Abstract
The intertidal communities on rocky shores are directly subjected to climatic changes in air and water temperatures and to derived effects of climate change, such as changes in freshwater run-off and ice dynamics. Global warming occurs at elevated rates in Greenland and results in changing species distributions with range expansions to the north and new species entering terrestrial habitats from the south. There is, however, no quantitative knowledge of past or present species distribution in the littoral zone of Southern Greenland, an area which represents an important gateway for northern range expansions of temperate species. This study provides baseline information on abundances of macroorganisms in the eulittoral Southern Greenland. This knowledge will pave the way for future studies on the impact of climate change and anthropogenic activities on these communities. Nine sites, situated at different exposure levels, were investigated. A total of 22 taxa were recorded, suggesting low species richness. Patellid limpets and predators such as dogwhelks, starfish and crabs were absent. Total standing stock ranged from 0 to 31,898 g m−2. Species composition and biomasses were related to locally generated wave exposure, oceanic swells and ice scouring. The high standing stock at sheltered sites indicated that neither light, temperature nor nutrients, constrained buildup of biomass in this environment. Inshore seasonal measurements of water and air temperatures were recorded for the first time in the region, displaying low water temperatures and high variation in air temperatures, indicating lack of insulating stable sea ice in the area. Possible impacts of ongoing temperature changes are discussed based on recorded temperatures and meteorological data from the past 30 years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intertidal zone of rocky shores is an intensively studied marine habitat. Ecologists studying community assembly rules have worked in this habitat in temperate regions for nearly a century, and as a result, the mechanisms influencing species composition and community dynamics along intertidal rocky shores are well-documented (Connell 1972; Paine 1994; Bertness et al. 2001; Little et al. 2009). Being directly exposed to changes in air and sea surface temperature, as well as changes in ice cover and freshwater input the intertidal zone is highly subjected to climate change. It is therefore considered an “early warning habitat” in which effects of a shifting climate can be detected prior to changes becoming detectable in other marine systems. Detailed knowledge of many intertidal communities and their direct susceptibility to changes in climate thus make the intertidal zone a useful model for studying biological effects of climate changes (Sagarin et al. 1999; Hawkins et al. 2008). Studies from the rocky intertidal, predominantly in temperate regions, have revealed how individual species respond to a warming climate by changing their distributional limits; for example, the mussel Mytilus edulis has contracted its southern distributional limit along the Atlantic coast of the US by 350 km since the 1960s as a result of mortality caused by increased summer temperatures (Jones et al. 2010; Zippay and Helmuth 2012). Not only do increasing temperatures change the southern distributional limit of temperate species in the Northern hemisphere; they also enable “southern” species to extend their northern range, as observed along the Pacific coast of the US (Barry et al. 1995; Zacherl et al. 2003; Blanchette et al. 2008) and in Great Britain (Mieszkowska et al. 2006; Herbert et al. 2007; Mieszkowska et al. 2007; Blanchette et al. 2008; Hawkins et al. 2009). These northward range expansions result in invasion of new species (Broitman et al. 2008) which can considerably influence ecosystem function and diversity through changes in biological interactions (Hawkins et al. 2009; Harley 2011; Kordas et al. 2011).
Climate models predict increasing Arctic temperatures at a rate of twice the global mean (Solomon et al. 2007). Rising temperatures and the resulting decrease in ice cover are expected to trigger a northward expansion of temperate and subarctic species. Ecological modeling suggests a significant change in distribution of intertidal macroalgae along North Atlantic coasts under the IPCC climate change scenarios (Jueterbock et al. 2013). Such changes are already observed in Svalbard where intertidal macrophytes are now advancing into inner fjord basins (Węsławski et al. 2010). A northward range shift of intertidal macroalgae species will lead to dramatic changes in community structure and ecosystem function along the Greenlandic coastline. Range expansions are bound by climatic conditions, but pelagic transport of propagules and larvae, which may depend on regional and local current regimes, is also a crucial parameter. South Greenland is hydrographically connected to the Northeast Atlantic through the North Atlantic drift and the Irminger Current, with may facilitate transport of northern temperate species. The southern tip of Greenland, the Cape Farewell area, thus seems to be a critical gateway to potential northward range expansions in this corner of the Northwest Atlantic (Labrador Sea, Davis Strait and Baffin Bay); at present, however, the rocky intertidal zone of Greenland with its composition of flora and fauna has not been quantitatively studied and studies of Arctic intertidal communities are sparse (Wulff et al. 2009; Węsławski et al. 2011). Only few present biomasses (Florczyk and Latala 1989; Hansen 1999; Espinosa and Guerra-Garcia 2005; Konar et al. 2009), but this baseline information is needed to evaluate predicted range shifts and to quantify the impact of climate change.
To enhance predictions and documentation of changes caused by drivers such as climate change, it is important to understand how past events affect contemporary community processes. The structure of North Atlantic ecosystems show signs of past large-scale events, such as the trans-Arctic interchange when the Bering strait opened, and there is a well-documented gradient in which the diversity of epilithic microphyte grazers decline across the Atlantic, moving from Western Europe across Iceland towards North America (Jenkins et al. 2008). The Greenlandic communities have never been considered in this context, and we still need to see whether they fit into the proposed schemes. Furthermore, the suggestions that the Arctic, as a whole, is home to no more than 100 macrobenthic intertidal species, and that fewer species are found off islands than off continental landmasses in the Arctic (Węsławski et al. 2011) need to be consolidated by data from the Greenlandic intertidal.
