Abstract
Arctic caribou and reindeer face an increase in human activity, tourism and infrastructure, which impact may depend on the potential for habituation. Habituation to nonlethal human disturbance in wild animals depends on their risk perception and is therefore hard to separate from effects of predation and hunting pressure. Having evolved under strong isolation with negligible predation and only recent (and local) hunting, the high-Arctic wild Svalbard reindeer represent an adequate model system for studies of habituation to humans. Here, we test for habituation by repeatedly provoking 739 flight responses in 29 radio-collared females throughout two summers in a nonhunted population where human activity level decreases with the distance to a small settlement (Ny-Ålesund). Following provocation by an approaching human on foot, reindeer escape distance (ED) before resuming normal activity ranged from 5 to 500 m and was highly variable among individuals (individual median ED = 23–100 m). Controlling for the effects of individual, observer, terrain ruggedness (positive effect) and having a calf (positive effect), ED increased with distance to Ny-Ålesund [from 32 to 57 m (w/o calf) and 38 to 70 m (with calf) across ~1 to 24 km distance to Ny-Ålesund]. ED also decreased with approach number during the two-month-long summer [average 44–34 m (w/o calf) and 55–43 m (with calf)]. The present study has demonstrated that the naïve Svalbard reindeer habituates to human presence at small spatiotemporal scales through individual learning, suggesting that wild predator-free ungulates may adapt rapidly to increased human activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How human disturbance affects the behaviour and performance of wild animals, and at which spatiotemporal scales these effects operate, is a central topic in conservation biology and wildlife management (Stankowich and Blumstein 2005). Many wildlife species are subjected to predation and/or hunting and perceive human disturbance as a form of predation risk, even if the disturbance is nonlethal and the risk is not real (Frid and Dill 2002; Stankowich and Blumstein 2005). In large herbivores, many studies have documented negative impacts of human infrastructure on behaviour, such as avoidance of roads and pipelines (e.g. moose Alces alces, Dussault et al. 2007; reindeer and caribou Rangifer tarandus [hereafter Rangifer], Leblond et al. 2011, 2013; mountain goats Oreamnos americanus, Singer 1978). On the other hand, flight responses to human disturbance often vary between levels of human activity (Stankowich and Blumstein 2005; Stankowich 2008), and because of habituation, animals in areas with frequent contact with humans typically show reduced flight responses compared to those in areas with rare human contact. While such habituation seems to occur in reindeer and caribou (e.g. Colman et al. 2001), they generally avoid humans and infrastructure (e.g. Wolfe et al. 2000; Dyer et al. 2001; Reimers and Colman 2006; Vistnes and Nellemann 2008; Leblond et al. 2011, 2013; Côté et al. 2013), and concerns have been raised that anthropogenic landscape change and increased tourism and disturbance (UNEP 2001; Johnson et al. 2005) may have contributed to population declines (Vors and Boyce 2009). To predict how the spatiotemporal increase in human activity will impact Rangifer population dynamics and range use, it is clearly important to understand how they habituate to nonlethal human disturbance.
Unfortunately, habituation is often difficult to disentangle from effects of predation and hunting (Stankowich 2008). In the high-Arctic archipelago of Svalbard, where tourism has tripled during the last two decades, the endemic subspecies of wild reindeer (Svalbard reindeer R. t. platyrhynchus) has evolved in the absence of significant predation and hunting. Only a handful of specimens have been reported taken by polar bears (Ursus maritimus) (Derocher et al. 2000; Sandal 2009), and to our knowledge, only one observation exists of a calf being predated by the Arctic fox (Vulpes lagopus) (Prestrud 1992). Reindeer hunting in Svalbard started with the whaling expeditions in the seventeenth century and increased with the introduction of land-based trappers, until hunting was banned in 1925—many local populations were then reduced to extinction. Currently, reindeer hunting is only allowed in parts of Nordenskiöld Land in central Spitsbergen, where some populations have been harvested at low rates (5–10 % annual outtake) during the last three decades. The annual harvest fluctuates around ~ 200 animals out of a total Svalbard reindeer population size roughly estimated to ~11,000 individuals (Governor of Svalbard 2012).
