Abstract
Climate-driven changes in the quality and availability of sea ice habitat (e.g., spatial extent, thickness, and duration of open water) are expected to affect Arctic species primarily through altered foraging opportunities. However, trophic interactions in Arctic marine systems are often poorly understood, especially in remote high-latitude regions. We used quantitative fatty acid signature analysis to examine the diets of 198 polar bears (Ursus maritimus) harvested between 2010 and 2012 in the subpopulations of Baffin Bay, Gulf of Boothia, and Lancaster Sound. The objective was to characterize diet composition and identify ecological factors supporting the high density of polar bears in these regions. Polar bears across the study area fed primarily on ringed seals (Pusa hispida, 41–56 %), although bearded seals (Erignathus barbatus, 11–24 %) and beluga whales (Delphinapterus leucas, 15–19 %) were also important prey. Harp seals (Pagophilus groenlandicus) were a major food source in Baffin Bay. Dietary diversity was greatest in Baffin Bay, perhaps because marine mammals were attracted to the nutrient-rich waters in and downstream from the North Water Polynya. Foraging patterns differed across age and sex classes of polar bear. In Baffin Bay, adult females had high levels of bearded seal in their diet, whereas adult males and subadults consumed high levels of harp seal. Seasonal variation in polar bear foraging was related to known migration patterns of marine mammals. Our results add to existing evidence that polar bears in these three separate subpopulations have a shared conservation status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sea ice is a fundamental characteristic of Arctic marine systems, serving as habitat and substrate for a wide range of species and biological processes. However, the Arctic region is experiencing rapid warming resulting in an overall reduction in sea ice extent and thickness (Screen and Simmonds 2010; Maslanik et al. 2011; Stroeve et al. 2011). The ecological consequences of ongoing and future sea ice declines are not well understood, and low species diversity in Arctic regions makes these ecosystems vulnerable to habitat-mediated changes in species assemblage (Chapin et al. 1997).
Apex predators can reveal broad-scale changes in the structure and functioning of ecosystems (Bowen 1997). As highly specialized apex predators, polar bears (Ursus maritimus) may be sensitive indicators of environmental change (Laidre et al. 2008; Peacock et al. 2011). Sea ice is the primary habitat for polar bears, which use it as a platform for hunting, traveling, and mating (Stirling and Derocher 1993; Amstrup 2003). Evidence to date suggests that polar bears across their circumpolar range feed primarily on ringed seals (Pusa hispida) and bearded seals (Erignathus barbatus; Stirling and Archibald 1977; Smith 1980; Thiemann et al. 2008a). Where available, polar bears will also feed on a variety of other species, including harp seals (Pagophilus groenlandicus; Derocher et al. 2002; Iverson et al. 2006; McKinney et al. 2013), beluga whales (Delphinapterus leucas; Lowry et al. 1987; Smith and Sjare 1990; Thiemann et al. 2008a), narwhals (Monodon monoceros; Smith and Sjare 1990), and walrus (Odobenus rosmarus; Kiliaan and Stirling 1978; Calvert and Stirling 1990; Thiemann et al. 2007).
Projected changes in sea ice conditions associated with global warming will unavoidably alter the accessibility of ice-associated prey and, in turn, the feeding ecology of polar bears. Satellite imagery has shown that sea ice extent and thickness has declined at a faster rate than initially modeled (Stroeve et al. 2007). Furthermore, projections indicate a continued accelerated decline until 2100 (Holland et al. 2006; Stroeve et al. 2007). Increased sea ice fragmentation has been observed in the southern portion of the polar bear’s range and has resulted in an overall decrease in optimal foraging habitat (Sahanatien and Derocher 2012). Thus, understanding current patterns of polar bear foraging and the link between prey and sea ice conditions is essential to understanding and predicting how Arctic marine ecosystem may be affected. Although several recent studies have examined the effects of sea ice changes on the ecology and behavior of polar bears in the southern and western parts of their North American range (e.g., Regehr et al. 2007; Molnár et al. 2011; Rode et al. 2014; Pilfold et al. 2015), the ecological consequences of ice cover changes in the High Arctic are largely unknown (Stirling and Derocher 2012). Initial increases in primary productivity have been hypothesized as first-year ice replaces thicker multi-year ice at high latitudes (specifically in the Canadian Archipelago–Gulf of Boothia and Lancaster Sound), but longer-term reductions in sea ice ultimately mean the loss of polar bear foraging habitat (Derocher et al. 2004; Hamilton et al. 2014). Baffin Bay is seasonally ice-free, and negative effects on polar bear body condition have been recorded as a result of declining summer sea ice and increasing periods of open water (Rode et al. 2012).
Fatty acid (FA) analysis has emerged as an important tool in understanding the feeding ecology of marine animals, including fish (Budge et al. 2002), sea birds (Iverson et al. 2007), pinnipeds (Beck et al. 2007; Meynier et al. 2010), cetaceans (Pomerleau et al. 2014), and polar bears (Iverson et al. 2006; Thiemann et al. 2008a; McKinney et al. 2013). Quantitative FA signature analysis (QFASA, Iverson et al. 2004) uses a statistical model to estimate the diet composition of individual predators by determining the combination of prey FA profiles (or “signatures”) that comes closest to matching the observed predator, after accounting for FA-specific patterns of metabolism. Because dietary fat is integrated into predator fat stores over weeks to months, the resulting diet estimates reflect patterns of feeding across ecologically relevant timescales (Iverson et al. 2004; Nordstrom et al. 2008).
