Abstract
Lipids play a crucial role in polar regions and are of particular importance in early life stages of Antarctic fish. This work presents the significance of lipids and fatty acids (FAs) in the early life stages of the icefish Chionodraco hamatus. Analysis of lipid classes (polar lipids, PL; cholesterol, Chol; and triacylglycerol, TAG) suggested different energy allocation strategies in preflexion (<25 mm) and postflexion larvae (>25 mm). Structural PL dominated lipid dynamics for preflexion larvae, indicating that small individuals allocate the majority of energy toward somatic growth. Conversely, postflexion larvae appear able to switch between growth (contribution of PL) and energy storage (contribution of TAG) strategies. The condition index ratio TAG/Chol varied from 0.2 to <2 with no differences between the two larval stages. Further, FA composition of the TAG and PL fractions suggests that both developmental stages share the same carnivorous diet and that C. hamatus relies on a few key prey items.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the pelagic ecosystem of the Southern Ocean, icefish (Channichthyidae, “white-blooded” fish) form a family within the perciform suborder Notothenioidei, the most predominant suborder of demersal Antarctic fishes (Kock 2005). Early life stages of most species of channichthyids have been described (Efremenko 1987; Kellermann and Schadwinkel 1991), and their distribution and relative abundance reviewed elsewhere (Loeb et al. 1993; La Mesa and Ashford 2008). At hatching, larvae of channichthyids are relatively large (13–17 mm), suggesting greater likelihood of survival compared to smaller larvae of other Antarctic fishes (Kock 2005). In addition, larval channichthyids exhibit relatively fast growth rates and feed primarily on young stages of euphausiids and larval nototheniids (Kellermann 1990; La Mesa et al. 2011). Although most channichthyids are demersal-benthic as adults, larvae and postlarvae are typically pelagic. The most common channichthyid within the pelagic communities of the Ross and Dumont d’Urville Sea is larval and juvenile Chionodraco sp. (North 1988; Granata et al. 2002). The genus Chionodraco includes C. hamatus, C. myersi and C. rastrospinosus, with C. hamatus and C. myersi previously identified from the Dumont d’Urville Sea (Koubbi et al. 2010). As the taxonomic identification of larval stages of Chionodraco sp. is still unclear (Kellermann 1990), most authors base larval identification on the co-occurrence of adults in the same area (Hubold 1990; Moteki and Ishimaru 2008). In the Dumont d’Urville Sea, adult C. hamatus is overwhelmingly more common than adult C. myersi (Koubbi et al. 2009), and some barcoded larvae have been identified as C. hamatus (Dettai, pers. comm.). Therefore, larvae of the genus Chionodraco are referred to as C. hamatus in this study.
The life history and biology for early life stages of C. hamatus remain poorly understood. This species has been reported to spawn during summer in the Ross Sea (Vacchi et al. 1996; La Mesa et al. 2003) and demonstrates nesting behavior (Ferrando et al. 2014). Hatching time is not known, although presumably occurs in summer/autumn because few reproductively active females have been observed in the coastal zone near the Dumont d’Urville station in January (Koubbi, pers. comm.).
In polar regions, lipids and their constituent FA play major roles in growth, movement, buoyancy and reproduction, and also represent the main energy reserves of many polar species (Sargent et al. 2002). Lipid class dynamics reflect the ability of fish larvae to withstand starvation events and can be used to determine a lipid-based condition index (Fraser 1989; Håkanson 1989; Giraldo et al. 2012). The lipid condition index is based on the principal assumption that larval condition in many marine organisms is dependent upon lipid energy reserves, which typically correspond to triacylglycerol (TAG) content. TAG content is dependent on larval size or body mass and must be standardized by cholesterol (Chol) content; Chol is a membrane lipid that is correlated with larval size but is independent of nutritional condition because it is not catabolized during starvation (Fraser 1989).
The relationship between the storage-class lipids (TAG) and the structural membrane lipids (polar lipids, PL) to total lipids (TL) also reflects the main metabolic strategy of larvae and the trade-off between energy allocation toward growth and/or lipid storage. Further, FA composition of the TAG fraction directly reflects FA composition of the diet, and differences in FA composition between different organisms or developmental stages therefore reflect dietary similarities/differences (Dalsgaard et al. 2003). In contrast, FA composition of the PL fraction is under genetic control (i.e., independent of diet) and can be used to determine ontogenetic changes within an organism (Giraldo et al. 2015a).