Having evolved in more or less absence of predation, the Svalbard reindeer are unusually tame and naïve for a wild large herbivore (Berger 2007). During summer, it is not uncommon for a still observer to have reindeer approaching at only a few metres distance. This overall tameness is reflected in their solitary behaviour (Tyler 1987), as grouping is regarded a costly anti-predator behaviour. The reindeer are also stationary, i.e. they do not undertake the long-distance migrations that are typical for many Rangifer populations and often related to anti-predator behaviour. However, some baseline and, perhaps, partly relict anti-predator behaviour is clearly present in the Svalbard reindeer (Berger 2007; Reimers and Eftestøl 2012). Studies on the population level have shown that vigilance and human-provoked flight distances are significantly lower in the population close to the major settlement, Longyearbyen, compared with more remote populations (Colman et al. 2001; Reimers et al. 2011; Reimers and Eftestøl 2012). This pattern indicates habituation to humans, but the effect of human presence per se is partly confounded with the lack of hunting (Colman et al. 2001) and low presence of polar bears (Reimers et al. 2011) compared with other investigated populations.
To test the hypothesis that reindeer habituate to nonlethal human disturbance, we applied 2 years of individual-based data on Svalbard reindeer flight responses along a spatial gradient in human activity level where predation risk (negligible) and hunting (banned) are similar. That is, the human activity decreases with distance to a small research settlement. The reindeer population originates from 12 individuals that were re-introduced in the area two decades before this study (Aanes et al. 2000). Because individual learning plays a major role in habituation (Geist 1971), we expected habituation effects to be evident on small spatiotemporal scales, predicting that reindeer flight distances should (1) decrease with human disturbance level (i.e. increase with distance to the settlement) and (2) decrease over time following repeated provocations.
Materials and methods
Study system
The study area is located at Brøggerhalvøya and Sarsøyra on the north-western coast of Spitsbergen, Svalbard (Fig. 1). Ny-Ålesund was established as a coal mining society during the early twentieth century and gradually became a settlement for research activities following the closing of the mines in the 1960s. The current population is ~35 citizens year-round and up to ~180 (including scientists) during summer. Human activities on land are generally confined to Ny-Ålesund and nearby areas on the northern and eastern part of Brøggerhalvøya. Presence by humans on the southern part of Brøggerhalvøya is mainly limited to some scooter traffic and the use of recreational cabins by the locals. Sarsøyra is hardly ever visited by humans, although a small cabin is used occasionally in winter. Accordingly, there is a gradual decline in human disturbance level with increasing distance from Ny-Ålesund.
Except for parts of central Spitsbergen, Svalbard reindeer hunting has been banned since 1925. However, the reindeer in the surroundings of Ny-Ålesund were hunted to local extinction before the ban, and the current reindeer population was founded by 12 wild individuals that were transferred by boat from Adventdalen to Brøggerhalvøya in 1978 (Aanes et al. 2000). The Brøggerhalvøya population irrupted and crashed from ~360 to ~80 individuals in winter 1994 (Aanes et al. 2000), when ~40 individuals migrated to Sarsøyra. The population sizes in Brøggerhalvøya and Sarsøyra were both estimated to ~160 individuals in winter 2000 (R. Aanes, unpubl.).
