentioned haul-outs; the number of animal doubles in the late autumn, when the females and their calves return from the Kara Sea. A walrus’ diet includes bivalve molluscs such as Nuculana pernula, Serripes groenlandicus, Mya truncata, Macoma calcarea, Hiatella arctica, Astarte sp. and Panomya arctica. Besides bivalves, mobile benthic crustaceans such as Saduria sp., Pagurus sp., Hyas sp., Sclerocrangon sp., gastropod molluscs from families Naticidae, Neptuneidae and Buccinidae and fish species of the genus Lycodes (Zoarcidae) are also important components of walrus forage (Svetocheva and Semenova 2017). The species composition of the walruses’ diet in the southeast Barents Sea is comparable with that in the Svalbard area of the Barents Sea (Gjertz and Wiig 1992) and around Greenland (Fisher and Steward 1997; Born et al. 2003).
The area around the islands is suitable for the walrus foraging because permanent dense aggregations of bivalve molluscs have been registered on shoals in the Pechora Sea for a long time (Denisenko et al. 2003; Menis 2003; Denisenko 2006a). As mentioned above, the most important walrus prey species are bivalve molluscs, but walruses can change their preferences (Fisher and Steward 1997; Svetocheva and Semenova 2017). Besides the direct removal of bottom invertebrates, walruses plow up to 5.6% of the sea bottom in their foraging areas and form deep ditches and pits (Nelson and Johnson 1987), disturbing populations of other invertebrate taxa such as polychaetes, sessile hydroids and bryozoans (Levermann et al. 2003). The question arises of whether and how large a population of walruses can negatively influence zoobenthic communities.
This study aimed to (1) obtain new information on zoobenthic distribution in the Pechora Sea around walrus haul-outs (2) check for temporal changes in the zoobenthos at chosen stations and (3) estimate the ecological status of zoobenthos populations around walrus haul-outs in the coastal areas off of Dolgiy and Matveev islands.
Materials and methods
Study area
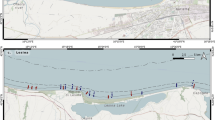
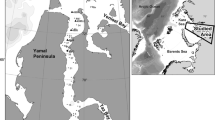
The study region was limited to shallow (< 20 m depth) areas around the Matveev and Dolgiy islands (Fig. 1), where one of the greatest local accumulations of walruses is registered in the southeastern part of the Pechora Sea. General geomorphologic and oceanographic descriptions of the Pechora Sea, as well as the oceanographic regime, are published elsewhere (Ilyin and Matishov 1992; Adrov and Denisenko 1996; Tarasov 1996; Dahle et al. 1998; Denisenko et al. 2003). Here, we mention the main features of the environments around the study area at the present time.
Oceanographic regime and bottom sediments
Shallow waters with depths not exceeding 20 m characterize the region of our observations (Fig. 1). The area is influenced by two main water masses: Arctic waters intruding from the Kara Gates and Yugorskiy Shar straits flowing northwards along the coast of Novaya Zemlya and coastal waters affected by freshwater runoff in the south (Ilyin and Matishov 1992; also concisely reviewed in Dahle et al. 1998). The temperature and salinity of both the surface and bottom water layers show distinct seasonal and spatial variations (Adrov and Denisenko 1996). The temperature of the bottom waters is generally below 0 °C from November until early June; it reaches its maximum values between August and September, from − 1 °C in the north to 6 °C in the southwest near the mainland (Denisenko et al. 2003). Rising bottom temperatures in the Barents Sea have been recorded since 2004 (Fig. 2A; for Pechora Sea see also Semushin et al. this issue).
A Long-term variation of the near-bottom temperature at the ‘Kola Transect’ (33°30′E crossing Barents Sea northward). Dotted line is 3 years average values of water temperature. URL: https://www.pinro.ru/ (date of observation 08–01-2018); B variation of the duration of ice-free period (days) around Dolgiy Island in the Pechora Sea and water temperature at the Kola Transect. URL: https://www.aari.ru/projects/ECIMO/ (dates of observations 2008–2018)
Ice cover usually appears in the study area at the beginning of October and mostly disappears in late June (Terziev et al. 1992). The rising temperatures observed in the Arctic over the last 10 years affect the ice-cover in the Pechora Sea, as the duration of the ice-free period in the Pechora Sea has been shown to positively correlate with water temperature in the Barents Sea (Fig. 2B; Adrov and Denisenko 1996; Denisenko 2013). The average near-bottom salinity in the area ranges from 34 in winter to 26–31 in late June; the decrease in salinity is associated with ice melting and extensive freshwater runoff from the Pechora River, which is responsible for roughly 80% of the total river water input in the whole Barents Sea (Milliman and Syvitski 1992).
In terms of sediment type, the Pechora Sea is relatively heterogeneous (Klenova 1960; Adrov and Denisenko 1996; reviewed in Dahle et al. 1998). In the study area, the sediments are represented mostly by fine sand and a mixture of silty sand and gravel (Table 1).
