Abstract
Tardigrades are found in most terrestrial and freshwater Antarctic ecosystems and are one of the most diverse and important groups of invertebrates in Antarctica. We developed a new laboratory system for rearing the Antarctic tardigrade Acutuncus antarcticus (Richters 1904), one of the most widespread and common Antarctic tardigrade species. To provide a description of the life history of this tardigrade, survival and reproduction of 68 individuals were observed and recorded daily at a constant temperature of 15 °C. The life-history data obtained are consistent with previous studies of other tardigrades. The exceptionally high hatching success obtained is suggested to be an important life-history characteristic of this species contributing to it often being a common and dominant species in the Antarctic habitats in which it occurs. Furthermore, high hatching success combined with very low variation in development time, under the protocol used in the current study, indicates that A. antarcticus may be a good model species for studies in developmental biology. Integrating data from this and previous studies, the importance of temperature on reproduction and growth in A. antarcticus was inferred. With terrestrial and freshwater ecosystems in some parts of Antarctica experiencing sometimes drastic contemporary climatic and environmental changes, studies of the effect of temperature on generation time and reproductive success in Antarctic tardigrades are urgently required, as these animals are important elements of community structure and function in polar ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctic terrestrial environments are amongst the most extreme and variable on Earth in terms of temperature, water availability and seasonality (Smith 1988; Peck et al. 2006). The harsh environments of Antarctica constrain its terrestrial ecosystems to be relatively simple, comprising a limited flora of bryophytes, lichens, algae and cyanobacteria, and an invertebrate fauna of micro-arthropods, nematodes, tardigrades, rotifers and protozoans (Convey et al. 2014). Terrestrial organisms living in these environments are known to demonstrate a variety of physiological and life-history traits that enable them to survive (e.g. Block et al. 2009). Nevertheless, knowledge is currently limited mainly to the micro-arthropod groups of mites and springtails, with some information also available on nematodes (Convey 1997; Hogg et al. 2014).
The limno-terrestrial microfauna of nematodes, tardigrades and rotifers are amongst the most diverse and dominant groups of invertebrates in Antarctica (Dastych 1984; Andrássy 1998; Convey and McInnes 2005; Adams et al. 2006; Maslen and Convey 2006; Sohlenius and Boström 2008; Velasco-Castrillón et al. 2014a). They play essential roles as consumers and decomposers in biological cycles in soil ecosystem processes (Wall 2005). Tardigrades are found in most terrestrial and freshwater Antarctic ecosystems, including some remote nunatak regions where even the otherwise ubiquitous nematodes are absent (Convey and McInnes 2005). In continental lakes and pools, in particular, tardigrades are one of the major community components (Gibson et al. 2007; Tsujimoto et al. 2014).
Amongst more than 1000 species of tardigrades recorded worldwide (Degma et al. 2014), 59 species have been recorded to date in Antarctica (Guidetti et al. 2014; Tsujimoto et al. 2014; Velasco-Castrillón et al. 2014b). Acutuncus antarcticus (Richters 1904) is known to be one of the most widespread Antarctic tardigrade species (Velasco-Castrillón et al. 2014b), being often the most common and dominant species in both terrestrial and lake environments (McInnes 1995; Gibson et al. 2007; Tsujimoto et al. 2014). Dougherty (1964) obtained a culture of Hypsibius arcticus (Murray 1907), which is now recognized as A. antarcticus (see Dastych 1991; Pilato and Binda 1997), from dried freshwater algal felt collected in the Cape Crozier region of Ross Island in continental Antarctica, and investigated the reproductive traits of five individuals and their offspring. While his data provided valuable information on reproduction in this species, the study was limited by the few individuals available and the relatively unstable temperature, nutrient and medium conditions under which the experiment took place. A recent culture study of this species has provided molecular profiles and morphological characteristics (Kagoshima et al. 2013), but detailed information on the species’ life history remains unavailable.
In order to provide a description of the life history of this Antarctic tardigrade, we developed a new laboratory rearing system. Using this system, survival and reproduction of 68 individuals of A. antarcticus were observed and recorded daily at a constant temperature of 15 °C. Life-history characteristics of Antarctic species are compared with those of other species of tardigrade reported in the available literature, including those of Dougherty (1964).