Data collection and analyses
Data on flight distances were obtained from n = 29 female reindeer that were captured and collared with VHF radio transmitters as adults during April 1999, October 1999 and April 2000 (Arnemo and Aanes 2009). The reindeer were sampled haphazardly within Brøggerhalvøya and Sarsøyra. We radio-tracked these individuals every second or third day during summers 1999 (n = 3 observers) and 2000 (n = 6 observers) as part of a habitat selection study (Hansen et al. 2009). Note that the reindeer were also unintendedly disturbed at irregular occasions between the radio-tracking dates due to parallel botanical studies covering the entire study area. Following radio-tracking and visual localisation of an animal, it was approached cautiously in order to get as close as possible before triggering a flight response. This was achieved by walking slowly and in sight by the animal, preferably giving the animal a downwind position, as scenting is important for recognition (Baskin and Skogland 1997). When a reindeer flight response (running or walking away) was triggered, the observer walked towards the original feeding or lying site and noted the GPS position and the escape distance (ED), i.e. the distance estimated by eye to the position where normal activity was resumed by the animal. ED was not noted for all observations in 1999. In total, we obtained n = 178 EDs from 13 individuals (nine with a calf) on Brøggerhalvøya and three individuals (two with a calf) on Sarsøyra (during July 13th–September 1st) in 1999, and n = 561 EDs from 10 individuals (five with a calf) on Brøggerhalvøya and 13 individuals (eight with a calf) on Sarsøyra (July 5th–August 30th) in 2000.
We analysed for habituation effects on ED (m, log-transformed) using a linear mixed model (function lmer in R package lme4; Bates et al. 2008). Observer id and animal id were treated as random intercepts and the following as fixed effects: year, with or w/o calf, terrain ruggedness (see Sappington et al. 2007; Hansen et al. 2009), approach number, and distance to Ny-Ålesund. The model was run using restricted maximum likelihood. 95 % confidence intervals of parameter estimates for fixed effects were obtained using function confint (method “Wald”) in R package stats. Note that there was no evidence for interaction effects based on stepwise removal of nonsignificant interaction terms from a global model with all possible two-way interactions. Replacing approach number with day number provided similar results (analyses not shown). Analyses were run in R for Windows versions 2.15.1 (R Development Core Team 2012).
Results
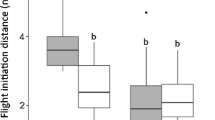
Following provocation by an approaching observer, female reindeer ED before resuming normal activity ranged between 5 and 500 m. Individual-level median ED varied between 23 and 100 m (year-specific estimates) and was positively correlated between years for individuals that were followed both summers (Spearman’s ρ = 0.725, n = 10 individuals, P < 0.05). ED was ≤20 m following 33 % of the provocations in NE Brøggerhalvøya (high human activity), versus 12 % in SW Brøggerhalvøya (low human activity) and only 8 % in Sarsøyra (virtually no human activity) (Fig. 2a). ED was >100 m following 0.6 % (NE Brøggerhalvøya), 11 % (SW Brøggerhalvøya) and 16 % (Sarsøyra) of the provocations. Accordingly, the linear mixed modelling results (Table 1) suggested that ED increased with distance to Ny-Ålesund [from 32 to 57 m (w/o calf) and from 38 to 70 m (with calf) at ~1 to 24 km distance from Ny-Ålesund] (Fig. 2b). ED also decreased with approach number during the two-month-long summer [from 44 to 34 m (w/o calf) and from 55 to 43 m (with calf)] (Fig. 2c). Note that replacing approach number with day number provided qualitatively similar results, and exploratory analyses indicated no evidence for nonlinear or threshold effects of approach/day number (Fig. 2c; analyses not shown). Finally, ED was higher in females with a calf versus those without a calf and increased with terrain ruggedness, while there was no effect of year (Table 1).