In this study, the lipid composition and dynamics of C. hamatus are analyzed to (1) determine lipid composition and the role of lipids in early life strategies of C. hamatus, and (2) provide an overview of the lipid class-specific FA composition of C. hamatus that can be used to determine trophic pathways in the East Antarctic pelagic ecosystem.
Materials and methods
Sampling
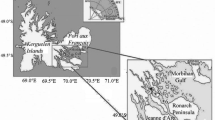
Field sampling was conducted in the Dumont d’Urville Sea (East Antarctica) during the austral summer 2010–2011 as part of the French IPEV-ICO2TA program (Integrated Coastal Ocean Observations in Terre Adélie) (Koubbi et al. 2011). Larval fish samples were collected from the RV “L’Astrolabe” using an Isaacs-Kidd midwater trawl (IKMT) at four stations along transects oriented from nearshore to across the continental shelf located from the Mertz Glacier Tongue to the Adelie Bank. All samples (n = 26) were immediately frozen in liquid nitrogen (−196 °C) and stored at −80 °C until further analysis. Back to our laboratory, samples were thawed on ice, weighted (degree of precision 0.01 mg) and measured to the nearest 0.1 mm with a digital caliper (standard length, SL) before analysis. Two larval developmental stages were differentiated based on flexion and corresponded to specimens smaller (preflexion, n = 16) and larger (postflexion, n = 10) than 25 mm. Preflexion larvae were defined as having a straight notochord with depleted yolk reserves. The second group corresponded to postflexion larvae and was characterized by an angled notochord with partially developed anal and dorsal fins. These two distinct larval stages were subsequently separated for further lipid analysis.
Lipid analysis
Lipid extraction followed the method of Bligh and Dyer (1959) as modified by Mayzaud et al. (2007). Samples were mechanically homogenized and twice-extracted with a one-phase solvent mixture of methanol/chloroform/water (2:1:0.8 v/v/v). Phases were separated overnight by addition of chloroform and NaCl 0.7 % (w/v) to a final solvent ratio of 2:2:1.8 methanol/chloroform/water (v/v/v). The total extract was vacuum-concentrated using a rotary evaporator and stored under nitrogen at −80 °C in the laboratory.
TL content was determined gravimetrically. Lipid classes were quantified using a chromatographic separation coupled with FID (Flame Ionization Detector) detection on an Iatroscan MK V TH 10. TL extracts were applied to SIII chromarods using a SAS A4100 autospotter programmed to deliver 1 µl of chloroform extract per rod. Analyses were performed in triplicate. Lipid classes were separated by chromatography using a double-development procedure with the following solvent systems: n-hexane/benzene/formic acid 80:20:1 (v/v/v) followed by n-hexane/diethyl ether/formic acid 97:3:1.5 (v/v/v). The FID was calibrated for each compound class using commercial standards. For FA analysis, lipid classes were isolated by thin-layer chromatography with the hexane/diethyl ether/acetic acid 170:30:2.5 (v/v/v) and the band of PL was then scraped off from the origin and eluted. Lipid classes were visualized using dichlorofluorescein, and identification was achieved by comparison with standard mixtures. Following identification, the TAG fraction was then scraped off and eluted. FAs from PL and TAG were subsequently converted into methyl esters with 7 % boron trifluoride in methanol (Morrison and Smith 1964). A control thin-layer chromatography was performed to verify the efficiency of the trans-methylation procedure.
Gas chromatography (GC) of all FA methyl esters (FAME) was carried out on a 30 m (length) × 0.32 mm (width) internal diameter quartz capillary column coated with Famewax (Restek) in a Perkin-Elmer XL Autolab GC equipped with FID. The column was operated isothermally at 185 °C for FAME. Helium was used as carrier gas at 7 psig. Injector and detector were maintained at 250 °C. Individual components were identified by comparing retention time data with those obtained from laboratory standards (capelin/menhaden oils 50:50). In addition, FAME samples were hydrogenated to confirm FA determination. The level of accuracy was ±3 % for major components, 1–9 % for intermediate components and up to ±25 % for minor components (<0.5 % of total FA). In order to have enough material for GC analysis, two to three preflexion larvae were pooled together according to their TAG/Chol ratio. Postflexion larvae were analyzed individually.