Materials and methods
Acutuncus antarcticus was collected by MT from Hamagiku-Ike Lake (formerly known as Abi-Ike Lake) in Skarvsnes (69°28′S 39°39′E), on the Sôya Coast of East Antarctica (Fig. 1), in January 2008, during the 49th Japanese Antarctic Research Expedition. Benthic samples were collected from the lake using a glove sampler (Ekman-Birge type, RIGO). The samples were placed into 2.5-ml tubes, stored at −70 °C and returned frozen to Japan. In January 2013, the frozen samples were wrapped with four Kimtowels (Nippon Paper Crecia Co., Ltd.) and thawed at 3 °C for 24 h. Thawed samples were placed into Petri dishes, and water was added. The benthic samples were sorted with tweezers in the dish, and then living individuals were retrieved using a pipette under a dissection microscope. Sub-samples were mounted on slides in Faure’s solution, and animals were identified under a phase-contrast microscope (Olympus BX53, 1000x).
Acutuncus antarcticus (Fig. 2) were reared under laboratory conditions established by modifying the method described in a study of Ramazzottius varieornatus (Horikawa et al. 2008). Animals were cultured in plastic plates 3.5 cm in diameter containing a thin layer of solid 1.5 % Bacto™ agar (Difco Laboratories Sparks, MD, USA) with 1.0 ml of Volvic® water and 2.0 μl of a suspension of the green alga Chlorella sp. (Raw Chlorella V-12, Chlorella Industry Co., Ltd., Tokyo, Japan). Dishes were covered with lids, placed in an incubator and cultivated at 3 °C in the dark (in order to prevent any overgrowth of the algae) for the first 4 months. Since the rate of population increase of A. antarcticus was quite slow at 3 °C, the dishes were cultivated at 10 °C for another 4 months in order to obtain a sufficient number of individuals prior to the present study. Animals were transferred to new culture dishes every 3 weeks.
Initially, we cultivated trial cohorts of 20 adults at constant temperatures of 10, 15, 20, 25 °C for 17 days in order to select an appropriate temperature for subsequent experimental observations. A rearing temperature of 5 °C was not tested since the preliminary culture work indicated that the reproductive rate was low, and additionally, higher reproductive performance at 10 than 4 °C has previously been reported in this species (Kagoshima et al. 2013). The numbers of eggs deposited over 17 days were 71, 168, 71, 0, respectively, at 10, 15, 20, 25 °C (all the individuals reared at 25 °C died within the study period). Based on this initial trial, a temperature of 15 °C was selected for the experiments described below.
Eggs deposited in the stock culture dishes cultivated at 10 °C were randomly selected independent of the oviposition date, transferred to new culture dishes and incubated at 15 °C. Sixty-eight juveniles hatched within a 24-h period (Fig. 3) were transferred to individual wells on culture plates (TPP® tissue culture plates, 12 wells, flat bottom with a layer of 300 μl of 1.5 % agar gel on the bottom, and 600 μl of Volvic® water and 1.8 μl of a suspension of Chlorella sp. added to each well), and reared at 15 °C in the dark. Individual tardigrades were inspected daily, and their survival and egg production were monitored. Animals were transferred to new culture dishes every week. This tardigrade typically develops several eggs within its ovaries before an oviposition event. Eggs from each clutch were isolated on the day of oviposition and transferred to individual wells on new culture plates. Subsequent egg hatching was monitored daily under a dissection microscope until 30 days after oviposition. Data on lifespan, timing and clutch size of each oviposition event, and egg maturation and hatching were recorded. The transparent body of A. antarcticus made it very difficult to identify exuviae inside the green algal medium in the narrow wells of the culture plates, and therefore no attempt was made to record the cycle and timing of moulting.
Results
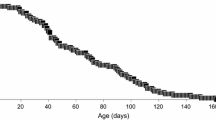
Under the rearing environment at 15 °C, A. antarcticus individuals survived for an average of 69.2 days (Table 1). Sixty-six of the 68 individuals had at least one oviposition event. The first oviposition occurred predominantly at the age of 9–10 days. The average number of oviposition events per individual was 7.5. In total, 508 clutches were recorded with clutch size varying from 1 to 10 eggs and oviposition interval ranging from 3 to 25 days. Of the 2342 eggs collected, 2286 (97.6 %) hatched. Hatching time showed little variability, with 50.6 % hatching in 8 days and an additional 39.7 % in 9 days.
There were a number of instances of individuals developing eggs but either re-absorbing them or the eggs not continuing to full maturation and oviposition. Two such individuals never successfully oviposited before dying, while a third successfully deposited two batches before failing to complete subsequent oviposition. During the process of normal egg formation, eggs develop in the posterior of the ovary and, as they become larger, they become packed in line in the ovary in the posterior part of the body prior to oviposition. Those individuals showing failure of oviposition tended to have the immature eggs located in other parts of the ovary in a disorganized fashion, suggesting a possible reproductive anomaly. Apparently well-developed eggs remaining within the bodies of three dead adults did not hatch, although eggs that had been deposited on the day before four individuals died hatched successfully.