This paper contains the first quantitative description of intertidal communities in Greenland based on an examination of the community composition at nine sites situated at different levels of wave exposure at the southernmost part of the Greenlandic west coast. This is a first step towards bridging the knowledge gap on intertidal communities in Greenland, providing baseline data on intertidal species composition, abundance and diversity in an area which is suspected to be an important stepping stone in climate-driven range shifts from the Northeast Atlantic towards the Northwest Atlantic.
Materials and methods
Study area
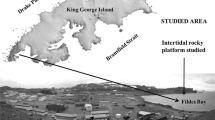
A total of nine sites were investigated in two different areas of Southern Greenland (Fig. 1a, b; Table 1), one of which was the Kitsissut islands about 10 km off the coast. These islands are extremely exposed to wind, oceanic swells and advected sea ice, except for the sheltered bay on the main island where samples were taken. The other area was the mainland coast near Cape Farewell where samples were collected both along the coast and in the fjords. Both Cape Farewell and Kitsissut are influenced by three water masses: (1) Fjord water receiving runoff from land, (2) cold low-saline polar/coastal water from the East Greenland Current and (3) the warmer, more saline Atlantic water entering the area via the Irminger Current; the latter is, however, most conspicuous at depth and more offshore (Ribergaard 2013). The properties of the surface water in the coastal zone are highly dependent on tidal mixing; on the east coast of Greenland, multi-year sea ice is transported south by the East Greenland Current, and the leading ice front typically passes Cape Farewell in January or February. This ice is present in the area for three to 5 months (Fig. 1c) (Buch 2002). Sea ice does not usually form locally in the fjords. Surface water salinity is around 28–32 in summer (Ribergaard 2013), and tides are semidiurnal with a maximal tidal amplitude of 2.7 m, resulting in an average air exposure in the mid intertidal of 6 h 50 min and a maximum air exposure of 8 h 20 min.
Sampling and sample processing
Sampling was carried out during July 2011 (Kitsissut) and the first half of September 2011 (Cape Farewell). Species abundance of macroalgae and benthic macrofauna were measured by coverage (expressed by the percent of a 25 × 25 cm area covered by the species), biomass (wet weight of whole individuals including exoskeletons on fauna) and number of individuals (for macrofauna) at each site. A horizontal transect was established at each site following the mean tidal level (MTL) and we restricted surveys to sites which consisted of boulders and solid bedrock. The MTL was located by raising a lath at the low water mark and by using a hand level to point out positions at the height representing half the maximum tidal amplitude from which the actual water level (calculated from tide tables) had been subtracted. Seven replicate samples were taken along the horizontal transect, with 0.5 meters interval. Samples were bordered by 25 × 25 cm (0.0625 m2) frames subdivided in 100 squares to ease coverage determination. The percentage of coverage of each algal species was determined within each 25 × 25 cm frame, and so was the percentage of bare rock. All invertebrates and algae attached within the frame were then collected for species identification and biomass determination. Coverage of barnacles and algae crusts was determined after the removal of macroalgae. Periwinkles and amphipods appearing after the removal of algae were collected using forceps.
Samples were sorted and preserved on the day of sampling or the following day. All macrofauna attached to the algae were carefully removed and transferred to 70 % ethanol. Water used for sample flushing was sieved through a 500 µm mesh to retrieve the macrofauna. Algae were shaken to remove excess water, and each species within a frame was weighed separately. Macrofauna were counted and identified to lowest possible taxon, i.e. species, using Hayward and Ryland (1995), and biomasses including exoskeletons of the individual species were determined using a 3-digit scale. The samples were then stored in 70 % ethanol. Macroalgal identification was performed according to Pedersen (2011).
The vertical range of habitat-forming macroalgae was measured laying out a vertical transect from the maximum low water level to the upper limit of the intertidal community. The transect was placed at a representative stretch of the site, and the presence of macroalgal species was registered on a 20 cm transversal line at 25 cm intervals along the transect. The vertical height of each observation was subsequently determined using the slope of the coastline.
Assessment of environmental gradients
Environmental stress parameters (ice scour, temperature and wave exposure) were measured at site CF1, CF2, CF3 and CF4 in the Cape Farewell area. Due to logistical limitations, environmental stress parameters were not assessed at all sites. Temperature loggers (Hobo professional v.3, Onset) were placed at MTL and retrieved after one and a half year.
Maximum water velocities were measured over a period of 14 days, during which time the area was hit by two storms with wind speeds of up to 34 m s−1. Spring-loaded dynamometers (Bell and Denny 1994) were calibrated and placed at the rock at the MTL by drilling a hole which fit a cylinder containing the spring. A thread—collar was glued onto the rock by means of marine epoxy, making it possible to unscrew and recover dynamometers at the end of the sampling period. Maximum water velocities were calculated according to Bell and Denny (1994).
Ice scour intensity was assessed at some sites (Table 1) by measuring the deformation of 15 stainless steel screws (4 mm) affixed to the rock at each site during the winter season. The average angle of deformation was used as a proxy for ice scouring intensity where 0° represent no bending of the screw and 90° maximum bending.
Relative wave exposure index (REI) was calculated based on Keddy (1982) and modified according to Fonseca et al. (2002). The transect lines were clipped at 10 km, as recommended by Fonseca (2010). Fetch lines were based on distances to the shoreline in 32 directions and were then reduced to eight lines by a weighted average of the closest nine lines. This accounts for irregularities of the coastline. Data on wind were retrieved from meteorological station number 4283 in Nanortalik (Fig. 1b) and provided by the Danish Meteorological Institute. Air temperatures from the past three decades were also obtained from this station.