a Frequencies of different escape distance (ED) intervals in female Svalbard reindeer in areas with contrasting human activity level (high = NE Brøggerhalvøya, low = SW Brøggerhalvøya, no = Sarsøyra). b ED plotted against the distance to Ny-Ålesund (with calf: triangles; without calf: circles). c ED plotted against approach number. The regression lines in a and b are from a linear mixed-effects model of ED (m, log-transformed) with distance to Ny-Ålesund, approach number, reproductive status (with calf: solid line; without calf: dashed line) and year as fixed effects, and animal id and observer id as random intercepts. The year effect was negligible, so only the 1999 regression lines are shown
Discussion
This study on a naïve wild ungulate has demonstrated patterns of flight responses that suggest habituation to humans at small spatiotemporal scales. Repeated provocations of individually marked reindeer showed that ED increased with distance from Ny-Ålesund and decreased during the course of the summer, lending support to the prevailing view from population-level studies that population differences in Svalbard reindeer vigilance and flight responses are due to habituation effects (Colman et al. 2001; Reimers et al. 2011). One common problem, however, with such population-level comparisons is that responses to nonlethal human disturbance are often confounded with effects of varying hunting or predation (Stankowich 2008). Accordingly, although reindeer were tamer in the nonhunted Adventdalen population (close to Longyearbyen, the major settlement and area for activity in Svalbard) compared with three populations facing lower human activity (Colman et al. 2001; Reimers et al. 2011), the latter populations were subject to hunting. Furthermore, comparison with two nonhunted populations hardly ever visited by humans showed inconsistent patterns relative to Adventdalen—i.e. flight distances were larger in Reinsdyrflya (Colman et al. 2001) but not in Edgeøya (Reimers et al. 2011). This may be due to an effect of higher polar bear abundance on the latter island, as supported by the higher reindeer vigilance there (Reimers and Eftestøl 2012). In our study area, a polar bear was only observed once during the entire field seasons, and there was no sign of the bear chasing reindeer or other indirect impact on the reindeer’s behaviour.
Animals that frequently experience nonlethal interactions with humans tend to habituate and even “ignore” humans, thereby reducing the flight distances (Denniston 1956; Cassirer et al. 1992; Louis and Le Beere 2000; Tarlow and Blumstein 2007; Stankowich 2008; but see, e.g. Côté et al. 2013 for very weak habituation to helicopter traffic). Although the disturbance frquency is relatively low compared with, e.g. Longyearbyen and surroundings, reindeer close to (i.e. within 3–5 km from) Ny-Ålesund are exposed to humans on foot, skis and snow mobiles on a daily basis more or less year-round. The frequency of encounters drops markedly at 5–10 km distance from Ny-Ålesund and is effectively zero in Sarsøyra, fitting well with our observations of changes in ED with distance. The decline in ED during the course of the summer (i.e. with approach number) further indicates that such habituation may operate on small spatiotemporal scales through individual learning mechanisms, apparently on the scale of days or weeks. Although changes in flight responses within or between seasons may be due to other factors than habituation that are hard to control for (e.g. Reimers and Colman 2006), most of these confounding factors (such as variation in predation pressure, insect harassment, between-season variation, calf development) are eliminated or controlled for in this study. Thus, there was no evidence for contrasting effects of approach number (or day number) on females with versus without a calf at heel (i.e. no significant interaction effect), indicating an overall habituation independent of reproductive status. However, caution is still needed when interpreting such temporal patterns, and it can be argued that the biological significance of this short-term temporal effect of repeated provocations is questionable due to the rather small effect size.
Clearly, estimating distances by eye introduces noise in the data. It is not unlikely that observers differ systematically in their precision and/or accuracy of estimated ED, but this should be accounted for in our mixed model procedure, by including observer as random factor. Likewise, we did not control for group size (no data), which influences ED in Svalbard reindeer (Reimers et al. 2011) as well as other ungulates (Stankowich 2008). However, there is no reason to believe that either group size or the precision/accuracy due to estimation by eye would change with distance to Ny-Ålesund, or during the course of the summer, and we therefore do not believe this affects our main results and conclusions.
Besides providing support for the habituation hypothesis, our results confirm several previously described patterns in Svalbard reindeer flight responses (Reimers et al. 2011). First, provoked reindeer ran longer distances the more rugged terrain, suggesting that the animals feel safer and in more control on level terrain (Reimers et al. 2010), where visibility is higher. Second, in accordance with differences in risk assessment due to reproductive allocation (Stankowich and Blumstein 2005), females with a calf had larger ED than those without calf, confirming the presence of a baseline anti-predator behaviour and some (very small) risk of calf predation by the Arctic fox (Prestrud 1992). The effect size of having a calf at heel was much smaller than that found in other populations (Reimers et al. 2011), which could result from different methods of approaching the animals rather than population differences. That is, the observers in the present study aimed at reducing animal disturbance to a minimum, i.e. approaching with care and with the wind, whereas previous studies have applied a more direct and provocative approach (Colman et al. 2001; Reimers et al. 2011; Reimers and Eftestøl 2012). This also means that our ED estimates are not directly comparable with other studies, in which the estimates are overall much higher.