Sampling
Two benthic surveys were carried out on the R/V Professor Vladimir Kuznetsov around Dolgiy and Matveev islands, close to the major summer haul-outs of the Pechora Sea population of Atlantic walrus (Odobenus rosmarus rosmarus). In July 2014, samples were collected at 18 stations. In 2016, six stations were chosen based on the results of processed materials from the previous survey. The 2016 expedition was carried out in the beginning of September, i.e., 1.5 months later than the 2014 expedition (see Usov et al. 2018). The sampling procedure was the same in both years. Quantitative samples were collected by van Veen grab (0.1 m2), while qualitative samples were taken by Sigsbee-Agassiz trawl (width 1 m) at a vessel speed of less than two knots. Trawl samples were taken to assess the macrozoobenthos species composition. This was necessary on hard bottoms where, due to the aggregation of settlements, grab sampling may not always allow to obtain exhaustive information on species composition. Overall, 54 and 18 quantitative samples were obtained in 2014 and in 2016, respectively (Table 1). Samples were sieved onboard, washed through a 1 mm nylon mesh and fixed with buffered 4% formaldehyde. The 2014 samples were sorted aboard the vessel, whereas in 2016 the sorting procedure was completed in the Zoological Institute of the Russian Academy of Sciences in Saint Petersburg, using identical methods as in 2014. At each station, depth, sediment texture, surface and near-bottom temperature and salinity were recorded.
Sorted animals were identified, counted, and weighed (wet formalin weight, g). Feeding modes of zoobenthic species were acquired from the literature (Kuznetsov 1984). This information was used to evaluate the contribution of specific trophic groups to the total biomass.
The biogeographic status of species was established as a combination of origin (Atlantic, Pacific, Arctic) and the area of species’ distribution within biogeographic zonation of oceans (boreal, Arctic, boreal-Arctic) (Golikov et al. 1990).
Calculations and statistics
The similarity of benthic fauna between stations was analyzed using a classification dendrogram based on the Czekanowski–Sørensen similarity index, averaged group clusterization procedure and multidimensional scaling (MDS) analysis. The one-way analysis of similarities test (ANOSIM) was used to estimate significant differences between the groups.
The similarity between stations in 2014 and in 2016 was checked by applying the Braun–Blanquet (1964) approach, excluding the species with frequency of occurrence less than 20% and more than 60%. This was performed to avoid possible bias in interannual comparison, because samples taken in September 2016 might contain new recruits of some species, which could shift relative proportions of these species in comparison to samples from July 2014.
Community diversity was calculated using the Shannon Index for species’ abundances:
where pi = ni/N, ni is the abundance of the ith species in a community (ind. m−2) and N is the total abundance of all species (ind. m−2).
The difference of evenness index (DE’; Denisenko 2006a, 2013; Denisenko et al. 2013) was applied to assess the status of macrozoobenthos populations based on calculations of both abundance and biomass:
where E′B is the evenness of species by biomass and E′A is the evenness of species by abundance in a sample (Pielou 1984). The DE’ index indicates the prevalence of macrozoobenthic species with r or K reproduction strategies. In fact, DE’ is a modification of the abundance–biomass curve method (ABC; Warwick 1986; Warwick et al. 1987). It allows estimation in a practical numerical form of the reproduction strategies in benthic assemblages. Previously, it was shown that evenness differences can be calculated based on a variety of indices that assess biodiversity (Denisenko 2006b).
Differences between the group means of the two sample sets (years 2014 and 2016) were assessed using a Student’s t test for unequal variances. Multiple linear regression was used to examine the relationships between DE’ values, the biomass of several key species and sediment parameters. For the regression analysis numeric codes were assigned to sediment types (Table 1). Calculations were performed using Paleontological Statistics version 3.17 (Hammer et al. 2001; https://folk.uio.no/ohammer/past) and Statistica 8.0 (StatSoft Inc., USA). A map of the study area was constructed using the Surfer 7 and MapViewer 7 programs (Colorado 1999).
All data are presented as mean value ± SE unless specified otherwise.
Results
General description of benthic fauna in the study area
A total of 238 species of macrozoobenthos were found in the study area. Among them, the most diverse taxonomic groups were polychaetes (34% of all species), molluscs (14%), crustaceans (10%) and bryozoans (17%). The remaining 25% comprised other taxonomic groups. Total abundance varied from 25 ± 3 to 2948 ± 738 ind. m−2 (Table 1, Fig. 3). Total biomass varied from 9.7 ± 2.4 to 381 ± 117 g wet weight m−2 (Table 1), with molluscs contributing the largest portion (55%) (Fig. 4). The second-largest group in the area was crustaceans, with a contribution of approximately 25% to the total biomass. The proportions of worms and other taxonomic groups in the total biomass were similar (12 and 10%, respectively). At 11 of 18 stations, molluscs dominated in biomass (Fig. 4). The most abundant were Mytilus edulis, with maximal biomasses of 86.1 and 68.2 g wet weight m−2 (Stations 10 and 20), Mya pseudoarenaria with a maximal biomass of 139.4 g wet weight m−2 (Station 15), Serripes groenlandicus with a maximal biomass of 98.3 g wet weight m−2 (Station 20) and Macoma calcarea with biomasses of more than 25 g wet weight m−2 at stations 6, 9 and 19 (26.8; 31.5; and 29.3 g wet weight m−2 respectively). Filter-feeders had the largest contribution (up to 60%) to total biomass of macrozoobenthos in the whole area and also predominated at most stations (Fig. 5). Among other trophic groups, the proportion of surface and sub-surface deposit feeders pooled together equalled ~ 15% of total biomass. Omnivorous species and predators formed quite a large proportion of the total biomass (20% and 15%, respectively).