Data analysis
Data analysis was carried out using R 3.0 (R core 2012) with reshape 2 for database handling (Wickham 2007), Vegan for ecological analysis (Oksanen et al. 2013) and R core functions for statistics. A species accumulation curve was calculated according to Ugland et al. (2003). In order to determine the pattern of ecological distances, expressed as Bray-Curtis dissimilarity, we used Principal Coordinates Analysis (PCoA) as implemented in Vegan package. Data were transformed by Wisconsin double transformation before calculating Bray-Curtis dissimilarity.
In situ temperature data retrieved from the temperature loggers placed at MTL were separated into air and water temperatures based on tides and sea level model for the area (available from the Danish Meteorological Institute).
Results
Biomass of macroorganisms, coverage of sessile organisms and abundance of macrofauna were quantified in 60, 0.0625 m2 quadrates covering nine distinct sites (Fig. 1; Table 1) at the MTL in Southern Greenland. The cumulative number of species registered across the nine sites was 22 taxa. Of these, 12 were macroalgae, and 10 were macrofauna (Fig. 3). The species accumulation curve approached its asymptote, indicating that most taxa of that particular habitat in the area were collected with the method employed (Fig. 2). The communities were characterized by the large canopy-forming algae Fucus evanescens, Fucus vesiculosus and Ascophyllum nodosum, and the dominant faunal species were the gastropod Littorina saxatilis, the amphipod Gammarus oceanicus and the cirriped Semibalanus balanoides (Fig. 3). Possible interactions among functional groups in the communities were examined by correlating the faunal functional groups, biofilm grazers (Littorina spp. and isopods), herbivorous shredders (amphipods) and filter feeders (cirripeds) to the macroalgal biomass within quadrates. The biomass of biofilm grazers was positively correlated to algae biomass (Pearson correlation coefficient ρ = 0.54, n = 60, p < 0.01) as was the biomass of herbivorous shredders (ρ = 0.82, n = 60, p < 0.01) and the total species richness for the fauna (ρ = 0.57, n = 60, p < 0.01). Coverage of filter feeders was not correlated to algal biomass (ρ = 0.01, n = 60, p = 0.92).
a Biomass measured at Kitsissut and Cape Farewell. b Coverage of macroalgae and cirripeds, and macrofaunal abundance Kitsissut and Cape Farewell. Data are shown in a box-and-whisker diagram. Bottom and top of the boxes are the first and third quartiles and the line inside the box shows the median. The ends of the whiskers represent the range of the data after excluding outliers. Outliers are plotted as individual points. The epithet spp is used when specimens can be assigned to the same genus but may belong to different species
Whole community composition was analyzed by PCoA ordination which showed a tendency for separation between areas (Fig. 4); this pattern was confirmed by ANOSIM (R = 0.1588, p < 0.001). The most notable differences between the two areas were the higher frequency of Littorina obtusata among the collected molluscs in Kitsissut compared to Cape Farewell (20 vs. 0.4 %) and the presence of the amphipod Hyale nilssoni being restricted to Cape Farewell. The two axes explained 55 % of the variation in the dataset, with eigenvalues of 6.25 and 6.049 for the first and the second axis, respectively. The gradient along the first axis reflected exposure, with the more exposed sites towards the right (Fig. 4). This is partly a consequence of the increased deformation of Fucus at the exposed sites. Generic level identifications, Fucus spp., were a consequence of the deformity or absence of key species characteristics, such as pairwise bladders or elongated receptacles, which may be needed for safe identification of F. vesiculosus and F. evanescens, respectively. This resulted in an increased occurrence of unidentifiable Fucus individuals classified as Fucus spp. at exposed sites.
Overall, intertidal biomass was not related to wave exposure level, expressed by REI; however, within each sampling area, biomass tended to increase with decreasing REI values (Fig. 5). The REI corresponded to the wave exposure, measured by dynamometers during local storms in the Cape Farewell area (Table 1). Total biomass ranged from 0 to 31,898 g m−2 at Cape Farewell and from 0 to 35,359 g m−2 at Kitsissut.
The vertical distribution of canopy forming macroalgal species at both sites is shown in Fig. 6: Cape Farewell (CF1–CF5) and Kitsissut (K1–K4), with decreasing wind fetch toward the right F. evanescens and F. vesiculosus showed a general vertical zonation pattern, i.e. at sites where both Fucus species were present, F. vesiculosus was found to inhabit the upper part of the littoral zone, whereas F. evanescens was concentrated in the lower part. A. nodosum was located between the two Fucus species at site CF4 which was the only site with an abundant A. nodosum population.
The vegetation belt of F. evanescens at Kitsissut tended to occur higher in the littoral zone as wave exposure increased, except at K3 where cracks and crevices sheltered a dense F. evanescens population in the upper part. No vertical zonation was observed at the most exposed sites, neither in the Cape Farewell area, nor at Kitsissut.
In situ temperatures were recorded by three temperature loggers retrieved from the MTL in the Cape Farewell area (site CF2, CF3 and CF4). The three intertidal temperature loggers recorded water temperatures between −1.8 and 8.8 °C (Fig. 7). Water temperatures differed between sites, with higher summer temperatures in the harbor of Aappilattoq (Fig. 7f). Air temperatures ranged between −13.2 and 32.9 °C, and seasonal variation was consequently around 40 °C. Maximum diurnal variation was 29.1 °C in the mid intertidal.
Three decades of temperature data from the weather station at Nanortalik showed a significant positive trend in mean winter (December to March) and summer (June to September) air temperatures from 1982 to 2011, with a statistically significant increase of 0.12 °C year−1 in summer (R 2 = 0.53, p < 0.01) and 0.14 °C year−1 in winter (R 2 = 0.36, p < 0.01) (Fig. 8).