The variation in flight response at small spatial and temporal scales demonstrated here suggests that habituation to humans may occur rapidly through individual learning mechanisms. Because of this “plastic” and overall tame behaviour, recent and future increase in terrestrial activity and tourism in Svalbard is unlikely to have a significant negative effect on the reindeer related to changes in their behaviour (Tyler 1991; Colman et al. 2001). If the patterns of habituation in this predator-free subspecies reflect traits and mechanisms that are representative for Rangifer in general, habituation to humans can help buffer wild Arctic reindeer and caribou against the effects of changes in landscape use, tourism and infrastructure.
References
Aanes R, Sæther B-E, Øritsland NA (2000) Fluctuations of an introduced population of Svalbard reindeer: the effects of density dependence and climatic variation. Ecography 23:437–443
Arnemo JM, Aanes R (2009) Reversible immobilization of free-ranging Svalbard reindeer (Rangifer tarandus platyrhynchus) with Medetomidine–Ketamine and Atipamezole. J Wildl Dis 45:877–880
Baskin LM, Skogland T (1997) Direction of escape in reindeer. Rangifer 17:37–40
Bates D, Maechler M, Dai B (2008) lme4: linear mixed-effects models using S4 classes. R package version 1.0-4 [computer program]. http://cran.r-project.org/web/packages/lme4/index.html. Accessed 14 Jan 2014
Berger J (2007) Carnivore repatriation and holarctic prey: narrowing the deficit in ecological effectiveness. Conserv Biol 21:1105–1116
Cassirer EF, Freddy DJ, Ables ED (1992) Elk responses to disturbance by cross-country skiers in Yellowstone National Park. Wildl Soc Bull 20:375–381
Colman JE, Jacobsen BW, Reimers E (2001) Summer response distances of Svalbard reindeer Rangifer tarandus platyrhynchus to provocation by humans on foot. Wildl Biol 7:275–283
Côté SD, Hamel S, St-Louis A, Mainguy J (2013) Do mountain goats habituate to helicopter disturbance? J Wildl Manage 77:1244
Denniston RH (1956) Ecology, behaviour, and population dynamics of the Wyoming or Rocky Mountain moose, Alces alces shirasi. Zoologica 41:105–118
Derocher AE, Wiig O, Bangjord G (2000) Predation of Svalbard reindeer by polar bears. Polar Biol 23:675–678
Dussault C, Ouellet J, Laurian C, Courtois R, Poulin M, Breton L (2007) Moose movement rates along highways and crossing probability models. J Wildl Manage 71:2338–2345
Dyer SJ, O’Neill JP, Wasel SM, Boutin S (2001) Avoidance of industrial development by woodland caribou. J Wildl Manage 65:531–542
Frid A, Dill L (2002) Human-caused disturbance stimuli as a form of predation risk. Conserv Ecol 6:16
Geist V (1971) A behavioural approach to the management of wild ungulates. In: Duffey E, Watt, ES (eds) The scientific management of animal and plant communities for conservation. The 11th symposium of the British Ecological Society, University of East Anglia, Norwich, Blackwell Scientific Publications, Oxford, pp 413–424
Governor of Svalbard (2012) Årsrapport for Sysselmannen på Svalbard 2011 (Annual report of the Governor of Svalbard 2011). Governor of Svalbard, Longyearbyen
Hansen BB, Herfindal I, Aanes R, Sæther B-E, Henriksen S (2009) Functional response in habitat selection and the tradeoffs between foraging niche components in a large herbivore. Oikos 118:859–872
Johnson CJ, Boyce MS, Case RL, Cluff HD, Gau RJ, Gunn A, Mulders R (2005) Cumulative effects of human developments on Arctic wildlife. Wildl Monogr 160:1–36