The clustering and MDS analyzes showed the presence of six benthic assemblages in the study area (Fig. 6). Assemblage I (Station 2), with a predominance of Ophiura robusta (Online Resource Table 1), was found on hard bottoms in the northern part of the study area. Assemblage II, with a predominance of crab (Hyas coarctatus) and barnacle (Semibalanus balanoides) species, occupied three stations (Stations 3, 7 and 11) with mixed sand and gravel bottoms. It includes 48 species with an average biomass of 1.1 ± 0.5 g wet weight m−2, n = 3. A relatively high average biomass (10.1 g wet weight m−2) and moderate species richness (49 species) are the main features of assemblage III (Station 15), with a predominance of the bivalve mollusc Mya pseudoarenaria. Assemblage IV was found at two stations (10 and 20) located on fine sand mixed with gravel. It is characterized by a biomass of 5.4 ± 1.4, n = 2 and quite high species richness (69 species). The largest assemblage, V, occupied 11 stations (Stations 4–6, 9, 12–14 and 17–19) and occurred on fine and silty sands. It is characterized by a high species richness at 143 species but a low average biomass (1.4 ± 0.8, n = 11). Astarte borealis and M. calcarea predominated in this assemblage. Assemblage VI was found at Station 8, which was characterized by silty sand sediments. The dominant species were the same as in previous communities, but the additional large gastropod Neptunea communis was found, which caused the separation of station 8 from other stations (Online Resource Table 1).
The ANOSIM R statistic indicated that the communities were significantly different (R = 0.753; p = 0.001, n = 18). The Shannon's Diversity Index in the study area varied from 1.0 to 4.8 (Table 1). The DE’ index showed that the macrozoobenthos was in a state of equilibrium at most stations, but at stations 5, 8, 10 and 15 the DE’ values were close to zero, indicating that those communities were in an unstable state (Table 1).
Interannual comparison
The comparison of macrozoobenthos characteristics at the six stations that were observed in both years gives ambiguous results. The discrepancy was observed both in total species number and in the proportions of the main taxonomic groups. In 2016, the species list of macrozoobenthos at the compared stations was by one third longer than in 2014; 166 species vs 119 species. In both years, the most diverse taxonomic groups were polychaetes, molluscs, crustaceans and bryozoans. But the proportion of the two first groups increased in 2016 from 34 to 40% for polychaetes and from 14 to 16% for molluscs. Thus, the share of crustaceans and bryozoans was reduced from 14 to 7% and from 17 to 14%, respectively. Clusterization by similarity of species composition at the compared stations indicated the presence of two sub-clusters distinguished at the level of about 20% similarity. Notably, samples collected in different years were grouped into different clusters (Fig. 7A). Checking the data using the Braun–Blanquet approach demonstrated the same result (Fig. 7B). The ANOSIM R statistic also indicated that the assemblages were separated significantly in both cases (R = 0.805; p = 0.002, n = 12 and R = 0.605; p = 0.001, n = 12, respectively). The largest difference in species composition was observed in such taxonomic groups as polychaetes, hydroids and crustaceans.
An analysis of the biogeographic composition showed a slight decrease in the proportion of Arctic species and widespread boreal species, whereas the proportion of Atlantic boreo-Arctic species increased from 2.0 to 5.3% (Fig. 8). However, this was not statistically confirmed (Student’s t test, t15 = 1.597, p = 0.141).
The biomass at the six stations varied within similar ranges in both years: from 62.6 ± 29.9 to 381 ± 117 g wet weight m−2 in 2014 and from 14.5 ± 6.6 to 363 ± 132 g wet weight m−2 in 2016. That said, a rearrangement of biomass values between the stations took place (Table 1). At most stations, the biomass had decreased slightly in 2016 compared to 2014, but at station 10 the opposite was observed; the biomass increased by 2.5 times its former value (Table 1). Changes in biomass were also pronounced within the taxonomic groups. The biomass of polychaetes decreased by an order of magnitude at stations 6 and 10 and increased 2–5 times at stations 8, 15 and 19. The biomass of crustaceans decreased by several orders at stations 15 and 20. The biomass of molluscs decreased several times at all stations except station 10, where it increased fourfold. A Student’s t test, however, did not confirm significant changes in the total biomass at the compared stations or in the proportions of biomass of the main taxonomic groups (Table 2).
The biomass of dominant species, constituting 80% of the total macrozoobenthos biomass, was practically the same at the compared stations in both data sets, but there was a rearrangement among the species biomass in 2016 compared to 2014. In 2016, bivalve molluscs (Serripes groenlandicus), crabs (Hyas coarctatus) and barnacles (Balanus balanus) lost their dominant positions and yielded their place to the polychaete Cistenides hyperborea and the gastropod Buccinum undatum (Table 3). Although the variations in the average biomass of the dominant species were considerable, for most species they were not significant due to the small number of compared stations and the extremely uneven distribution of the species between the stations (Table 3).