Few studies have attempted to quantify the trophic relationships between polar bears and their prey in the Canadian High Arctic. Previous work by Thiemann et al. (2008a) found that polar bears in Baffin Bay, Gulf of Boothia, and Lancaster Sound had similar FA profiles that were distinct from bears elsewhere in the Canadian Arctic. However, previous dietary estimates based on QFASA were ambiguous likely because low sample sizes (e.g., zero prey from Gulf of Boothia) limited the ability of the QFASA model to reliably distinguish harp seals from other potential prey in these regions (Thiemann et al. 2008a).
The objective of this study was to characterize polar bear diet composition in these same three High Arctic subpopulations using an expanded and improved prey data set. Given that Baffin Bay, Gulf of Boothia, and Lancaster Sound contain some of the highest densities of polar bears in their circumpolar range (Obbard et al. 2010), we also sought to identify possible ecological and environmental correlates of polar bear foraging habits. Polar bears in the High Arctic have not yet experienced major declines in sea ice conditions comparable to those in more southerly regions. Moreover, predictable polynyas (areas of open water) in northern Baffin Bay and the shallow continental shelf of the Arctic Archipelago may serve as important habitat for migratory and resident marine mammals. We hypothesized that polar bears in the High Arctic would exhibit diverse and seasonally variable diets related to marine mammal migration routes and sea ice conditions. We also hypothesized that polar bear diets will be affected by sex and age as larger male polar bears have a greater ability to successfully capture larger prey (Derocher et al. 2005, 2010). The ability to exploit diverse marine mammal prey has been identified as a possible factor delaying the effects of sea ice loss (Rode et al. 2014). Thus, a better understanding of polar bear foraging ecology within these regions is essential to better assess current and future effects of climate change in the Arctic.
Materials and methods
Sample collection
Polar bears
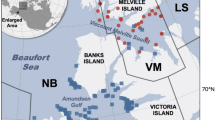
We analyzed adipose tissue samples from 198 individual polar bears in three subpopulations: Baffin Bay, Gulf of Boothia, and Lancaster Sound (Fig. 1). The samples were collected by local Inuit subsistence hunters between July 1, 2010, and June 30, 2012 (i.e., the 2010–2011 and 2011–2012 harvest seasons). Hunters assessed the age of bears, and samples included male and female adults (5+ years), subadults (3–4 years), and independent 2-year-olds (Table 1). Harvest management is based on a 2:1 male:female ratio, and it is illegal to hunt females with dependent cubs. Adipose tissue samples (ca. 6 cm × 3 cm) were taken through the full depth of the subcutaneous adipose layer over the rump. Samples were individually wrapped in aluminum foil, sealed in a Whirl–Pak, and stored at −20 °C until analysis.
Polar bear prey
A total of 390 blubber samples were collected from potential polar bear prey, including ringed seals (n = 206), bearded seals (n = 43), harp seals (n = 9), harbor seals (P. vitulina, n = 17), beluga whales (n = 57), narwhals (n = 37), and walrus (n = 21). Full-depth blubber samples were collected during Inuit subsistence hunts from nine polar bear subpopulations (Baffin Bay, Davis Strait, Foxe Basin, Gulf of Boothia, Lancaster Sound, Northern Beaufort Sea, Southern Beaufort Sea, Southern Hudson Bay, and Western Hudson Bay) in Nunavut, Canada, from 2004 to 2012. The collection of seal and whale samples included all sex and age classes. Samples were wrapped in aluminum foil, sealed in labeled Whirl–Pak bags, and stored at −20 °C until analysis. Data from Thiemann et al. (2008b) for 247 marine mammals [bearded seals (n = 31), beluga whales (n = 105), harp seals (n = 101), and narwhals (n = 10)] were also included, for a total prey library of 637 samples.
Laboratory analysis
Sample analysis
A subsample of approximately 0.5 g was taken from the center of larger polar bear adipose tissue pieces, and lipid quantitatively extracted according to Iverson et al. (2001). Prey samples were subsampled through the full depth of blubber, since studies have found significant vertical stratification in blubber of cetaceans and pinnipeds (Koopman et al. 1996; Best et al. 2003). Fatty acid methyl esters (FAME) were derived from isolated lipid extracts using sulfuric acid as a catalyst (Budge et al. 2006). FAME were analyzed in duplicate using temperature-programmed gas chromatography on a PerkinElmer Autosystem II capillary gas chromatograph (GC) with a flame ionization detector (FID), using a polar column (Agilent Technologies, DB-23; 30 m × 0.25 mm ID (Budge et al. 2006). FA data were reported as the mass% of total FA ± 1 SEM and expressed by the shorthand nomenclature of A:Bn-X, where A represents the length of the carbon chain, B represents the number of double bonds, and X indicates the position of the first double bond relative to the terminal methyl group.
QFASA modeling
We used quantitative FA signature analysis (QFASA; Iverson et al. 2004) to estimate the diet composition of individual polar bears. Briefly, the QFASA model estimates diet by comparing the predator FA profile, or “signature,” to the average FA signatures of its potential prey. To account for FA-specific patterns of metabolism in the predator, we used calibration coefficients developed by Thiemann et al. (2008a) from captive feeding studies on mink (Mustela vison); a terrestrial carnivore fed a marine-based diet. The combination of prey FA profiles that minimizes the statistical distance between the prey and the calibrated predator represents the relative contribution of each prey type to the predator’s diet, on a lipid biomass basis.