Leblond M, Frair J, Fortin D, Dussault C, Ouellet J-P, Courtois R (2011) Assessing the influence of resource covariates at multiple spatial scales: an application to forest-dwelling caribou faced with intensive human activity. Landsc Ecol 26:1433–1446
Leblond M, Dussault C, Ouellet J-P (2013) Avoidance of roads by large herbivores and its relation to disturbance intensity. J Zool 289:32–40
Louis S, Le Beere M (2000) Adjustment in flight distance from humans by Marmota marmota. Can J Zool 78:556–563
Prestrud P (1992) Arctic foxes in Svalbard: population ecology and rabies. PhD thesis, Univ Oslo, Oslo
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Reimers E, Colman JE (2006) Reindeer and caribou (Rangifer tarandus) response towards human activities. Rangifer 26:55–71
Reimers E, Eftestøl S (2012) Response behaviour of Svalbard reindeer towards humans and humans disguised as polar bears on Edgeøya. Arct Antarct Alp Res 44:483–489
Reimers E, Røed KH, Flaget Ø, Lurs E (2010) Habituation responses in wild reindeer exposed to recreational activities. Rangifer 30:45–59
Reimers E, Lund S, Ergon T (2011) Vigilance and fright behaviour in the insular Svalbard reindeer (Rangifer tarandus platyrhynchus). Can J Zool 89:753–764
Sandal T (2009) Blant rein og bjørn (in Norwegian). Villreinen 24:68–69
Sappington JM, Longshore KM, Thompson DB (2007) Quantifying landscape ruggedness for animal habitat analysis: a case study using bighorn sheep in the Mojave Desert. J Wildl Manage 71:1419–1426
Singer FJ (1978) Behaviour of mountain goats in relation to U.S. Highway 2, Glacier National Park Montana. J Wildl Manage 42:591–597
Stankowich T (2008) Ungulate flight responses to human disturbance: a review and meta-analysis. Biol Conserv 141:2159–2173
Stankowich T, Blumstein DT (2005) Fear in animals: a meta-analysis and review of risk assessment. Proc R Soc Lond B Biol Sci 272:2627–2634
Tarlow EM, Blumstein DT (2007) Evaluating methods to quantify anthropogenic stressors on wild animals. Appl Anim Behav Sci 102:429–451
Tyler NJC (1987) Natural limitation of the abundance of the high Arctic Svalbard reindeer. Dissertation, University of Cambridge
Tyler NJC (1991) Short-term behavioural responses of Svalbard reindeer Rangifer tarandus platyrhynchus to direct provocation by a snowmobile. Biol Conserv 56:179–194
UNEP (2001) GLOBIO—global methodology for mapping human impacts on the biosphere. United Nations Environmental Programme, Nairobi
Vistnes I, Nellemann C (2008) The matter of spatial and temporal scales: a review of reindeer and caribou response to human activity. Polar Biol 31:399–407
Vors LS, Boyce MS (2009) Global declines of caribou and reindeer. Glob Change Biol 15:2626–2633
Wolfe SA, Griffith B, Wolfe CAG (2000) Response of reindeer and caribou to human activities. Polar Res 19:63–73
Acknowledgments
The study was funded by the Norwegian Research Council (Arctic Field Grant and POLARPROG Grant Nr 216051 to BBH), the Norwegian Polar Institute and Centre for Biodiversity Dynamics (co-funded through the Norwegian University of Science and Technology). We thank S. Henriksen for collecting data in 1999 and O. G. Støen, H. Skoglund, W. L. G. Johansen, M. Ericson, J. P. Ikonen, P. Kuss and H. Landsem for valuable help in the field. We are also grateful to the personnel at the Sverdrup Station in Ny-Ålesund for logistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hansen, B.B., Aanes, R. Habituation to humans in a predator-free wild ungulate. Polar Biol 38, 145–151 (2015). https://doi.org/10.1007/s00300-014-1572-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1572-0