The changes also affected the trophic composition of the macrozoobenthos. The proportion of filter feeders compared to other trophic groups in the study area had decreased slightly in 2016 in comparison to 2014. Omnivorous species and predators stayed in the second position in both years and did not show much change. In 2016, the proportion of deposit feeders sharply increased; this change in sub-surface deposit feeders was statistically significant (Student’s t test; Table 4).
The average value of the Shannon's Diversity Index increased in 2016 in comparison to 2014 (Student’s t10 = 2.227, p = 0.050). In 2016, all DE’ values were negative, indicating that benthic communities experienced no ecological stress. In general, the variation of the DE’ index was not large between the studied stations in different years (Table 2). In contrast to 2014, however, in 2016 positive values close to zero—reflecting a starting deviation from the equilibrium state towards ecologically stressed conditions—were not recorded at the compared stations (Table 1).
The relationship between DE’, sediment characteristics and the biomass of several mass benthic species is described best by the full factorial multiple linear model (Table 5). DE’ values depended on the bottom sediments’ composition (F = 5.817, p = 0.027), on the biomass of the mobile crab Hyas coarctatus (omnivorous feeder; p = 0.008) and on the biomass of bivalve mollusc Macoma calcarea (suspension/surface deposit feeder; p = 0.001; Table 5). The latter species is one of the most dominant species in the study area. At the same time, DE’ values did not depend on the biomass of other abundant molluscs important to the walrus diet (e.g. Serripes groenlandicus).
Discussion
The Pechora Sea is regarded as quite vulnerable (Shavykin and Ilyin 2010), but it is still characterized by low anthropogenic impact and has negligible background contamination, which was recorded only at a local level (Mitskevich and Telitsina 2002; Samokhina 2009) outside the location of our study. Therefore, we assume that the area is influenced only by natural stressors as was shown earlier (Skibinskiy et al. 2003).
We speculated that changes in water temperature and the presence of large benthic consumers—walruses, which increased in number from 500 in 2011 (Anufriev et al. 2017) to 2000 in 2017 (Bogomolova et al.2017)—could cause possible changes in the Pechora Sea’s macrozoobenthos. The average water temperature in the Barents Sea undergoes fluctuations in quasi-periodic cycles, with a positive general trend observed in the recent 40 years (Hovland et al. 2013). An observed increase of 2 °C in the average annual water temperature at the Kola Transect (33º30′E, crossing the Barents Sea to the North) in recent decades indicates a possible increase in water temperature in the Pechora Sea as well, as a high correlation (R > 0.75) has been shown between Kola Transect temperatures and those from other standard hydrological sections in the Barents Sea, including its southeastern part, the Pechora Sea (Adrov and Denisenko 1996; Denisenko 2013). The absolute amplitudes of multi-year variations in water temperature tend to increase from west to east (Adrov and Denisenko 1996; Denisenko 2013). According to observations in 2014, the maximum water temperatures in the study area were already three times higher at southern stations (Table 1) than the mean of the annual values registered in 1990 (Adrov and Denisenko 1996; Denisenko 2013). In 2016, the bottom temperature in the study area was four times higher than the mean long-term values (its maximum exceeded 12 °C; Table 1). It is known (Kaufman 1977; Golikov 1980) that a water temperature of 8–9 °C is necessary for boreal species to begin breeding. The successful reproduction and prolongation of the larval presence as the water temperature increased led to the spread of Atlantic boreal Arctic species from the western to the eastern regions of the Barents Sea, increasing the number of recruits in benthic populations. This could be a reason for the increase in proportion of Atlantic species in the samples from 2016, which were collected during a period of maximum warming of the bottom waters.
At the same time, the species richness of the macrozoobenthos in the study area—as well as Shannon's Diversity Index—remained similar to that observed in the beginning of 1990s (Denisenko et al. 2003). Likewise, the values of the DE' Index in 2014 were very much like those recorded in the area in 1958–1959 (Denisenko 2006b). Therefore, long-term comparison supports the absence of any strong impact in the area. On the other hand, our observations of the macrozoobenthos in 2016 demonstrated sharp changes in several benthic characteristics in comparison to previous data. The increase in the number of species in samples, the difference in species composition and the increase of Shannon's Diversity Index in 2016 could be explained by the difference in sampling time. The 2016 samples were collected almost two months later than those in 2014, in September, after the new recruits of benthic invertebrates would have settled. However, applying the Braun–Blanquet (1964) approach showed the same results as the application of common similarity indices. The species composition in 2016 and 2014 remained different. This fact indicates the beginning of a reorganization in benthic fauna. Although the changes in biogeographic composition were not significant, the penetration of species of Atlantic origin could be a possible second reason for the discrepancy in species richness at compared stations. The expansion of boreal and Atlantic boreo-Arctic species usually accompanies an increase in water temperature. An increase in the near-bottom water temperature has been observed in the Pechora Sea since 2004 (our estimation of the data by Knipovich Polar Research Institute of Marine Fisheries and Oceanography in Murmansk [PINRO, www.pinro.ru]; Semushin et al. this issue). This process was accompanied by the spread of Atlantic waters towards the east-north-east. The increasing water heat content (Loeng et al. 2005; Sando et al. 2010) favors the development of boreal and boreo-Arctic species of Atlantic origin and the oppression of Arctic species; this may be a reason for the observed shift of the biogeographic boundary (Antipova et al. 1989; Denisenko 1990; Josefson et al. 2013). Although the increase of Athlantic species was not statistically significant,but in proportion it was considerable. We suppose that the increase in Atlantic boreo-Arctic species is a result of the successful spawning and spreading of their juveniles owing to the increase in water temperature, which in turn promoted the successful replenishment of benthic populations with more numerous recruits.