Statistics
Linear regression of log-transformed values was used to assess the relationship between TL, WW and SL. Contribution of main lipid classes (i.e., Chol, PL and TAG) to TL was assessed using linear regression on standardized values (µg lipid class per mg WW). Correspondence analysis (CA) (Benzécri et al. 1973) has been widely applied to analyze ecological data (Giraldo et al. 2015b) and was chosen here to describe the total inertia in the multi-dimensional FA data as a sample of fewer dimensions that best visualizes patterns in the data. The FA matrix was transformed to relative frequencies and scaled such that each row (or column) can be viewed as a row (or column) of conditional probability distribution. Distances between profiles were computed with \(X^{2}\) metrics. This distance gives symmetry to the two sets of data such that each factorial axis of the cloud of variables corresponds to a factorial axis of the cloud of observations. Thus, it was possible to represent both descriptors and observations on the plane defined by the factorial axes. The percent variance explained is given for each analysis. Symbol size is proportional to the cosine2, illustrating the quality of representation for each point. Computations were performed in the statistical software package R ver. 3.1.0 (R Core Team 2014). Data were transformed to normalize (Zar 1999).
Results
Size, weight and lipid components
The SL of C. hamatus (n = 26) ranged from 19.3 to 35.1 mm with WW from 31 to 249 mg. The regression between WW and SL was described by a log–log function (Eq. 1):
TL content varied from 1.1 to 2.1 % (of WW) and was positive correlated with WW and SL, as described by the following equations (Eqs. 2 and 3):
Chol and PL contents were used to indicate the mass of an individual. A relatively good linear fit (Fig. 1) shows that these lipid components are indeed indicative of larval weight (Eqs. 4 and 5):
PL were the major constituent in both larval stages (96.2 ± 1.7 and 94.1 ± 2.5 % for preflexion and postflexion, respectively) followed by Chol (1.96 ± 0.6 and 2.70 ± 1.1 %) and TAG (1.5 ± 0.2 and 3.0 ± 1.5 %). In some samples, free FA and diacylglycerols were identified but represented <1 % of TL. Wax esters were not present.
Lipid dynamics
The contribution of the main lipid classes (i.e., PL, Chol and TAG) to TL content was analyzed by standardizing lipids (µg) by WW (mg). In preflexion larvae, PL and Chol were the dominant constituent influencing changes in TL (linear regression F = 24,470 and 30.84, respectively; df = 14, p < 0.001), while TAG levels were low relative to other lipid fractions and did not contribute significantly to TL content (linear regression, F = 0.55, df = 14, p = 0.46) (Fig. 2). Similarly, PL dominated TL in postflexion larvae, but all lipid classes contributed significantly to changes in TL (linear regression F = 2039, 37.15, 34.6 for PL, Chol and TAG, respectively, df = 8, p < 0.001) (Fig. 3).
Condition index TAG/Chol
Although there was a slightly better fit between PL and WW than between Chol and WW, Chol was used to standardize TAG content because PL can be mobilized during starvation and might therefore be less stable. The TAG/Chol ratio varied between 0.18 and 1.74 in preflexion larvae and from 0.22 to 2.20 in postflexion larvae. No significant differences were detected for TAG/Chol ratios (Wilcoxon test, W = 45, p > 0.05) between the two larval stages.
Fatty acid signature
Polar lipids
Overall, saturated (~26 %) and polyunsaturated FA (PUFA, ~45 %) dominated the FA composition of the PL fraction of each larval stage (Table 1). For all developmental stages, saturated FAs were dominated by palmitic acid (16:0) and, to a lesser extent, by stearic acid (18:0). Monoenoic acids (~23 %) were dominated by oleic (18:1n-9), vaccenic (18:1n-7) and palmitoleic (16:1n-7), while PUFAs were dominated by EPA (20:5n-3) and DHA (22:6n-3). Correspondence analysis explained 52 % of the variance between the FA of preflexion and postflexion larvae. Individuals were distributed around the centroid of the analysis with no clear differences between the two stages (Fig. 4).