Discussion
Studies of the life-history traits of tardigrades have been limited until very recently (Schill 2013). Using daily observation of growth and reproduction of individual tardigrades, Suzuki (2003) successfully revealed the detailed life history of Milnesium sp., and the life-history traits of a small number of temperate and tropical species in culture have been described in the last decade (Altiero et al. 2006; Horikawa et al. 2008; Lemloh et al. 2011; Schill 2013). The life-history data described here for A. antarcticus in culture at 15 °C under stable conditions fall within the ranges reported in previous studies of eight species (Table 1), notwithstanding the differences in food type and temperature conditions applied in each study. The hatching success of A. antarcticus eggs obtained in the current study was exceptionally high compared to most previous studies. High hatching success could be an important life-history characteristic contributing to this species being one of the most widespread and often common and dominant species within its Antarctic habitats of occurrence, where there is a general requirement to reproduce in a very limited period of time during the summer (Convey 1997). Furthermore, egg development duration was relatively constant, with more than 90 % of the eggs hatching at 8–9 days after oviposition. The very high hatching success combined with the stable development time under the protocol used here indicates that A. antarcticus may be a good model species for studies in developmental biology, as with Hypsibius dujardini (Gabriel et al. 2007) and Milnesium tardigradum (Schill and Fritz 2008).
In comparison with Dougherty’s (1964) study, in which cultures were maintained at the lower temperature range of 4–7 °C, the first oviposition was earlier and development was faster in the current study. Dougherty (1964) reported a range of 2–13 eggs per clutch with a minimum oviposition interval of 8 days, in comparison with 1–10 eggs and a minimum interval of 3 days in the present study. Hohberg (2006) noted the significant influence of temperature on clutch size, egg development, survival rate, body growth and generation time in Paramacrobiotus richtersi (reported as Macrobiotus richtersi in Hohberg 2006), cultivating cohorts of 20 eggs at each of 8, 12, 16 and 20 °C. Our simple preliminary survey on the cultivation of A. antarcticus at different temperatures over 17 days at the beginning of this study also demonstrated a wide variation in reproductive output at the different temperatures. Temperature is also one of the most important factors controlling birth and growth rate in several species of rotifer (Galkovskaja 1987; Sarma and Rao 1991; Pérez-Legaspi and Rico-Martínez 1998). The effect of temperature on longevity is well-known in model invertebrate species such as the fruit fly Drosophila melanogaster (Lamb 1968) and the nematode Caenorhabditis elegans (Klass 1977).
In all species of tardigrade, moulting continues periodically throughout the lifetime and is often associated with vitellogenesis (Kinchin 1994). In some species, eggs are laid freely in the substratum, while in others they are enclosed in the cast exuvia of the adult (Marcus 1928). Milnesium sp. is known to deposit eggs in the old exuviae in the space between the old and new cuticles (Suzuki 2003), after which ecdysis occurs within a few hours. In species characterized by free-laying oviposition, the temporal relationship between oviposition and moulting is unknown. During the current study, eggs of A. antarcticus were deposited freely in the medium although deposition in exuviae has also been reported in this species (Dougherty 1964; McInnes 1995; Kagoshima et al. 2013).
Despite periodically producing eggs, failure of oviposition was observed in three individuals during the study. Egg re-absorption has been noted in cultures of some tardigrade species in which animals were observed not feeding under the culture conditions (Baumann 1970; Bertolani 1983). It was not possible to confirm whether the individuals failing to oviposit here were feeding or non-feeding. Egg viability was high even for those laid the day before the parent animals died, while those remaining inside the body of dead adults did not hatch. It is known that eggs of Milnesium sp. experience extreme constriction as they pass through the opening of the cloaca during oviposition (Suzuki 2003). Although not examined in the current study, tardigrade eggs may require such an extreme physical stimulus in order to initiate embryological development.
The present study advances understanding of the life history of Antarctic tardigrades. Terrestrial and freshwater ecosystems in some parts of Antarctica are currently experiencing sometimes drastic climatic and environmental changes (Quayle et al. 2002; Turner et al. 2009). Understanding how individual species respond to these changes is recognized as a key research challenge in order to predict how ecosystem structure and function might be altered (Convey 2011). In the relatively simple terrestrial and freshwater ecosystems of Antarctica, tardigrades are an important component of the biota. Studies demonstrating the effect of temperature on the generation time and reproductive success of Antarctic tardigrades are urgently required as they can affect substantially the community structure and function in these ecosystems.