We used diet simulations based on the methods of Iverson et al. (2004) to test the ability of the QFASA model to distinguish between prey types (Appendix A). Polar bear diets were estimated using 30 dietary FAs derived solely or primarily from the diet (Iverson et al. 2004). The only difference between our modeling FA set and that of Thiemann et al. (2008a) was the exclusion of 20:1n-11, which appeared to contribute to the overlap among prey species as detected by principal component analysis (PCA). Diet simulations (Appendix A) revealed better separation of prey species when 20:1n-11 was excluded from the analysis. When available, prey species from the specific subpopulation were used to model predator diets of that same region. However, when necessary, samples collected from another region were used to increase the sample size (Appendix B). Regional differences in prey FA signature within a prey species were small compared to species-specific differences, and thus, prey species were pooled across geographic regions. As well, multiple diet simulations were conducted to determine the most accurate combination of prey species which would give the best resolution in the simulation. All diet simulations and QFASA estimates were performed in R (R version 2.1.0, The R Foundation for Statistical Computing, 2005).
Statistical analyses
We used PCA and multivariate analysis of variance (MANOVA) to test for geographic (three regions), sex, age class (adults and subadults), and seasonal differences in polar bear FA profiles. The PCA used a set of 38 FA (32 dietary FA and 6 extended dietary FA; sensu Iverson et al. 2004) transformed by calculating the log of the ratio of each FA to 18:0 (Budge et al. 2002, 2006). We also restricted the sample:variable ratio to 5:1 as suggested by Budge et al. (2008) and used MANOVA on PCA scores to compare FA across subpopulations.
We used randomization–permutation analyses (Good 2000; Anderson 2001a, b; Thiemann et al. 2008a) to test for geographic, sex, age class, and seasonal differences in polar bear diet composition. We tested sex and age class differences within each region; a two-way MANOVA was performed. A two-way MANOVA was also used to test spatial and seasonal variation while accounting for sex effects on polar bear diet composition. Seasons were defined as fall (September–November), winter (December–February), and spring (March–May) based on the date the bear was harvested. Summer (June–August) was excluded from all the seasonal analyses, due to a small sample size. A consequence of the integrative timeframe of QFASA (i.e., indicative of diet over the preceding weeks to months) is that point diet estimates may reflect feeding across two seasons. However, predator FA profiles respond rapidly to changes in diet (Nordstrom et al. 2008) and seasonal differences in diet estimates are reliable indicators of temporal change. All statistical comparisons were performed in R (R version 2.15.3, The R Foundation for Statistical Computing, 2013).
Results
Polar bear FA composition
PCA revealed no clear separation among polar bear subpopulations (Fig. 2). The FA with the highest loadings on PC1 were 16:3n-4, 16:4n-3, 16:4n-1, 18:4n-3, 18:4n-1, 20:5n-3, on PC2 were 20:1n-9, 22:1n-11, 22:1n-9, 22:1n-7, 22:4n-3, and on PC3 was 18:3n-6. A MANOVA carried out on PC scores showed a significant regional difference between the three polar bear subpopulations (MANOVA, Wilks’ λ = 0.73, F (6,384) = 10.81, p < 0.001). Only PC3 had a significant effect on the regional variation. Another PCA was performed on each subpopulation separately and MANOVA conducted on PC scores for effects of sex, age class, and season. Sex and age class had no significant effect on polar bear FA in any region (MANOVA, p > 0.06 in all cases). There was no significant seasonal effect on polar bear FA signatures in Gulf of Boothia (MANOVA, Wilks’ λ = 0.97, F (3,57) = 0.66, p = 0.579). However, season did have a significant effect on Baffin Bay (MANOVA, Wilks’ λ = 0.71, F (3,48) = 1.16, p < 0.001) and Lancaster Sound polar bear FA signature (MANOVA, Wilks’ λ = 0.88, F (3,73) = 3.34, p < 0.05).
Polar bear diet composition
Geographic differences in polar bear diet
There was a significant regional difference in polar bear diet composition between the three subpopulations (two-way permutation MANOVA, p < 0.001; Fig. 3). Ringed seal had the highest dietary contribution across the study area. However, polar bears in Gulf of Boothia consumed a higher proportion of ringed seal compared to Baffin Bay and Lancaster Sound (permutation ANOVA, p = 0.020). Ringed seal consumption was also the most frequent, being present in nearly all bears across the three regions (Baffin Bay 95 % of bears, Gulf of Boothia 100 % of bears, and Lancaster Sound 93 % of bears). Bearded seal was found in higher proportions and most frequent in the diets of bears in Gulf of Boothia and Lancaster Sound (permutation ANOVA, p < 0.001; 89 and 90 % of bears, respectively) compared to bears in Baffin Bay (63 % of bears). In contrast, harp seal consumption was highest (21 ± 2 %) and most frequent (84 % of bears) in diets of polar bears in Baffin Bay, but present as a minor component in Gulf of Boothia (6 ± 1 % of diet, 69 % of bears) and Lancaster Sound (10 ± 1 % of diet, 68 % of bears; permutation ANOVA, p < 0.001). Beluga whales had the third highest dietary contribution in all three subpopulations; however, there was no significant difference in consumption between the three regions (Baffin Bay 19 ± 4 %, Gulf of Boothia 15 ± 3 %, Lancaster Sound 15 ± 3 %; permutation ANOVA, p = 0.235). Beluga whale consumption was less frequent (Baffin Bay 55 % of bears, Gulf of Boothia 48 % of bears, and Lancaster Sound 43 % of bears), though present in high levels (>50 %) in some individuals (Baffin Bay 18 %, Gulf of Boothia 13 %, and Lancaster Sound 13 % of bears) with the majority of their diet consisting of beluga whale consumption.