Ice scouring intensity was assessed at four sites in the Cape Farwell area and ranged between 0 in the harbor of Aappilattoq (site CF4) and 90 degrees of deformation at Ikigaat (site CF1), the latter of which was also the most wind-exposed site, as shown by the REI values (Table 1).
Discussion
Extreme diurnal and seasonal changes in temperature and light, together with scouring by sea ice, make the Arctic intertidal an extremely physically disturbed habitat. It has been suggested that the Arctic intertidal as a whole is home to less than 100 intertidal species (summarized by Węsławski et al. 2011). Our observations in Southern Greenland confirm the notion of a species-poor Arctic intertidal, with only 22 taxa registered in the MTL across the nine sampling sites (Fig. 3). The number of observed species was influenced by sample efforts which were concentrated in the mid intertidal. The relative flat species accumulation curve indicates that the majority of species were sampled in this habitat. In a study from Nova Scotia, an area also influenced by sea ice, was total species richness in the mid intertidal 16 and 30 species on exposed and sheltered shores, respectively (Scrosati and Heaven 2007). The low number of species in our study can, most likely, be attributed to the harsh environment, with ice scour being a particularly important parameter in the investigated area. The ice is advected into the region each year, and the variable deformation of stainless steel screws between sites indicated large local variation in ice scour intensity, which may, to some degree, be related to differences in wave exposure (Table 1). The impact of ice scouring was also clear from the deformation of fucoid algae at most of the visited sites.
Cape Farewell and Kitsissut segregated in the ordination due to different proportions of species (Fig. 4). Studies indicate that L. obtusata is more frequently found on Fucus than L. saxatilis in subarctic environments (Johannesson and Ekendahl 2002), and this seems to also be the case at the Kitsissut sites which had a more extensive coverage of Fucus compared to the sites visited at Cape Farewell (Fig. 6).
The diversity of epilithic microphagous grazers was very low and no patellid limpets were present in the MTL in the studied areas. The absence of limpets has also been observed in other subarctic locations in Greenland (MK. Sejr pers. obs.). Theoretically, the lack of limpet grazing should allow the fucoid algae to occupy more exposed areas because grazing directly determines the degree to which fucoid algae survive exposure (Hawkins et al. 2008). This is seen in northern Europe where fucoid algae extend further out onto exposed shores compared to southern Europe (Spain and Portugal) where limpet grazing is more intense and restricts fucoids to sheltered and estuarine refuges (Ballantine 1961).
The lack of predators was another conspicuous trait of the investigated intertidal community. The dogwhelk Nucella lapillus was not found in the MTL community in Southern Greenland even though it has been reported from the MTL of the southern part of Iceland (Espinosa and Guerra-Garcia 2005) and from Nova Scotia (Scrosati and Heaven 2007); other potentially important predators, such as crabs and starfish were also absent and these findings are consistent with reports of low predator abundance in the intertidal of the Godthåbsfjord, SW Greenland (64°N) (Blicher et al. 2013). The lack of predators in the Southern Greenlandic MTL adds to the many examples of physical stress altering the relative importance of biological interactions in structuring species assemblages in the intertidal (Menge 1976). Specifically, it is expected that the influence of predation decreases as physical stress increases, and that strong physical stress induces a replacement of competitive species interactions by facilitative interactions (Leonard 2000). This was exemplified in our dataset by the positive correlations between algal biomass and functional faunal groups (and species richness), indicating that the moderation of physical stressors, such as extreme temperatures, desiccation or wave exposure by algae could improve the overall chances of success for some intertidal fauna species.
Whether the lack of predators is mainly an “island effect” or due to the harsh environment remains speculative. Nucella populations are abundant along South Icelandic shores (M. Blicher, pers. obs.), an area which could be an obvious source of predator colonization to Greenland. Coastal sea surface temperatures in Southern Iceland are above 0 °C during winter and are generally 4–8 °C above temperatures measured in this study along the coast of Cape Farewell (Hanna et al. 2006); this would suggest that sea surface temperature could be an important factor for colonization and proliferation of predators in the Greenlandic intertidal. Historical records stress this point by showing increasing abundance and a northward expansion of starfish in west Greenland, following an episodic inflow of warmer Atlantic water during the 1930s (Jensen 1949).
Species composition and total biomasses were related to water velocities which were about twice as high as velocities measured by the same method in Nova Scotia, Eastern Canada (Scrosati and Heaven 2007); this shows that Cape Farewell is one of the windiest locations on Earth (Moore et al. 2008). The impact of exposure on species composition was evident in the ordination, displaying a gradient with the most exposed sites towards the right (Fig. 4). The more exposed sites had a higher occurrence of unidentifiable Fucus individuals, and A. nodosum also responded strongly to exposure, being observed only at the two most sheltered sites (CF4 and CF5) at Cape Farewell but not at the sheltered Kitsissut sites. Thus, A. nodosum seems vulnerable to the oceanic swells at the Kitsissut islands, since locally generated wind exposure, as expressed by REI values, was smaller than REI at sites CF4 and CF5. The effect of swells was also observed in the Cape Farwell area where A. nodosum was scattered with biomasses <1,496 g m−2 at the sheltered coastal site (CF5), whereas it was dominant and habitat forming within the fjord system in the sheltered natural harbor of Aappilattoq (site CF4) which is unaffected by oceanic swell. The ordination displays segregation of areas along the second axis; this must be driven by a factor other than exposure, but it is not clear, based on the collected data, whether this segregation is caused by differences in the buildup of ice or other parameters.