References
Adams B, Bardgett RD, Ayres E, Wall DH, Aislabie J, Bamforth S, Bargagli R, Cary C, Cavacini P, Connell L, Convey P, Fell J, Frati F, Hogg I, Newsham N, O’Donnell A, Russell N, Seppelt R, Stevens MI (2006) Diversity and distribution of Victoria Land biota. Soil Biol Biochem 38:3003–3018
Altiero T, Rebecchi L, Bertolani R (2006) Phenotypic variations in the life history of two clones of Macrobiotus richtersi (Eutardigrada, Macrobiotidae). Hydrobiologia 558:33–40
Andrássy I (1998) Nematodes in the sixth continent. J Nematode Morph Syst 1:107–186
Baumann H (1970) Lebenslauf und Lebensweise von Macrobiotus hufelandi Schultze (Tardigrada). Veroff Ubersee-Mus Bremen 4:29–43 (Article in German)
Bertolani R (1983) Tardigrada. Oogenesis, oviposition, and oosorption. In: Adiodi KG, Adyodi RG (eds) Reproductive biology of invertebrates, vol 1. Wiley, Chichester, pp 431–441
Block W, Smith RIL, Kennedy AD (2009) Strategies of survival and resource exploitation in the Antarctic fellfield ecosystem. Biol Rev 84:449–484
Convey P (1997) How are the life history strategies of Antarctic terrestrial invertebrates influenced by extreme environmental conditions? J Thermal Biol 22:429–440
Convey P (2011) Antarctic terrestrial biodiversity in a changing world. Polar Biol 34:1629–1641
Convey P, McInnes SJ (2005) Exceptional tardigrade-dominated ecosystems in Ellsworth Land, Antarctica. Ecology 86:519–527
Convey P, Chown SL, Clarke A, Barnes DKA, Cummings V, Ducklow H, Frati F, Green TGA, Gordon S, Griffiths H, Howard-Williams C, Huiskes AHL, Laybourn-Parry J, Lyons B, McMinn A, Peck LS, Quesada A, Schiaparelli S, Wall D (2014) The spatial structure of Antarctic biodiversity. Ecol Monogr 84:203–244
Dastych H (1984) The Tardigrada from Antarctic with descriptions of several new species. Acta Zool Cracov 27:377–436
Dastych H (1991) Redescription of Hypsibius antarcticus (Richters, 1904), with some notes on Hypsibius arcticus (Murray, 1907) (Tardigrada). Mitt Hamb Zool Mus Inst 88:141–159
Degma P, Bertolani R, Guidetti R (2009–2014) Actual checklist of Tardigrada species (ver. 26, 10-07-2014). http://www.tardigrada.modena.unimo.it/miscellanea/ActualchecklistofTardigrada.pdf
Dougherty E (1964) Cultivation and nutrition of micrometazoa. II. An Antarctic strain of the tardigrade Hypsibius arcticus (Murray, 1907) Marcus, 1928. Trans Am Microsc Soc 83:7–11
Gabriel WN, McNuff R, Patel SK, Gregory TR, Jeck WR, Jones CD, Goldstein B (2007) The tardigrade Hypsibius dujardini, a new model for studying the evolution of development. Dev Biol 312:545–559
Galkovskaja A (1987) Planktonic rotifers and temperature. Hydrobiologia 147:307–317
Gibson JAE, Comer L, Agius JT, McInnes SJ, Marley NJ (2007) Tardigrade eggs and exuviae in Antarctic lake sediments: insights into Holocene dynamics and origins of the fauna. J Limnol 66(s1):65–71
Guidetti R, Rebecchi L, Cesari M, McInnes SJ (2014) Mopsechiniscus franciscae, a new species of a rare genus of Tardigrada from continental Antarctica. Polar Biol 37:1221–1233
Hogg ID, Stevens MI, Wall DH (2014) Invertebrates. In: Cowan D (ed) Antarctic terrestrial microbiology: physical and biological properties of Antarctic soils. Springer, Berlin, pp 55–78
Hohberg K (2006) Tardigrade species composition in young soils and some aspects on life history of Macrobiotus richtersi J. Murray, 1911. Pedobiologia 50:267–274
Horikawa DD, Kunieda T, Abe W, Watanabe M, Nakahara Y, Yukuhiro F, Sakashita T, Hamada N, Wada S, Funayama T, Katagiri C, Kobayashi Y, Higashi S, Okuda T (2008) Establishment of a rearing system of the extremotolerant tardigrade Ramazzottius varieornatus: a new model animal for astrobiology. Astrobiology 8:549–556
Kagoshima H, Imura S, Suzuki AC (2013) Molecular and morphological analysis of an Antarctic tardigrade, Acutuncus antarcticus. J Limnol 72(s1):15–23
Kinchin I (1994) The biology of tardigrades. Portland Press Ltd, London, p 186
Klass MR (1977) Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing lifespan. Mech Ageing Dev 6:413–429