Diet composition of polar bears (n = 198) in each of the three subpopulations (Baffin Bay, Gulf of Boothia, and Lancaster Sound). Prey contribution is represented as each species’ proportional contribution to polar bear diet. Data are shown as mean ± SE. Diet composition of Gulf of Boothia and Lancaster Sound polar bears were not modeled using harbor seals because the species is not available in these two regions
Sex and age class differences in diet
Sex and age class had no effect on polar bear diet composition in Gulf of Boothia (two-way permutation MANOVA, p = 0.833) or Lancaster Sound (two-way permutation MANOVA, p = 0.231). In Baffin Bay, there was a significant interaction between sex and age class in overall diet composition (two-way permutation MANOVA, p = 0.006; Fig. 4a). In Baffin Bay, bearded seal consumption was significantly higher in adult females than in adult males and younger age classes (permutation ANOVA, p < 0.001) and most adult females consumed bearded seal (90 % of bears). The relatively high mean levels of bearded seal seem to be influenced by 3 of 10 individuals consuming over 50 % bearded seal. There was a significant interaction between sex and age class for harp seal consumption, as adult females had significantly less harp seal in their diets than did adult males and subadults (permutation ANOVA, p = 0.028). Harp seal was most frequent in the diet of adult males (96 % of bears) and subadult females (100 %). Sex and age class did not significantly affect the consumption of ringed seal (permutation ANOVA, p = 0.217) or beluga (permutation ANOVA, p = 0.163) in Baffin Bay. However, beluga whales were found more often in the diet of adult males (77 % of bears) and subadult females (83 % of bears) than in adult females (10 % of bears) or subadult males (33 % of bears).
Seasonal differences in polar bear diet
Season had no significant effect on overall diet composition in Baffin Bay (two-way permutation MANOVA, p = 0.669) or Lancaster Sound (two-way permutation MANOVA, p = 0.056). In Gulf of Boothia, there were too few samples to compare fall diets, but winter and spring diets differed (two-way permutation MANOVA, p = 0.023; Fig. 4b). Ringed seals were consistently the primary prey in all three High Arctic regions throughout the year. In Gulf of Boothia, bearded seal consumption was higher in the winter than in the spring (permutation t test, p = 0.017). Beluga whale became the most abundant prey next to ringed seal in the spring but declined in the winter (permutation t test, p = 0.022). Beluga whale was also more frequent in the diet of polar bears in the spring (61 % of bears) compared to winter (25 % of bears). Walrus was a minor component in Gulf of Boothia diets during the spring (permutation t test, p = 0.006).
Discussion
This study quantified the diet composition of polar bears in Baffin Bay, Gulf of Boothia, and Lancaster Sound, where polar bears feed on diverse prey species, many of which make seasonal shifts in habitat use among the three regions. Our results help explain the relative similarities in FA profiles previously observed among bears in these three regions (Thiemann et al. 2008a, c) and suggest that distinctive and diverse foraging opportunities may support the apparently high density of polar bears in the Canadian High Arctic. Although sea ice conditions may not yet be strongly affected by climate change in Gulf of Boothia and Lancaster Sound, overall ice cover in Baffin Bay has declined by approximately 9 % per decade since 1979 (Perovich and Richter-Menge 2009). Seasonal variation in diet suggests a link between sea ice conditions and the availability of particular polar bear prey common throughout the three regions.
Geographic variation in polar bear FAs and diet
The similarity of polar bear FA profiles across the three subpopulations we studied (Fig. 2) is consistent with previous studies (Thiemann et al. 2008a) and is possibly a consequence of bears using a shared resource pool. Shared resource use may derive from similarities in available prey (e.g., prey migrating throughout the area) or from polar bear movement between adjacent areas (i.e., between Gulf of Boothia and Lancaster Sound, as well as Lancaster Sound and Baffin Bay) to common foraging areas. Genetic similarities of polar bears in the Canadian Archipelago (Peacock et al. 2015) are indicative of some degree of genetic interchange among the subpopulations studied here. Our results are also consistent with previous conclusions that polar bears in our study area have FA profiles distinct from bears in other parts of the Canadian Arctic (Thiemann et al. 2008a). The distinctiveness of these High Arctic polar bears may be a consequence of their diverse diets, which include comparatively high levels of both beluga whale and harp seal.
Dietary diversity was highest in Baffin Bay (Fig. 3), at least partly because of access to harbor seals along the nearshore shallow waters of Baffin Island (Mansfield 1967). Ringed seals are the primary prey of polar bears throughout their circumpolar range, including our study area, because of their ubiquitous distribution and high abundance (Stirling and Archibald 1977; Kingsley et al. 1985; Stirling and Øritsland 1995). Bearded seals are also a common prey species for polar bears (Smith 1980; Derocher et al. 2002; Iverson et al. 2006; Thiemann et al. 2008a), including those in this study (Fig. 3). Predation on other species may vary as a function of marine mammal migratory patterns that affect the seasonal availability of some prey. Thus, polar bears likely exploit locally abundant prey as seasonal conditions allow. Moreover, some individual bears may specialize in capturing larger prey species more frequently (Thiemann et al. 2011).