Vertical zonation of macroalgae
The main structuring element in the intertidal community is the large brown algae belonging to the Fucales, and a distinct vertical zonation between different Fucales species in the intertidal is well-described for temperate areas (Schonbeck and Norton 1980; Karez and Chapman 1998). According to Pedersen (2011), four Fucales species prevail in Greenland; A. nodosum, Fucus distichus, F. evanescens and F. vesiculosus. The typical vertical zonation of these species in the Greenlandic littoral is F. vesiculosus in the upper part, A. nodosum in the middle part and F. evanescens in the lower littoral and upper subtidal (Rosenvinge 1893; Christensen 1981; Pedersen 2011), whereas F. distichus is typically found in rock pools both in Greenland and Norway (Rueness 1977; Pedersen 2011).
The height of each vegetation zone is largely determined by exposure to waves with an upward shift when exposure increases (Lüning 1990; Lobban and Harrison 1994). In the investigated region, this implies an upward shift in F. evanescens, leaving an increasingly narrow band of the upper littoral zone for F. vesiculosus which eventually disappears with increasing wave exposure. Such distributional patterns were observed at Kitsissut where F. evanescens tended to occur higher in the littoral zone when wave exposure increased, except at K3 where cracks and crevices sheltered a dense F. evanescens population relatively high in the intertidal zone. In the Cape Farewell area, F. vesiculosus disappeared at the coastal sites (CF1 and CF5) at the impact of oceanic swell. The zonation at the most protected in-fjord location (site CF4) displayed the classical zonation pattern, i.e. F. vesiculosus in the upper part, A. nodosum in the middle part and F. evanescens in the lower littoral. Even though the zonation pattern observed in the present study generally confirmed previous observations and general theoretical predictions, it also stressed that the zonation of the habitat-forming macroalgae cannot be predicted by REI index alone, but are also influenced by ocean swell, ice scouring and local physical parameters, such as roughness of the substratum.
Biomasses
There are few quantitative studies of biomasses in the Arctic intertidal. Konar et al. (2009) measured macroalgal biomasses in the MTL from latitudes of 20°N to 60°N and found the highest biomasses at high latitudes in Alaska. The biomasses range from about 800–4,800 g m−2 which are lower than the biomasses measured in the present study (range 0–35,000 g m−2). Comparable biomasses are found in Iceland with biomasses of up to 25,000 g m−2 in the MTL on a southern shore, whereas a more northwesterly site had biomasses of around 1,800 g m−2 (recalculated according to Espinosa and Guerra-Garcia 2005). In Greenland, there are few previous reports of littoral biomasses: In the Disko Bay area, biomasses range from 300 to 3,800 g m−2 (recalculated according to Hansen 1999), and average densities of A. nodosum are 19,000 g m−2 in Kobbefjord, close to Nuuk (Juul-Pedersen et al. 2013). The present data show that temperature, light and nutrient regime in subarctic intertidal habitats do not limit the buildup of biomass, provided that the shore is sufficiently sheltered in terms of waves and ice (Fig. 5). Consequently, exposure to waves and ice scouring seems to be the limiting element in the region.
The effect of wave exposure is well-described in the intertidal of temperate regions where the overall picture is one of gradual change from exposed shores dominated by sessile invertebrates to sheltered areas dominated by macroalgae (Little et al. 2009). In south Greenland, REI did not correlate to the standing stock in the dataset as a whole, but when sites within the two separate areas were ranked according to REI, a negative effect of wave exposure on total standing stock became evident (Fig. 5). The difference between areas seemed to reflect the effect of another exposure parameter besides the locally generated waves, namely oceanic swell that also exerts an important acceleration force, hampering the buildup of biomass. This was the case for the sites at Kitsissut where sampling was carried out in an embayment which, although sheltered from local waves (low REI values), was indeed affected by oceanic swell. Thus, the observed differences in standing stock between the Kitsissut sites reflect different exposure to swell rather than to local waves (REI), stressing the importance of different wave forces for structure of the intertidal community.
Temperature regime
The increasing trend in the 29 years of air temperature measurements from the region suggests changing physical conditions during the past three decades. This is the first quantitative study of the intertidal community in the region, and it is not clear whether the observed changes in climate have already impacted community structure. Changes in temperature of a similar magnitude are linked to changes in species composition of the rocky intertidal zones in other areas, e.g. California, Great Britain and Australia (Barry et al. 1995; Mieszkowska et al. 2006; Jones et al. 2009). In the Arctic, changes in seawater temperatures and ocean circulation have caused boreal crustaceans to advance into Arctic fjord basins, cold-water Arctic species to retreat and M. edulis to be reintroduced after 1000 years of absence (Berge et al. 2005; Beuchel et al. 2006; Weslawski et al. 2010; Węsławski et al. 2011).
Minimum air temperatures recorded in the MTL in this study border on the median lower lethal temperatures for M. edulis, L. saxatilis and S. balanoides from the British Isles which are estimated to −8.2, −16.4 and −14.6 °C, respectively (Davenport and Davenport 2005). Assuming that lethal values are representative for Greenlandic populations, we speculate that increasing temperatures could have caused a decrease in freezing-induced mortality of several intertidal species, potentially leading to increasing biomass and range expansion. Organisms in the eulittoral of South Greenland are exposed to extreme diurnal temperature variations which at the same time seem to differ between the three sites (Fig. 7). These differences are most likely due to the specific orientation of each logger to direct sunlight. The fluctuating temperatures during winter underline the important lack of insulating stable sea ice (Scrosati and Eckersley 2007) which is found in many regions of Greenland, but which did not build up in our study area except during a few days in March in the harbor of Aappilattoq, following a period of air temperatures below—13 °C (Fig. 7c, f). The eulittoral was therefore directly exposed to winter air temperatures and consequently subjected to the ongoing climatic changes in winter air temperatures (Fig. 8).