Lamb MJ (1968) Temperature and lifespan in Drosophila. Nature 220:808–809
Lemloh ML, Brümmer F, Schill RO (2011) Life history traits of bisexual tardigrades: Paramacrobiotus tonollii and Macrobiotus sapiens. J Zool Syst Evol Res 49(Suppl 1):58–61
Marcus E (1928) Spinnentiere oder Arachnoidea IV: Bärtierchen (Tardigrada). Springer, Jena
Maslen NR, Convey P (2006) Nematode diversity and distribution in the southern maritime Antarctic—clues to history? Soil Biol Biochem 38:3141–3151
McInnes SJ (1995) Tardigrades from Signy Island, South Orkney Islands, with particular reference to freshwater species. J Nat Hist 29:1419–1445
Michalczyk L, Welnicz W, Frohme M, Kaczmarek L (2012) Redescriptions of three Milnesium Doyere, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3154:1–20
Peck L, Convey P, Barnes DKA (2006) Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability. Biol Rev 81:75–109
Pérez-Legaspi IA, Rico-Martínez R (1998) Effect of temperature and food concentration in two species of littoral rotifers. Hydrobiologia 387(388):341–348
Pilato G, Binda MG (1997) Acutuncus, a new genus of Hypsibiidae (Eutardigrada). Entomol Mitt Zool Mus Hamb 12:159–162
Quayle WC, Peck LS, Peat H, Ellis-Evans JC, Richard Harrigan P (2002) Extreme responses to climate change in Antarctic lakes. Science 295:645
Sarma SSS, Rao TR (1991) The combined effects of food and temperature on the life history parameters of Brachionus patulus Müller (Rotifera). Int Revue Hydrobiol 76:225–239
Schill RO (2013) Life-history traits in the tardigrade species Paramacrobiotus kenianus and Paramacrobiotus palaui. J Limnol 72(s1):160–165
Schill RO, Fritz GB (2008) Desiccation tolerance in embryonic stages of the tardigrade. J Zool 276:103–107
Smith RIL (1988) Recording bryophyte microclimate in remote and severe environments. In: Glime JM (ed) Methods in bryology. Hattori Botanical Laboratory, Nichinan, pp 275–284
Sohlenius B, Boström S (2008) Species diversity and random distribution of microfauna in extremely isolated habitable patches on Antarctic nunataks. Polar Biol 31:817–825
Suzuki AC (2003) Life history of Milnesium tardigradum Doyere (Tardigrada) under a rearing environment. Zool Sci 20:49–57
Tsujimoto M, McInnes SJ, Convey P, Imura S (2014) Preliminary description of tardigrade species diversity and distribution pattern around coastal Syowa Station and inland Sør Rondane Mountains, Dronning Maud Land, East Antarctica. Polar Biol 37:1361–1367
Turner J, Bindchadler R, Convey P, Di Prisco G, Fahrbach E, Gutt J, Hodgson D, Mayewski P, Summerhayes C (eds) (2009) Antarctic climate change and the environment. Scientific Committee on Antarctic Research, Cambridge
Velasco-Castrillón A, Schultz MB, Colombo F, Gibson JAE, Davies KA, Austin AD, Stevens MI (2014a) Distribution and diversity of microfauna from East Antarctica: assessing the link between biotic and abiotic factors. PLoS ONE 9:e87529
Velasco-Castrillón A, Gibson JAE, Stevens MI (2014b) A review of current Antarctic limno-terrestrial microfauna. Polar Biol 37:1517–1531
Wall DH (2005) Biodiversity and ecosystem functioning in terrestrial habitats of Antarctica. Antarct Sci 17:523–531
Acknowledgments
We thank Daiki Horikawa and Hiroshi Kagoshima for useful advice in establishing the rearing method and data collection. Peter Convey, Sandra McInnes and three anonymous reviewers provided helpful comments and advice on the manuscript. This study was supported by Grant-in-Aid for Scientific Research No. 23247012 to SI from the Japan Society for the Promotion of Science, and also contributes to the SCAR AnT-ERA research programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsujimoto, M., Suzuki, A.C. & Imura, S. Life history of the Antarctic tardigrade, Acutuncus antarcticus, under a constant laboratory environment. Polar Biol 38, 1575–1581 (2015). https://doi.org/10.1007/s00300-015-1718-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1718-8