Beluga whales comprised a large portion of the diets of polar bears in this study. Belugas may be most accessible to polar bears during winter months because of possible sea ice entrapments while traveling through the sea ice (Lowry et al. 1987; Heide-Jørgensen et al. 2003). Other sources may include natural carrion, or hunting remains from the Inuit harvest.
Harp seals migrate northward as the sea ice retreats in summer, reaching Baffin Bay in early July and Lancaster Sound during the summer months (Finley et al. 1990; DFO 2011). As the sea ice begins to reform in late September, harp seals migrate back to southern areas (Finley et al. 1990). Polar bears prey on harp seals during the summer in Svalbard (Derocher et al. 2002) and an increase in harp seals and hooded seals in the diets of polar bears in East Greenland (McKinney et al. 2013). The harp seal population in eastern Canada has increased over the past four decades (DFO 2011) and represents an important prey species for polar bears, particularly in Davis Strait (Iverson et al. 2006). We found the highest level of harp seal consumption in Baffin Bay, where polar bears would have access to harp seals during the spring and fall movement and summer residency (Sergeant 1991). Harp seals were less abundant in the diets of bears in Lancaster Sound and Gulf of Boothia where they are less accessible to polar bears during the ice-free season.
Each of the three subpopulations studied here supports a high density of polar bears (Obbard et al. 2010). The North Water Polynya is the largest recurring polynya in the Canadian Arctic extending from northern Baffin Bay to eastern Lancaster Sound (Stirling 1997; Born et al. 2004; Heide-Jørgensen et al. 2013). A variety of marine mammals are attracted to the biologically productive open water (Stirling 1980; Laidre et al. 2008; Heide-Jørgensen et al. 2013). The similarity of polar bear diets in the High Arctic may be a consequence of bears from Baffin Bay and Lancaster Sound targeting this common feeding area. Moreover, the shallow continental shelf waters and small polynyas throughout Lancaster Sound and the Gulf of Boothia (Stirling 1980; Welch et al. 1992; Stirling 1997) may contribute to a higher diversity and availability of prey and ultimately higher densities of polar bears in these areas.
Sex and age variation in polar bear diet
Sex and age class had a significant influence on polar bear diets in Baffin Bay, although ringed seal consumption was consistently high in all sex and age classes (Fig. 4a). In past studies, adult male polar bears have often been found to have a higher level of bearded seal in their diet (Iverson et al. 2006; Thiemann et al. 2007, 2008a). The larger body size of adult male bears (Atkinson et al. 1996; Derocher et al. 2005, 2010) potentially allows them to capture larger prey, like bearded seals. Our results showed a reverse trend in Baffin Bay where adult females fed more on bearded seal than adult males. It may be that female bears are targeting younger, smaller bearded seals which may be spatially segregated from dominant adult seals (Young et al. 2010). Alternatively, adult male bears may be targeting harp seal and beluga whale, and thus, a smaller proportion of bearded seal is found in their diet.
In Gulf of Boothia and Lancaster Sound, there was no difference in diets between sexes or age classes of polar bears. The high level of large prey in the diets of adult males is consistent with studies from other regions (Iverson et al. 2006; Thiemann et al. 2007, 2008a), although larger prey species were also frequently present in the diet of adult females and subadults. Subordinate individuals in all three subpopulations may scavenge the remains of kills made by larger adult male bears, and previous studies have suggested that scavenging is an important opportunistic food source for less experienced bears (Stirling and McEwan 1975; Derocher et al. 2002), especially the Canadian Archipelago (Smith and Sjare 1990).
Seasonal variation in polar bear diet
Seasonal differences in ringed seal, bearded seal, and beluga whale consumption suggest that polar bears in our study area may capture prey species as they become temporally available. The shift in beluga whale consumption is the largest seasonal difference in the Gulf of Boothia and the increase in consumption likely proportionally reduces ringed seal and bearded seal consumption in early spring (i.e., March and April).
As sea ice retreats in summer, beluga whales use shore leads to migrate from the North Water Polynya through Lancaster Sound and south toward summer feeding grounds in the Gulf of Boothia (Smith and Martin 1994; Richard et al. 2001; Heide-Jørgensen et al. 2003). Among Baffin Bay and Lancaster Sound polar bears, beluga whale contribution was highest during winter and fall, suggesting that polar bear foraging is associated with beluga migration patterns and potentially, entrapment events (Stewart et al. 1995). Harvest seasons in some communities (such as Clyde River and Arctic Bay) occur in the fall, and thus, polar bears may have additional access to beluga whale carcasses to scavenge. In the Gulf of Boothia, polar bear predation on beluga whales increased in early spring which likely reflects the frequency of overwinter mortality.