The increase of species number in 2016—166 species versus 119 species found in 2014—at the compared stations explains the increase in Shannon's Diversity Index in 2016. In addition to the new records of the Atlantic species, considerable changes were observed in the trophic structure of the benthos and the composition of dominant species, not only in number but also in their biomass. The latter changes cannot be explained by the increase in water temperature alone.
The abrupt increase of biomass of deposit feeders, the decline of filter feeders and the reorganization of biomass among dominant species is most likely a result of strong disturbances in bottom sediments, which may be associated with two aspects of mammalian activity. The first is the direct consumption of benthic organisms by walruses, while the second is disturbance and re-sedimentation due to the plowing of bottom sediments by walrus tusks during foraging (Nelson and Johnson 1987). The influence of the re-deposition of sediment on zoobenthos is well known (Herman et al. 2001; Widdows et al. 2006; Denisenko 2010; Denisenko 2013; Denisenko et al. in press). In shallow areas, the process of sediment re-deposition is also enhanced by the intensive water mixing induced by currents and wind impact, which changes the sediment texture (Wu 1983; Herman et al. 2001; Dolotov et al. 2008). We can assume that after the excavation of forage species by walruses, the habitats are disturbed and most bottom organisms are injured. The traumatization of benthic animals is also supported by the presence of a large proportion of carnivores and omnivores in communities in the study area. The re-deposition of sediments is accompanied by an increase in concentration of inorganic suspended matter in the water column.
The disturbance of sediments by walruses is reinforced by wind impact, which intensifies water mixing at shallow depths (Wu 1983). Wind roses for 2014 and 2016 were constructed using the Gidromet database (URL: https://rp5.ru/Пoгoда_в_Bapaндee; date of last access 12–02-2018). In 2016, south-west winds blowing along the archipelago prevailed in the wind diagram (Fig. 9), which could induce changes in the sediment structure compared to 2014. The observed homothermy in the water column in 2016 may result from wind and wave action. The wind roses supported our assumption about the influence of wind on re-deposition of sediments. The amplification of wind effects can be related to the decrease in ice-coverage duration, which correlates with the increase in water temperature (Ozhigin et al. 2003). Besides capes and straits between islands, high turbidity accompanied by a high amount of inorganic matter (our observations, Usov et al. 2018) was also recorded at stations near the walrus haul-outs. Suspended inorganic bottom material and high turbidity have negative impacts on some filter feeders. This was likely the reason for the decreasing of biomass of the filter feeding barnacles Balanus balanus in 2016. This species inhabits coarse sediment habitats near the northern part of a walrus haul-out on Matveev Island, but these mammals avoid sites with rocky bottoms. Records of the littoral species of barnacle Semibalanus balanoides in sublittoral habitats could be a result of the transportation of stones by walruses from the sea coast to deeper areas. The observed changes in biomass of this dominant species indicate a combined influence of re-deposition of bottom sediments via plowing by walruses and wind action in 2016.
The multiple linear regression model indicated that DE’ inversely depended on both the degree of coarseness of sediments and biomass of the dominant molluscs. In 2016, changes were observed in both drivers. The decrease in mollusc biomass could be a result of their consumption by walruses. Bottom sediments could be influenced by the plowing of the bottom by walruses, after which re-deposition occurs; coarse particles are laid down before fine ones due to different deposition rates (Wood and Armitage 1997). Both changes provoke the enhancement of DE’ values, which became negative at all stations in 2016. The presence of slightly stressed benthic communities in the area in 2014 may have been caused by both the growing population of Atlantic walrus in the area and an increase in water temperature in the Barents Sea. At the same time, the absence of a statistically significant change in the macrozoobenthic biomass as well as the decline of DE’ values in 2016 indicate that the study area was stable and the system remained in a state of equilibrium.
Several factors affect macrozoobenthos communities around walrus haul-outs in the southeast Pechora Sea. We suggest that walruses play a role in regulating the abundance and structure of macrozoobenthos. At the same time, environmental conditions such as increasing water temperatures and the wind-induced mixing of water masses also contribute to the disturbance of the benthos. Nevertheless, bottom ecosystems in the study area have a high potential for recovery. The latter conclusion is supported by the elevated Shannon's Diversity Index and low Ecological Stress Index estimated at the study sites.