This work provides the first inshore water temperature recordings of seasonal sea surface temperatures in the region, revealing low temperatures with small amplitude compared to sea surface temperatures of southern Iceland (Hanna et al. 2006). Maximum temperatures also tended to be lower than open water observations (SST) in the more northerly Godthåbsfjord system in west Greenland (64°N) (Vesteegh et al. 2012). This is probably due to the close contact to the East Greenland Current and possibly also glacier runoff in the fjords.
Models based on sea surface temperatures predict a significant poleward expansion of F. vesiculosus, A. nodosum and Fucus serratus during the next century (Jueterbock et al. 2013). Whether or not the macroalgae can track the newly opened niches depends on both their dispersal potential and their adaptation abilities. F. vesiculosus and A. nodosum are already present in Greenland. These species bear air vesicles which allow flotation of thalli, and their northward migration is expected to not be limited by dispersal. A. nodosum is, however, known to have long generation times of 50–70 years (Olsen et al. 2010), and clearance experiments in Iceland show that it takes at least 8–10 years to build up A. nodosum climax communities in sheltered habitats (Ingolfsson and Hawkins 2008).
There are no reports on F. serratus from the Greenlandic intertidal even though present water temperatures should be suitable for the species in Southern Greenland (Jueterbock et al. 2013). The remoteness of Greenland could be a barrier, preventing F. serratus from extending its range in response to warming since the poorly-dispersing F. serratus lacks flotation vesicles. The species has, however, recently colonized shores of Iceland and the Faroe Islands through shipping activities (Coyer et al. 2006), and such activities also carry the potential of introducing this species in Greenland.
The predicted range expansion of intertidal macroalgae species towards the north (Müller et al. 2009; Jueterbock et al. 2013) will lead to a dramatic alternation in shore ecology, but the colonization success also depends on the adaptability of the species to high Arctic light conditions. Getting through the polar night requires storage of photosynthates and nutrients, and the occurrence of F. evanescens on Svalbard demonstrates that this species is able to physically adapt to short day lengths and the polar night (Svendsen 1959; Weslawski et al. 1997; Fredriksen and Kile 2012). Likewise, F. vesicolusus is found in the high Arctic in Upernavik (72°N) and Uumannaq (70°N) (M. Blicher pers.obs). These species, and possibly also other fucoids, have the physiological plasticity which could enable gain of habitable area towards the north as temperatures rise.
Conclusion
This quantitative survey documents the species composition of macroorganisms in the Southern Greenlandic MTL. This area is characterized by low species richness, as well as the absence of patellid limpets and potentially important predators, such as dogwhelks, crabs and starfish, illustrating the role of enhanced physical stress in reducing the importance of biological interactions in structuring species assembly. High standing stocks were observed at sheltered sites, showing that light, temperature and nutrient regimes in subarctic Greenland do not limit the buildup of biomass of the habitat forming macroalgae. The key structuring physical factors were wave exposure and ice scouring.
Measurements of air temperature in the area showed an increasing trend of 0.12–0.14 °C year−1 over the past 30 years. We speculate that especially increasing winter temperatures may result in decreased mortality in some intertidal species of SW Greenland, potentially leading to increasing biomass and range expansion. Baseline studies, such as the present, are urgently needed if we are to identify the response of the rocky intertidal zone to continued climatic changes in subarctic and Arctic regions.
References
Ballantine WJ (1961) A biologically defined exposure scale for the comparative description of rocky shores. Field Stud J 1:1–19
Barry JP, Baxter CH, Sagarin RD, Gilman SE (1995) Climate-related, long-term faunal changes in a California rocky intertidal community. Science 267:672–675
Bell EC, Denny MW (1994) Quantifying wave exposure—a simple device for recording maximum velocity and results of its use at several field sites. J Exp Mar Biol Ecol 181:9–29
Berge J, Johnsen G, Nilsen F, Gulliksen B, Slagstad D (2005) Ocean temperature oscillations enable reappearance of blue mussels Mytilus edulis in Svalbard after a 1000 year absence. Mar Ecol Prog Ser 303:167–175
Bertness MD, Gaines SD, Hay ME (2001) Marine community ecology. Sinauer Associates Inc, Massachusetts
Beuchel F, Gulliksen B, Carroll ML (2006) Long-term patterns of rocky bottom macrobenthic community structure in an Arctic fjord (Kongsfjorden, Svalbard) in relation to climate variability (1980–2003). J Marine Syst 63:35–48
Blanchette CA, Miner CM, Raimondi PT, Lohse D, Heady KEK, Broitman BR (2008) Biogeographical patterns of rocky intertidal communities along the Pacific coast of North America. J Biogeogr 35:1593–1607
Blicher ME, Sejr MK, Høgslund S (2013) Population structure of Mytilus edulis in the intertidal zone in a subarctic fjord, SW Greenland. Mar Ecol Prog Ser 487:89–100