Implications for conservation
Our results provide further support for the designatable units for conservation first suggested by Thiemann et al. (2008c). Currently, polar bears in Canada are considered a single unit in terms of conservation status but are distributed among 13 subpopulations for harvest management (COSEWIC 2008). Ecological factors such as primary productivity, prey distribution, and sea ice conditions vary spatially and temporally across the Canadian Arctic and the rate at which subpopulations will be affected by climate change is predicted to vary (Stirling and Derocher 2012). Thiemann et al. (2008c) recommended five distinct designatable units: Beaufort Sea, Central Arctic, High Arctic, Hudson Bay/Foxe Basin, and Davis Strait. Baffin Bay, Gulf of Boothia, and Lancaster Sound would comprise part of the Central Arctic Unit. The similarity of polar bear diets, the likely interchange of prey and possibly polar bears (Peacock et al. 2015), and the regional similarity of projected sea ice conditions (Hamilton et al. 2014) reinforce the hypothesis that bears in these three subpopulations share a common conservation status driven by regionally specific ecological factors.
This study identified the high diversity and seasonal variability of polar bear diets in the Canadian High Arctic. Recurring areas of open water and the associated abundance of marine mammals may contribute to the distinct diets and high density of polar bears in this region. Continued use of harvest-based sampling and improved understanding of polar bear movement patterns and habitat use, will clarify the ecological factors supporting these subpopulations and how they may be shaped by future environmental change.
References
Amstrup SC (2003) Polar bear, Ursus maritimus. In: Feldhamer GA, Thompson BC, Chapman JA (eds) Wild mammals of North America: biology, management, and conservation, 2nd edn. Johns Hopkins Unversity Press, Baltimore, pp 587–610
Anderson MJ (2001a) Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58:626–639
Anderson MJ (2001b) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Atkinson SN, Stirling I, Ramsay MA (1996) Growth in early life and relative body size among adult polar bears (Ursus maritimus). J Zool 239:225–234
Beck CA, Iverson SJ, Bowen WD, Blanchard W (2007) Sex differences in grey seal diet reflect seasonal variation in foraging behaviour and reproductive expenditure: evidence from quantitative fatty acid signature analysis. J Anim Ecol 76:490–502
Best NJ, Bradshaw CJA, Hindell MA, Nichols PD (2003) Vertical stratification of fatty acids in the blubber of southern elephant seals (Mirounga leonina): implications for diet analysis. Comp Biochem Physiol Part B 134:253–263
Born EW, Teilmann J, Acquarone M, Riget FF (2004) Habitat use of ringed seals (Phoca hispida) in the North Water Area (North Baffin Bay). Arctic 57:129–142
Bowen WD (1997) Role of marine mammals in aquatic ecosystems. Mar Ecol Prog Ser 158:267–274
Budge SM, Iverson SJ, Bowen WD, Ackman RG (2002) Among- and within-species variability in fatty acid signatures of marine fish and invertebrates on the Scotian Shelf, Georges Bank, and southern Gulf of St. Lawrence. Can J Fish Aquat Sci 59:886–898
Budge SM, Iverson SJ, Koopman HN (2006) Studying trophic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Mar Mamm Sci 22:759–801
Budge SM, Springer AM, Iverson SJ, Sheffield G, Rosa C (2008) Blubber fatty acid composition of bowhead whales, Balaena mysticetus: implications for diet assessment and ecosystem monitoring. J Exp Mar Bio Ecol 359:40–46
Calvert W, Stirling I (1990) Interactions between polar bears and overwintering walruses in the central Canadian High Arctic. Bears Their Biol Manag 8:351–356
Chapin FS, Walker BH, Hobbs RJ, Hooper DU, Lawton JH, Sala OE, Tilman D (1997) Biotic control over the functioning of ecosystems. Science 277:500–504
COSEWIC (2008) COSEWIC Assessment and update status report on the polar bear Ursus maritimus in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa
Derocher AE, Wiig Ø, Andersen M (2002) Diet composition of polar bears in Svalbard and the western Barents Sea. Mar Mamm Sci 25:448–452
Derocher AE, Lunn NJ, Stirling I (2004) Polar bears in a warming climate. Integr Comp Biol 44:163–176
Derocher AE, Andersen M, Wiig Ø (2005) Sexual dimorphism of polar bears. J Mamm 86:895–901
Derocher AE, Andersen M, Wiig Ø, Aars J (2010) Sexual dimorphism and the mating ecology of polar bears (Ursus maritimus) at Svalbard. Behav Ecol Sociobiol 64:939–946
DFO (2011) Current status of northwest Atlantic harp seals, (Pagophilus groenlandicus). DFO Can Sci Advis Sec Sci Advis Rep 2011/050
Finley KJ, Bradstreet MSW, Miller GW (1990) Summer feeding ecology of harp seals (Phoca groenlandica) in relation to Arctic cod (Boreogadus saida) in the Canadian High Arctic. Polar Biol 10:609–618
Good P (2000) Permutation tests: a practical guide to resampling methods for testing hypotheses, 2nd edn. Springer, New York
Hamilton SG, Castro de la Guardia L, Derocher AE, Sahanatien V, Tremblay B, Huard D (2014) Projected polar bear sea ice habitat in the Canadian Arctic Archipelago. PLoS One 9:e113746