Triacylglycerols
Monoenoic (~28 %) and PUFA (~36 %) dominated the TAG fraction of both preflexion and postflexion larvae (Table 1). For all developmental stages, saturated FAs were dominated by palmitic acid (16:0), monoenoic acids were dominated by oleic (18:1n-9), palmitoleic (16:1n-7) and vaccenic acid (18:1n-7), and PUFAs were dominated by EPA (20:5n-3) and DHA (22:6n-3). Correspondence analysis explained 73.49 % of variance in FA signature of the TAG fraction of preflexion and postflexion larvae. However, all samples fell within the centroid of the analysis, indicating no marked differences in FA composition between the two larval stages.
Discussion
Larval development in many marine organisms is largely dependent upon energy reserves, which typically correspond with TAG content. Starvation and predation represent major causes of mortality in larval fishes, particularly once yolk reserves are depleted, and larvae must switch to deriving energy from exogenous sources (Leggett and Deblois 1994). Predation risk is lower in individuals with larger body size, and fish larvae are therefore confronted with a trade-off between allocating energy reserves to optimize growth, reduce predation risk and increase the opportunity to consume larger prey items, versus storing TAG to increase the likelihood of survival during starvation events. In this study, the dominance of polar structural lipids (PL) compared to TAG content in preflexion larvae (<25 mm) of C. hamatus indicates that small individuals primarily allocate energy reserves toward somatic growth. Conversely, the important contribution of both TAG and PL to TL dynamics of older individuals (>25 mm, postflexion larvae) suggests an ability to allocate energy toward both growth and lipid storage reserves because the benefits of allocating energy toward growth decrease with increase in body size. Overall, the results of this study highlight a marked ontogeny of energy allocation that likely decreases predation risk for small larvae and favors lipid storage in postlarval C. hamatus.
Ratios of TAG/Chol suggested no significant differences in condition factor of pre- and postflexion larvae, indicating that both stages were in good condition and that prey availability during the summer 2011 was sufficient to meet the basal requirements of C. hamatus individuals from this study.
PL are known to be strongly regulated and appear to be directly related to membrane requirements and specific energetic pathways (reviewed by Sargent et al. 2002; Dalsgaard et al. 2003). Although the lipid class dynamics of preflexion and postflexion C. hamatus larvae were different, neither of these developmental stages exhibited any detectable ontogeny in the FA pattern of PL and TAG fractions. High levels of DHA with a mean EPA/ DHA ratio of 0.6 ± 0.05 reflect the role of polyunsaturated n-3 FA in the maintenance of biological membranes in C. hamatus larvae. In contrast to PL, the FA composition of TAG is related to trophic interactions in marine organisms (Dalsgaard et al. 2003; Mayzaud et al. 2011). In this study, there were no significant differences between the pattern of FA trophic markers for pre- and postlarval C. hamatus, indicating similar diet across larval stages. Specifically, early life stages of C. hamatus were characterized by low contribution of Calanus-type copepod markers (∑C20:1, C22:1) and a relatively high value of the ratio C18:1n-9/C18:1n-7, a typical carnivory index in marine trophic ecology (Hagen et al. 2002). This finding is consistent with previous work demonstrating that young stages of euphausiids (furcilia to juveniles) and fish larvae constitute the most important food source for channichthyids larvae (Kock 2005).
Early life stages of the pelagic fish, Pleuragramma antarctica (Antarctic Silverfish), one of the most important key species of Antarctica (along with the krill Euphausia superba) (Corsolini et al. 2002), may be of particular importance in the diet of C. hamatus. Early life stages of C. hamatus have been previously reported as specialized predators in the Ross Sea, feeding on a relatively narrow niche width, with P. antarctica larvae as their overwhelmingly dominant prey (La Mesa et al. 2011). Compared to the carnivory index previously described for P. antarctica larvae (1.34 and 1.06) (Mayzaud et al. 2011; Tavernier et al. 2012), the relative high value (1.93) of the ratio C18:1n-9/C18:1n-7 reported for C. hamatus is in good agreement with the previous studies. Moreover, stable nitrogen signatures (δ15N), mainly used to establish trophic relationships, have been reported as 10.5 ‰ for C. hamatus larvae (SL = 31–38 mm) (Cherel et al. 2011) and 6.7 ‰ for larval P. antarctica (Giraldo et al. 2011). Considering that, on average, a ~3 ‰ enrichment in δ15N values accompanies each trophic step, these results also support the hypothesis that larval P. antarctica could be the main prey item for C. hamatus larvae. The hatching time of C. hamatus and P. antarctica is thought to coincide. Further, C. hamatus and P. antarctica co-occur spatially and have a similar geographic distribution in the western Ross Sea (La Mesa et al. 2010) and offshore of the Antarctic Peninsula (White and North 1987). Future studies could use FA as dietary biomarkers to test the hypothesis that survival of larval C. hamatus is closely linked to spatial and temporal dynamics of young stages of euphausiids and P. antarctica larvae.