References
Adrov NM, Denisenko SG (1996) Oceanographic characteristics of the Pechora Sea. In: Matishov GG, Tarasov GA, Denisenko SG, Denisov VV, Galaktionov KV (eds) Biogeocenoses of glacial shelf of the western Arctic seas. Kola Science Centre RAS, Apatity, pp 166–179 (In Russian)
Anisimova NA, Jørgensen LL, Lyubin PA, Manushin IE (2010) Mapping and monitoring of benthos in the Barents Sea and Svalbard waters: Results from the joint Russian-Norwegian benthic programme 2006-2008. IMR-PINRO Joint Report Series 1-2010. ISSN 1502-8828. 114 pp
Antipova TV, Denisenko NV, Semenov VN (1989) Distribution of benthic species and issues of biogeographic regionalization of the Northern Seas. In: Matishov GG, Scarlato OA (eds) Life and environments of the Polar Seas. Nauka, Leningrad, pp 147–157 (In Russian)
Anufriev VV, Glotov AS, Zolotoy SA (2017) Monitoring of the Atlantic walrus (Odobenus rosmarus rosmarus) in the “Nenetskiy” State Nature Reserve. Proc of the Karelian Research Center RAS 4:15–25 (In Russian)
Bogomolova YuM, Mizin EA, Kochnev AA (2017) Matveev Island – the most important area of protection of the Atlantic walruses in the Pechora Sea. Wildlife of the Arctic: Biodiversity conservation and ecosystems state assessment. Book of Abstracts of the International conference. KMK Scientific Press, Moscow, pp 31–33 (In Russian)
Boltunov AE, Belikov SE, Gorbunov YA, Menis DT, Semenova VS (2010) The Atlantic walrus in the southeastern Barents Sea and adjacent areas: review of present-day status. WWF-Russia and Marine Mammal Council, Russia (In Russian and English)
Born EW, Rysgaard S, Ehlmé G, Sejr M, Acquarone M, Levermann N (2003) Underwater observations of foraging free-living Atlantic walruses (Odobenus rosmarus rosmarus) and estimates of their food consumption. Polar Biol 26:348–357
Braun-Blanquet J (1964) Pflanzensoziologie. Springer-Verlag, Wien and New York, Grundzüge der Vegetationskunde
Brotskaya VA, Zenkevich LA (1939) Quantitative estimation of the bottom fauna of the Barents Sea. Trans All Union Res Inst Mar Fish Oceanogr (VNIRO). Moscow 4:3–150 (In Russian)
Clarke KR (1990) Comparison of dominance curves. J Exp Mar Biol Ecol 138:143–157
Dahle S, Denisenko S, Denisenko N, Cochrane S (1998) Benthic fauna in the Pechora Sea. Sarsia 83:183–210
Denisenko NV (1990) Distribution and ecology of bryozoans of the Barents Sea. Kola Science Centre RAS, Apatity (In Russian)
Denisenko SG (2006a) Long-term fluctuations of macrozoobenthos in the Pechora Sea. Bull Geogr Sci 138(2):37–48 (In Russian)
Denisenko SG (2006b) Shannon’s information measure and its use for biodiversity assessment (as exemplified by marine macrozoobenthos). In: Sirenko BI, Vasilenko SV (eds) Marine invertebrates of Arctic, Antarctic, and subantarctic. Exploration of the fauna of the seas. ZIN RAS, St.–Petersburg 56(64):35– 46 (In Russian)
Denisenko NV (2010) The description and prediction of benthic biodiversity in high Arctic and freshwater-dominated marine areas: The southern Onega Bay (the White Sea). Mar Pollut Bull 61:224–233
Denisenko SG (2013) Biodiversity and bio-resources of macrozoobenthos in the Barents Sea. Nauka, St.–Petersburg (In Russian)
Denisenko SG, Denisenko NV, Lehtonen KK, Andersin A-B, Laine AO (2003) Macrozoobenthos of the Pechora Sea (SE Barents Sea): community structure and spatial distribution in relation to environmental conditions. Mar Ecol Prog Ser 258:109–123
Denisenko NV, Denisenko SG, Lehtonen KK (in press) Distribution of macrozoobenthos in an Arctic estuary (Pechora Bay, SE Barents Sea) during the spring flood period. Polar Biol. https://doi.org/10.1007/s00300-018-02452-6
Dolotov YuS, Filatov NN, Schevchenko VP, Petrov MP, Tolstikov AV, Zdorovennov RE, Platonov AV, Filippov AS, Bushuev KL, Kutcheva IP, Denisenko NV, Stain R, Saukel C (2008) Multidisciplinary studies in Onega Bay of the White Sea and the estuary of the Onega River during the summer period. Oceanology 48:255–267
Fisher KI, Steward REA (1997) Summer foods of Atlantic walrus, Odobenus rosmarus rosmarus, in northern Foxe Basin. Northwest Territories. Can J Zool 75(7):1166–1175
Gjertz I, Wiig O (1992) Feeding of walrus Odobenus rosmarus in Svalbard. Polar Res 28:57–59
Golikov AN (1980) Molluscs Buccinidae of the World Ocean. Key books of fauna of the USSR, Nauka, Leningrad (In Russian)
Golikov AN, Dolgolenko MA, Maximovich NV, Scarlato OA (1990) Theoretical approaches to marine biogeography. Mar Ecol Prog Ser 63:289–301