Heide-Jørgensen MP, Richard P, Dietz R, Laidre KL, Orr J, Schmidt HC (2003) An estimate of the fraction of belugas (Delphinapterus leucas) in the Canadian High Arctic that winter in West Greenland. Polar Biol 26:318–326
Heide-Jørgensen MP, Burt LM, Hansen RG, Nielsen NH, Rasmussen M, Fossette S, Stern H (2013) The significance of the North Water Polynya to arctic top predators. Ambio 42:596–610
Holland MM, Bitz CM, Tremblay B (2006) Future abrupt reductions in the summer Arctic sea ice. Geophys Res Lett 33:1–5
Iverson SJ, Lang SL, Cooper MH (2001) Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 36:1283–1287
Iverson SJ, Field C, Bowen WD, Blanchard W (2004) Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecol Monogr 74:211–235
Iverson SJ, Stirling I, Lang SLC (2006) Spatial and temporal variation in the diets of polar bears across the Canadian Arctic: indicators of changes in prey populations and environment. In: Boyd IL, Wanless S, Camphuysen CJ (eds) Top predators in marine ecosystems. Cambridge, New York, pp 114–133
Iverson SJ, Springer AM, Kitaysky A (2007) Seabirds as indicators of food web structure and ecosystem variability: qualitative and quantitative diet analyses using fatty acids. Mar Ecol Prog Ser 352:235–244
Kiliaan HPL, Stirling I (1978) Observations on overwintering walruses in the eastern Canadian High Arctic. J Mamm 59:197–200
Kingsley MCS, Stirling I, Calvert W (1985) The distribution and abundance of seals in the Canadian High Arctic, 1980–82. Can J Fish Aquat Sci 42:1189–1210
Koopman HN, Iverson SJ, Gaskin DE (1996) Stratification and age-related differences in blubber fatty acids of the male harbour porpoise (Phocoena phocoena). J Comp Physiol B 165:628–639
Laidre KL, Stirling I, Lowry LF, Wiig Ø, Heide-Jørgensen MP, Ferguson SH (2008) Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol Appl 18:S97–S125
Lowry LF, Burns JJ, Nelson RR (1987) Polar bear, Ursus maritimus, predation on belugas, Delphinapterus leucas, in the Bering and Chukchi seas. Can Field-Nat 101:141–146
Mansfield AW (1967) Distribution of the harbor seal, Phoca vitulina Linnaeus, in Canadian Arctic waters. J Mamm 48:249–257
Maslanik J, Stroeve J, Fowler C, Emery W (2011) Distribution and trends in Arctic sea ice age through spring 2011. Geophys Res Lett 38:L13502
McKinney MA, Iverson SJ, Fisk AT, Sonne C, Rigét FF, Letcher RJ, Arts MT, Born EW, Rosing-Asvid A, Dietz R (2013) Global change effects on the long-term feeding ecology and contaminant exposures of East Greenland polar bears. Glob Change Biol 19:2360–2372
Meynier L, Morel PCH, Chilvers BL, Mackenzie DDS, Duignan PJ (2010) Quantitative fatty acid signature analysis on New Zealand sea lions: model sensitivity and diet estimates. Am Soc Mamm 91:1484–1495
Molnár PK, Derocher AE, Klanjscek T, Lewis MA (2011) Predicting climate change impacts on polar bear litter size. Nat Commun 2:186
Nordstrom C, Wilson L, Iverson SJ, Tollit D (2008) Evaluating quantitative fatty acid signature analysis (QFASA) using harbour seals Phoca vitulina richardsi in captive feeding studies. Mar Ecol Prog Ser 360:245–263
Obbard ME, Thiemann GW, Peacock E, DeBruyn TD (eds) (2010) Polar bears: proceedings of the 15th working meeting of the IUCN/SSC Polar Bear Specialist Group, 29 June–3 July 2009, Copenhagen, Denmark. Occassional Paper of the IUCN Species Survival Commission, No. 43. International Union for Conservation of Nature, Gland, Switzerland and Cambridge, UK
Peacock E, Derocher AE, Thiemann GW, Stirling I (2011) Conservation and management of Canada’s polar bears (Ursus maritimus) in a changing Arctic. Can J Zool 89:371–385
Peacock E, Sonsthagen SA, Obbard ME, Boltunov A, Regehr EV, Ovsyanikov N, Aars J, Atkinson SN, Sage GK, Hope AG, Zeyl E, Bachmann L, Ehrich D, Scribner KT, Amstrup A, Belikov S, Born EW, Derocher AE, Stirling I, Taylor MK, Wiig Ø, Paetkau D, Talbot SL (2015) Implications of the circumpolar genetic structure of polar bears for their conservation in a rapidly warming Arctic. PLoS One 10:e112021
Perovich DK, Richter-Menge JA (2009) Loss of sea ice in the Arctic. Ann Rev Mar Sci 1:417–441
Pilfold NW, Derocher AE, Stirling I, Richardson E (2015) Multi-temporal factors influence predation for polar bears in a changing climate. Oikos. doi:10.1111/oik.02000
Pomerleau C, Lesage V, Winkler G, Rosenberg B, Ferguson SH (2014) Contemporary diet of bowhead whales (Balaena mysticetus) from the eastern Canadian Arctic inferred from fatty acid biomarkers. Arctic 67:84–92
R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
R Development Core Team (2005). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://R-project.org
Regehr EV, Lunn NJ, Amstrup SC, Stirling I (2007) Effects of earlier sea ice breakup on survival and population size of polar bears in Western Hudson Bay. J Wildl Manage 71:2673–2683
Richard PR, Heide-Jørgensen MP, Orr JR, Dietz R, Smith TG (2001) Summer and autumn movements and habitat use by belugas in the Canadian High Arctic and adjacent areas. Arctic 54:207–222