References
Benzécri JP, Benzécri F, Birou A, Blumenthal S, De Bœck A (1973) L‘analyse de données, vol 2. L‘analyse des correspondances. Dunod, Paris
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37(8):911–917
Cherel Y, Koubbi P, Giraldo C et al (2011) Isotopic niches of fishes in coastal, neritic and oceanic waters off Adélie Land, Antarctica. Polar Sci 5:286–297
Corsolini S, Romeo T, Ademollo N, Greco S, Focardi S (2002) POPs in key species of marine Antarctic ecosystem. Microchem J 73(1):187–193
Dalsgaard J, St John M, Kattner G, Müller-Navarra D, Hagen W (2003) Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol 46:225–340
Efremenko VN (1987) Species composition and distribution of mesopelagic fish eggs and larvae in the Ross Sea. Biological and oceanographic investigations of the Pacific sector of Antarctica, pp 186–193
Ferrando S, Castellano L, Gallus L, Ghihliotti L, Masini MA, Pisano E, Vacchi M (2014) A Demonstration of nesting in two Antarctic Icefish (Genus Chionodraco) Using a fin dimorphism analysis and ex situ videos. PLoS One 9(3):e90512. doi:10.1371/journal.pone.0090512
Fraser AJ (1989) Triacylglycerol content as a condition index for fish, bivalve, and crustacean larvae. Can J Fish Aquat Sci 46:1868–1873
Giraldo C, Cherel Y, Vallet C, Mayzaud P, Tavernier E, Moteki M, Hosie G, Koubbi P (2011) Ontogenic changes in the feeding ecology of the early life stages of the Antarctic silverfish (Pleuragramma antarcticum) documented by stable isotopes and diet analysis in the Dumont d’Urville Sea (East Antarctica). Polar Sci 5(2):252–263
Giraldo C, Cherel Y, Vallet C et al (2012) Lipid components as a measure of nutritional condition in fish larvae (Pleuragramma antarcticum) in East Antarctica. Mar Biol 160:877–887
Giraldo C, Mayzaud P, Tavernier E, Boutoute M, Penot F, Koubbi P (2015a) Lipid dynamics and trophic patterns in Pleuragramma antarctica life stages. Antarct Sci 27(5):429–438
Giraldo C, Stasko A, Choy ES, Rosenberg B, Majewski A, Power M, Swanson H, Loseto L, Reist JD (2015b) Trophic variability of Arctic fishes in the Canadian Beaufort Sea: a fatty acids and stable isotopes approach. Polar Biol. doi:10.1007/s00300-015-1851-4
Granata A, Cubeta A, Guglielmo L et al (2002) Ichthyoplankton abundance and distribution in the Ross Sea during 1987–1996. Polar Biol 25:187–202
Hagen W, Kattner G, Friedrich C (2002) The lipid compositions of high-Antarctic notothenioid fish species with different life strategies. Polar Biol 23(11):785–791
Håkanson JL (1989) Analysis of lipid components for determining the condition of anchovy larvae, Engraulis mordax. Mar Biol 102:143–151
Hubold G (1990) Seasonal patterns of ichthyoplankton distribution and abundance in the southern Weddell Sea. In: Kerry KR, Hempel G (eds) Antarctic ecosystems. Springer, Berlin, pp 149–158
Kellermann A (1990) Catalogue of early life stages of Antarctic notothenioid fishes. Ber Polarforsch 67:45–136
Kellermann A, Schadwinkel S (1991) Winter aspects of the ichthyoplankton community in Antarctic Peninsula waters. Polar Biol 11:117–127
Kock K-H (2005) Antarctic icefishes (Channichthyidae): a unique family of fishes. A review, part I. Polar Biol 28:862–895
Koubbi P, Duhamel G, Hecq JH et al (2009) Ichthyoplankton in the neritic and coastal zone of Antarctica and Subantarctic islands: a review. J Mar Syst 78:547–556
Koubbi P, Ozouf-Costaz C, Goarant A et al (2010) Estimating the biodiversity of the East Antarctic shelf and oceanic zone for ecoregionalisation: example of the ichthyofauna of the CEAMARC (Collaborative East Antarctic Marine Census) CAML surveys. Polar Sci 4:115–133