Broitman BR, Mieszkowska N, Helmuth B, Blanchette CA (2008) Climate and recruitment of rocky shore intertidal invertebrates in the eastern north Atlantic. Ecology 89:81–90
Buch E (2002) Present oceanographic conditions in Greenland waters. Danish Meteorological Institute, Copenhagen
Christensen T (1981) Havbundens planter. In: Böcher TW, Nielsen CO, Schou A (eds) Danmarks Natur, Book 10. Politikens Forlag, Copenhagen, pp 184–192
Connell JH (1972) Community interactions on marine rocky intertidal shores. Annu Rev Ecol Sys 3:169–192
Coyer JA, Hoarau G, Skage M, Stam WT, Olsen JL (2006) Origin of Fucus serratus (Heterokontophyta; Fucaceae) populations in Iceland and the Faroes: a microsatellite-based assessment. Euro J Phycol 41:235–246
Davenport J, Davenport JL (2005) Effects of shore height, wave exposure and geographical distance on thermal niche width of intertidal fauna. Mar Ecol Prog Ser 292:41–50
Espinosa F, Guerra-Garcia JM (2005) Algae, macrofaunal assemblages and temperature: a quantitative approach to intertidal ecosystems of Iceland. Helgoland Mar Res 59:273–285
Florczyk I, Latala A (1989) The phytobenthos of the Hornsund fjord, SW Spitsbergen. Polar Res 7:29–41
Fonseca MS, Malhotra A (2010) WEMo: wave exposure model for use in ecological forecasting 4.0. Center for coastal fisheries and habitat research, North Carolina
Fonseca M, Whitfield PE, Kelly NM, Bell SS (2002) Modeling seagrass landscape pattern and associated ecological attributes. Ecol Appl 12:218–237
Fredriksen S, Kile MR (2012) The algal vegetation in the outer part of Isfjorden, Spitsbergen: revisiting Per Svendsen’s sites 50 years later. Polar Res. doi:10.3402/polar.v31i0.17538
Hanna E, Jonsson T, Olafsson J, Valdimarsson H (2006) Icelandic coastal sea surface temperature records constructed: putting the pulse on air-sea-climate interactions in the northern North Atlantic. Part I: Comparison with HadISST1 open-ocean surface temperatures and preliminary analysis of long-term patterns and anomalies of SSTs around Iceland. J Clim 19:5652–5666
Hansen L (1999) The intertidal macrofauna and macroalgae at five Arctic localities (Disko, West Greenland). In: Brandt A, Thomsen HA, Heide-Jørgensen MP, Kristensen RM, Ruhberg H (eds) The 1998 Daish-German excursion to Disko Islands. Alfred Wegener Institut für Polar und Meeresforschung, Bremerhaven, West Greenland, pp 92–109
Harley CDG (2011) Climate change, keystone predation, and biodiversity loss. Science 334:1124–1127
Hawkins SJ, Moore PJ, Burrows MT, Poloczanska E, Mieszkowska N, Herbert RJH, Jenkins SR, Thompson RC, Genner MJ, Southward AJ (2008) Complex interactions in a rapidly changing world: responses of rocky shore communities to recent climate change. Clim Res 37:123–133
Hawkins SJ, Sugden HE, Mieszkowska N, Moore PJ, Poloczanska E, Leaper R, Herbert RJH, Genner MJ, Moschella PS, Thompson RC, Jenkins SR, Southward AJ, Burrows MT (2009) Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Mar Ecol Prog Ser 396:245–259
Hayward PJ, Ryland JS (1995) Handbook of the Marine Fauna of North-West Europe. Oxford University Press, Oxford
Herbert RJH, Southward AJ, Sheader M, Hawkins SJ (2007) Influence of recruitment and temperature on distribution of intertidal barnacles in the English Channel. J Mar Biol Assoc UK 87:487–499
Ingolfsson A, Hawkins SJ (2008) Slow recovery from disturbance: a 20 year study of Ascophyllum canopy clearances. J Mar Biol Assoc UK 88:689–691
Jenkins SR, Moore P, Burrows MT, Garbary DJ, Hawkins SJ, Ingolfsson A, Sebens KP, Snelgrove PVR, Wethey DS, Woodin SA (2008) Comparative ecology of north Atlantic shores: do differences in players matter for process? Ecology 89:3–23
Jensen A (1949) Concerning a change of climate during recent decades in the Arctic and Subarctic regions, from Greenland in the west to Eurasia in the east, and contemporary biological and geophysical changes. Book 14 (8). Det Kgl. Danske Videnskabernes Selskab, Copenhagen
Johannesson K, Ekendahl A (2002) Selective predation favouring cryptic individuals of marine snails (Littorina). Biol J Linn Soc 76:137–144
Jones SJ, Mieszkowska N, Wethey DS (2009) Linking thermal tolerances and biogeography: mytilus edulis (L.) at its southern limit on the east coast of the United States. Biol Bull 217:73–85
Jones SJ, Lima FP, Wethey DS (2010) Rising environmental temperatures and biogeography: poleward range contraction of the blue mussel, Mytilus edulis L., in the western Atlantic. J Biogeogr 37:2243–2259
Jueterbock A, Tyberghein L, Verbruggen H, Coyer JA, Olsen JL, Hoarau G (2013) Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecol Evol 3:1356–1373
Juul-Pedersen T, Arendt EK, Mortensen J, Rysgaard S, Søgaard HD, Retzel A, Nygaard R, Burmeister AD, Sejr MK, Blicher ME, Krause-Jensen D, Marna N, Merzouk A, Labansen AL, Geertz-Hansen O, Boye T, Simon M (2013) Nuuk Basic, the marine basic programe 2010. In: Jensen LM, Rasch M (eds) Nuuk ecological research operations, 6th annual report, 2012. Aarhus University, DCE, Aarhus, pp 47–67
Karez R, Chapman ARO (1998) A competitive hierarchy model integrating roles of physiological competence and competitive ability does not provide a mechanistic explanation for the zonation of three intertidal Fucus species in Europe. Oikos 81:471–494