Gurevich VJ (1976) The studies of organic components in the bottom sediments of the Barents and White seas. In: Tokin IB (ed) Biology of the Barents and White Seas. Kola Branch of USSR Academy of Sciences, Apatity, pp 30–49 (In Russian)
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4(1): 9 pp
Haug T, Nilssen KT (1995) Observations of walrus (Odobenus rosmarus rosmarus) in the southeastern Barents and Pechora Seas in February 1993. Polar Res 14:83–86
Herman PMJ, Middelburg JJ, Heip CHR (2001) Benthic community structure and sediment processes on an intertidal flat: results from the ECOFLAT project. Continent Shelf Res 21:2055–2071
Hovland EK, Dierssen HM, Ferreira AS, Johnsen G (2013) Dynamics regulating major trends in Barents Sea temperatures and subsequent effect on remotely sensed particulate inorganic carbon. Mar Biol Prog Ser 484:17–32
Ilyin GV, Matishov GG (1992) Oceanographic conditions in the Pechora Sea in July. In: Matishov GG (ed) An international American-Norwegian-Russian ecological expedition in the Pechora Sea, Novaya Zemlya, Vaygach, Kolguyev and Dolgiy islands, July 1992 (RV ‘Dalnie Zelentzy’). MMBI report, Kola Science Centre RAS, Apatity, pp 7–11 (In Russian)
Josefson AB, Mokievsky V, Bergmann M, Blicher ME, Bluhm B, Cochrane S, Denisenko NV, Hasemann Ch, Jørgensen LL, Klages M, Schewe I, Sejr MK, Soltwedel Th, Wesławski JM, Włodarska-Kowalczuk M (2013) Marine invertebrates. In: Melforte M (ed) Arctic Biodiversity Assessment. Status and trends in Arctic biodiversity, CAFF, Akureyri, pp 277–309
Kaufman ZS (1977) Patterns of the sexual cycles of the White Sea invertebrates as adaptation to habitation in high latitudes environments. Nauka, Leningrad (In Russian)
Klenova MV (1960) Geology of the Barents Sea. Acad Sci USSR, Moscow, Leningrad (In Russian)
Krasnov YuV, Goriayev YuI, Shavykin AA, Nikolayeva NG, Gavrilo MV, Chernook BE (2002) Atlas of birds of the Pechora Sea: distribution, abundance, dynamics, problems of preservation. Kola Science Centre RAS, Apatity (In Russian)
Kuznetsov AP (1984) Ecology of the bottom communities of shelf zones of the World Ocean (Trophic structure of marine bottom fauna). Nauka, Moscow (In Russian)
Larsen T, Boltunov A, Denisenko N, Denisenko S, Gavrilo M, Mokievskiy V, Nagoda D, Spiridonov V (2004) The Barents Sea ecoregion. A biodiversity assessment, WWF, Oslo
Levermann N, Galatius A, Ehlme G, Rysgaard S, Born EW (2003) Feeding behavior of free-ranging walruses with notes on apparent dextrality of flipper use. BMC Ecol 3:9. https://doi.org/10.1186/1472-6785-3-9
Loeng H, Brander K, Carmack E, Denisenko S, Drinkwater K, Hansen B, Kovacs K, Livingston P, McLaughlin F, Sakshaug E (2005) Marine Systems. Arctic Climate Impact Assessment (ACIA). Cambridge University Press, Cambridge, pp 453–538
Loring DH, Naes K, Dahle S, Matishov GG, Ilyin G (1995) Arsenic, trace metals, and organic microcontamination in sediments from the Pechora Sea, Russia. Mar Geol 2:153–167
Lydersen C, Chernook VI, Glazov DM, Trukhanova IS, Kovacs KM (2012) Aerial survey of Atlantic walruses (Odobenus rosmarus rosmarus) in the Pechora Sea, August 2011. Polar Biol 35:1555–1562. https://doi.org/10.1007/s00300-012-1195-2
Menis TD (2003) Characteristics of marketable bivalve in the Pechora Sea. In: Anonimous (ed) Proceedings of the reporting session of the Northern Branch of PINRO after research work in 2001-2002. SevPINRO, Arkhangelsk, pp 131–139 (In Russian)
Milliman JD, Syvitski JPM (1992) Geomorphic/tectonic control of sediment discharge to the oceans: the importance of small mountainous rivers. J Geol 100:525–544
Mitskevich IV, Telitsina LA (2002) Assessment impact of bottom sediments pollutions byhydrocarbons on macrozoobenthos state in the White and the Pechora Seas. In: Anonimous (ed) Materials of fishery research in the basins of the European North. Scientific publications. SevPINRO, Arkhangelsk, pp 5–11(In Russian)
Nelson CH, Johnson KR (1987) Whales and Walruses as Tillers of the Sea Floor. Scien Americ 256:112–118
Ozhigin VK, Drobysheva SS, Ushakov NG, Yaragina NA, Titov OV, Karsakov AL (2003) Interannual variability in the physical environment, zooplankton, capelin (Mallotus villosus) and North-East Arctic cod (Gadus morhua) in the Barents Sea. ICES Mar Sci Symposia 219:283–293
Pielou ES (1984) The interpretation of ecological data. Wiley, New York