Koubbi P, O’Brien C, Loots C, Giraldo C, Smith M, Tavernier E, Vacchi M, Vallet C, Chevallier J, Moteki M (2011) Spatial distribution and inter-annual variations in the size frequency distribution and abundances of Pleuragramma antarcticum larvae in the Dumont d’Urville Sea from 2004 to 2010. Polar Sci 5(2):225–238
La Mesa M, Ashford J (2008) Age and growth of ocellated icefish, Chionodraco rastrospinosus DeWitt and Hureau, 1979, from the South Shetland Islands. Polar Biol 31:1333–1342
La Mesa M, Caputo V, Rampa R, Vacchi M (2003) Macroscopic and histological analyses of gonads during the spawning season of Chionodraco hamatus (Pisces, Channichthyidae) off Terra Nova Bay, Ross Sea, Southern Ocean. Polar Biol 26:621–628
La Mesa M, Catalano B, Russo A, Greco S, Vacchi M, Azzali M (2010) Influence of environmental conditions on spatial distribution and abundance of early life stages of Antarctic silverfish, Pleuragramma antarcticum (Nototheniidae), in the Ross Sea. Antarct Sci 22:243–254
La Mesa M, Catalano B, Greco S (2011) Larval feeding of Chionodraco hamatus (Pisces, Channichthyidae) in the Ross Sea and its relation to environmental conditions. Polar Biol 34:127–137
Leggett WC, Deblois E (1994) Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages? Neth J Sea Res 32:119–134
Loeb VJ, Kellermann AK, Koubbi P, North AW, White MG (1993) Antarctic larval fish assemblages: a review. Bull Mar Sci 53:416–449
Mayzaud P, Boutoute M, Perissinotto R, Nichols P (2007) Polar and neutral lipid composition in the pelagic tunicate Pyrosoma atlanticum. Lipids 42(7):647–657
Mayzaud P, Chevallier J, Tavernier E, Moteki M, Koubbi P (2011) Lipid composition of the Antarctic fish Pleuragramma antarcticum. Influence of age class. Polar Sci 5:264–271
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 5(4):600–608
Moteki M, Ishimaru T (2008) Development of feeding and swimming functions in larvae of Chionodraco rastrospinosus (Channichthyidae). Cybium 32:247–251
North A (1988) Distribution of fish larvae at South Georgia: horizontal, vertical, and temporal distribution and early life history relevant to monitoring year-class strength and recruitment SCCAMLR. Sel Sci Pap 4:1987
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013
Sargent JR, Tocher DR, Bell JG (2002) The lipids. Fish Nutr 3:181–257
Tavernier E, Mayzaud P, Boutoute M, Vallet C, Koubbi P (2012) Lipid characterization of Pleuragramma antarcticum (Nothoteniidae) larvae off East Antarctica (139 E–145.10 E) during summer. Polar Biol 35(6):829–840
Vacchi M, Williams R, La Mesa M (1996) Reproduction in three species of fish from the Ross Sea and Mawson Sea. Antarct Sci 8:185–192
White MG, North AW (1987) Postlarval Notothenioidei and midwater fish collected during the SIBEX cruise by British Antarctic Survey, 1985. In: Proc Congr Europ Ichthyol, Stockholm, vol 1985, pp 405–411
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Englewood Clifs
Acknowledgments
This project is a contribution to the Zone Atelier Antarctique du CNRS. Field surveys were funded by the French Polar Institute (IPEV). The authors thank the anonymous reviewers for their valuable comments to improve the manuscript and Shannon MacPhee from Fisheries and Oceans Canada for proofreading and editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giraldo, C., Boutoute, M., Mayzaud, P. et al. Lipid dynamics in early life stages of the icefish Chionodraco hamatus in the Dumont d’Urville Sea (East Antarctica). Polar Biol 40, 313–320 (2017). https://doi.org/10.1007/s00300-016-1956-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-1956-4