Rode KD, Peacock E, Taylor M, Stirling I, Born EW, Laidre KL, Wiig Ø (2012) A tale of two polar bear populations: ice habitat, harvest, and body condition. Popul Ecol 54:3–18
Rode KD, Regehr EV, Douglas DC, Durner G, Derocher AE, Thiemann GW, Budge SM (2014) Variation in the response of an Arctic top predator experiencing habitat loss: feeding and reproductive ecology of two polar bear populations. Glob Change Biol 20:76–88
Sahanatien V, Derocher AE (2012) Monitoring sea ice habitat fragmentation for polar bear conservation. Anim Conserv 15:397–406
Screen JA, Simmonds I (2010) The central role of diminishing sea ice in recent Arctic temperature amplification. Nature 464:1334–1337
Sergeant DE (1991) Harp seals, man and ice. Can Spec Publ Fish Aquat Sci 114:153
Smith TG (1980) Polar bear predation of ringed and bearded seals in the land-fast sea ice habitat. Can J Zool 58:2201–2209
Smith TG, Martin AR (1994) Distribution and movements of belugas, Delphinapterus leucas, in the Canadian High Arctic. Can J Fish Aquat Sci 51:1653–1663
Smith TG, Sjare B (1990) Predation of belugas and narwhals by polar bears in nearshore areas of the Canadian High Arctic. Arctic 43:99–102
Stewart DB, Akeeagok A, Amarualik R, Panipakutsuk S, Taqtu A (1995) Local knowledge of beluga and narwhal from four communities in Arctic Canada. Can Tech Rep Fish Aquat Sci 2065
Stirling I (1980) The biological importance of polynyas in the Canadian Arctic. Arctic 33:303–315
Stirling I (1997) The importance of polynyas, ice edges, and leads to marine mammals and birds. J Mar Syst 10:9–21
Stirling I, Archibald WR (1977) Aspects of predation of seals by polar bears. J Fish Res Board Can 34:1126–1129
Stirling I, Derocher AE (1993) Possible impacts of climatic warming on polar bears. Arctic 46:240–245
Stirling I, Derocher AE (2012) Effects of climate warming on polar bears: a review of the evidence. Glob Change Biol 18:2694–2706
Stirling I, McEwan EH (1975) The caloric value of whole ringed seals (Phoca hispida) in relation to polar bear (Ursus maritimus) ecology and hunting behavior. Can J Zool 53:1021–1027
Stirling I, Øritsland NA (1995) Relationships between estimated of ringed seal (Phoca hispida) and polar bear (Ursus maritimus) populations in the Canadian Arctic. Arctic 2612:2594–2612
Stroeve J, Holland MM, Meier W, Scambos T, Serreze M (2007) Arctic sea ice decline: faster than forecast. Geophys Res Lett 34:1–5
Stroeve JC, Maslanik J, Serreze MC, Rigor I, Meier W, Fowler C (2011) Sea ice response to an extreme negative phase of the Arctic Oscillation during winter 2009/2010. Geophys Res Lett 38:L02502
Thiemann GW, Budge SM, Iverson SJ, Stirling I (2007) Unusual fatty acid biomarkers reveal age- and sex-specific foraging in polar bears (Ursus maritimus). Can J Zool 85:505–517
Thiemann GW, Iverson SJ, Stirling I (2008a) Polar bear diets and Arctic marine food webs: insights from fatty acid analysis. Ecol Monogr 78:591–613
Thiemann GW, Iverson SJ, Stirling I (2008b) Variation in blubber fatty acid composition among marine mammals in the Canadian Arctic. Mar Mamm Sci 24:91–111
Thiemann GW, Derocher AE, Stirling I (2008c) Polar bear Ursus maritimus conservation in Canada: an ecological basis for identifying designatable units. Oryx 42:504–515
Thiemann GW, Iverson SJ, Stirling I, Obbard ME (2011) Individual patterns of prey selection and dietary specialization in an Arctic marine carnivore. Oikos 120:1469–1478
Welch HE, Bergmann MA, Siferd TD, Martin KA, Curtis MF, Crawford RE, Conover RJ, Hop H (1992) Flow through the marine of the energy ecosystem Lancaster Sound region, Arctic Canada. Arctic 45:343–357
Young BG, Loseto LL, Ferguson SH (2010) Diet differences among age classes of Arctic seals: evidence from stable isotope and mercury biomarkers. Polar Biol 33:153–162
Acknowledgments
We are especially grateful to the Hunters and Trappers Associations and Organizations of Nunavut for collecting fat samples from polar bears and marine mammals harvested during their subsistence hunts. A. Coxon and P. Frame (Government of Nunavut–Department of Environment) helped coordinate the collection, organization, and shipment of polar bear samples. Thanks to B. Dunn, B. Young (Fisheries and Oceans Canada), D. Muir, and X. Wang (Environment Canada) for providing additional marine mammal seal samples. S. Budge and C. Barry (Dalhousie University) conducted the gas chromatography. I. Stirling and A. Derocher provided helpful comments on an earlier version of the manuscript. This project was funded by the Natural Sciences and Engineering Research Council (NSERC, Canada), Environment Canada (Grants and Contributions), Kenneth M. Molson Foundation, Nunavut General Monitoring Plan, Northern Scientific Training Program, and York University, Faculty of Graduate Studies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Galicia, M.P., Thiemann, G.W., Dyck, M.G. et al. Characterization of polar bear (Ursus maritimus) diets in the Canadian High Arctic. Polar Biol 38, 1983–1992 (2015). https://doi.org/10.1007/s00300-015-1757-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1757-1