Samokhina LA (2009) Assessment of benzo(a)pyrene influence on quantitative characteristics of zoobenthos in the White Sea and the southeastern Barents Sea. Bull Pomor Univ 2:76–82 (In Russian)
Sando AB, Nilsen JEO, Lohmann Gao Y, K, (2010) Importance of heat transport and local air-sea heat fluxes for Barents Sea climate variability. J Geophysic Res 115:C07013. https://doi.org/10.1029/2009JC005884
Semenova V, Boltunov A, Nikiforov V (in press) Key habitats and movement patterns of Pechora Sea walruses studied by satellite telemetry. Polar Biol. https://doi.org/10.1007/s00300-018-02451-7
Semushin AV, Novoselov AP, Sherstkov VS, Levitsky AL, Novikova YV (2018) Long-term changes in the ichthyofauna of the Pechora Sea in response to ocean warming. Polar Biol. https://doi.org/10.1007/s00300-018-2405-3
Shavykin AA, Ilyin GV (2010) An assessment of the integral vulnerability of the Barents Sea from oil contamination. MMBI KSC RAS, Murmansk (In Russian)
Skibinskiy LE, Ivanov GI, Romankevich EA, Ponomarenko TV (2003) Ecological status of the Pechora Sea. In: Romankevich EA and Lisitzin AP, Vinogradov ME (eds) The Pechora Sea. System studies (hydrophysics, optics, biology, chemistry, geology, ecology, social and issues). More, Moscow, pp 365–392 (In Russian)
Sukhotin AA, Krasnov YV, Galaktionov KV (2008) Subtidal populations of the blue mussel Mytilus edulis as key determinants of waterfowl flocks in the southeastern Barents Sea. Polar Biol 31(11):1357–1363
Svetocheva ON, Semenova VS (2017) Feeding ecology of Atlantic walrus in the south-eastern part of the Barents Sea. In: Commercial species and their biology. Trans All Union Res Inst Mar Fish Oceanogr (VNIRO) 168:34–39 (In Russian)
Tarasov GA (1996) Peculiarities of distribution of main types of bottom sediments. In: Matishov GG, Tarasov GA, Denisenko SG, Denisov VV, Galaktionov KV (eds) Biogeocenoses of glacial shelf of the western Arctic seas. Kola Research Centre RAS, Apatity, pp 66–80 (In Russian)
Terziev FS, Zatuchnaya BM, Shevchenko AV, Vlasenko PV, Berezina ON (eds) (1992) Hydrochemical and oceanographic basics of forming of biological productivity. Gidrometeoizdat, St.–Petersburg (In Russian)
Usov N, Khaitov V, Smirnov V, Sukhotin A (2018) Spatial and temporal variation of hydrological characteristics and zooplankton influenced by freshwater runoff in Pechora Sea. Polar Biol. https://doi.org/10.1007/s00300-018-2407-1
Warwick RM (1986) A new method for detecting pollution effects on marine macrobenthos communities. Mar Biol 92:557–562
Warwick RM, Pearson TH, Ruswahyuni H (1987) Detection of pollution effects on marine macrobenthos: further evaluation of the species abundance biomass method. Mar Biol 95:193–200
Widdows J, Brinsley MD, Pope ND, Staff FJ, Bolam SG, Somerfield PJ (2006) Changes in biota and sediment erodability following the placement of fine dredged material on upper intertidal shores of estuaries. Mar Ecol Prog Ser 319:27–41
Wood PJ, Armitage PD (1997) Biological effects of fine sediment in the lotic environment. Environm Manag 21:203–217
Wu J (1983) Sea-Surface Drift Currents Inducted by Wind and Waves. J Physic Oceanogr 13:1441–1451
Acknowledgements
The authors are grateful to the captain and crew of the R/V Professor Vladimir Kuznetsov of the Zoological Institute RAS for their support in sampling procedure. Drs. V. Skvortsov and K. Bijagov are gratefully thanked for their help during sampling. The study was financed by the Ministry of Education and Science of the Russian Federation (N AAAA-A17-117030310207-3, AAAA-A17-117021300220-3), the “Influence of temperature oscillations and acidification of sea waters on bioresources and productivity of ecosystems of the Arctic seas” program of the Presidium of the RAS and the “Century-long changes in the bottom ecosystems of the Russian Arctic seas, the current state and forecast” grant of the Russian Foundation for Basic Research (# 18-05-60157).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
V. V. Petryashov—Deceased.
This article belongs to the special issue on the "Ecology of the Pechora Sea", coordinated by Alexey A. Sukhotin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Denisenko, S.G., Denisenko, N.V., Chaban, E.M. et al. The current status of the macrozoobenthos around the Atlantic walrus haul-outs in the Pechora Sea (SE Barents Sea). Polar Biol 42, 1703–1717 (2019). https://doi.org/10.1007/s00300-018-02455-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-02455-3