Keddy PA (1982) Quantifying within-lake gradients of wave energy—interrelationships of wave energy, substrate particle-size and shoreline plants in Axe Lake, Ontario. Aquat Bot 14:41–58
Konar B, Iken K, Edwards M (2009) Depth-stratified community zonation patterns on Gulf of Alaska rocky shores. Mar Ecol Evol Persp 30:63–73
Kordas RL, Harley CDG, O’Connor MI (2011) Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J Exp Mar Biol Ecol 400:218–226
Leonard GH (2000) Latitudinal variation in species interactions: a test in the New England rocky intertidal zone. Ecology 81:1015–1030
Little CG, Williams GA, Trowbridge CD (2009) The biology of rocky shores. Oxford University Press Inc., Oxford
Lobban CS, Harrison PJ (1994) Seaweed ecology and physiology. Cambridge University Press, Cambridge
Lüning K (1990) Seaweeds. Their environment, biogeography, and ecophysiology. Wiley, New York
Menge BA (1976) Organization of the New England rocky intertidal community: role of predation, competition, and environmental heterogeneity. Ecol Monogr 46:355–393
Mieszkowska N, Kendall MA, Hawkins SJ, Leaper R, Williamson P, Hardman-Mountford NJ, Southward AJ (2006) Changes in the range of some common rocky shore species in Britain—a response to climate change? Hydrobiologia 555:241–251
Mieszkowska N, Hawkins SJ, Burrows MT, Kendall MA (2007) Long-term changes in the geographic distribution and population structures of Osilinus lineatus (Gastropoda : Trochidae) in Britain and Ireland. J Mar Biol Assoc UK 87:537–545
Moore GWK, Pickart RS, Renfrew IA (2008) Buoy observations from the windiest location in the world ocean. Geophys Res Lett, Cape Farewell. doi:10.1029/2008GL034845
Müller R, Laepple T, Barsch I, Wiencke C (2009) Impact of oceanic warming on the distribution of seaweeds in polar and cold-temperate waters. Bot Mar 52:617–638
Oksanen J, Guillaume BF, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2013) Vegan: Community ecology package. R package version 2.0-7. http://CRAN.R-project.org/package=vegan. Accessed 18 Mar 2013
Olsen JL, Zechman FW, Hoarau G, Coyer JA, Stam WT, Valero M, Aberg P (2010) The phylogeographic architecture of the fucoid seaweed Ascophyllum nodosum: an intertidal ‘marine tree’ and survivor of more than one glacial-interglacial cycle. J Biogeogr 37:842–856
Paine RT (1994) Marine rocky shores and community ecology: an experimentalist’s perspective. Ecology Institute, Oldendorf/Luhe
Pedersen PM (2011) Grønlands havalger. Epsilon, Copenhagen
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/. Accessed 18 Mar 2013
Ribergaard MH (2013) Oceanographic investigations off west Greenland 2012. NAFO Scientific Council Documents, Copenhagen
Rosenvinge LKR (1893) Grønlands havalger. Meddel Grønland 3:765–981
Rueness J (1977) Norsk Algeflora. Universitetsforlaget, Oslo
Sagarin RD, Barry JP, Gilman SE, Baxter CH (1999) Climate-related change in an intertidal community over short and long time scales. Ecol Monogr 69:465–490
Schonbeck MW, Norton TA (1980) Factors controlling the lower limits of fucoid algae on the shore. J Exp Mar Biol Ecol 43:131–150
Scrosati R, Eckersley LK (2007) Thermal insulation of the intertidal zone by the ice foot. J Sea Res 58:331–334
Scrosati R, Heaven C (2007) Spatial trends in community richness, diversity, and evenness across rocky intertidal environmental stress gradients in eastern Canada. Mar Ecol Prog Series 342:1–14
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (2007) Climate change 2007: the physical science basis. Working Group I, contribution to the fourth assessment report of the IPCC Intergovernmental panel on climate change. Cambridge University Press, Cambridge
Svendsen P (1959) The algal vegetation of Spitsbergen. Norsk polarinst skr 116:1–51
Ugland KI, Gray JS, Ellingsen KE (2003) The species-accumulation curve and estimation of species richness. J Anim Ecol. doi:10.1046/j.1365-2656.2003.00748.x
Vesteegh EAA, Blicher ME, Mortensen J, Rysgaard S, Als TD, Wanamaker AD (2012) Oxygen isotope ratios in the shell of Mytilus edulis: archives of glacier meltwater in Greenland? Biogeosciences 9:5231–5241
Węsławski JM, Wiktor J Jr, Zajaczkowski M, Futsaeter G, Moe KA (1997) Vulnerability assessment of Svalbard intertidal zone for oil spills. Estuar Coast Shelf Sci 44:33–41
Węsławski JM, Wiktor J Jr, Kotwicki L (2010) Increase in biodiversity in the Arctic rocky littoral, Sorkappland, Svalbard, after 20 years of climate warming. Mar Biodiv 40:123–130
Węsławski JM, Kendall MA, Włodarska-Kowalczuk M, Iken K, Kędra M, Legezynska J, Sejr MK (2011) Climate change effects on Arctic fjord and coastal macrobenthic diversity—observations and predictions. Mar Biodiv 41:71–85
Wickham H (2007) Reshaping data with the reshape package. J Stat Softw 21:1–20
Wulff A, Iken K, Quartino ML, Al-Handal A, Wiencke C, Clayton MN (2009) Biodiversity, biogeography and zonation of marine benthic micro-and macroalgae in the Arctic and Antarctic. Bot Mar 52:491–507
Zacherl D, Gaines SD, Lonhart SI (2003) The limits to biogeographical distributions: insights from the northward range extension of the marine snail, Kelletia kelletii (Forbes, 1852). J Biogeogr 30:913–924
Zippay ML, Helmuth B (2012) Effects of temperature change on mussel, Mytilus. Integr Zool 7:312–327
Acknowledgments
We would like to thank Jens Deding, Poul Møller Pedersen and Tom Djurhuus for skillful assistance with fieldwork and logistics. We thank Palle Bo Nielsen from the Danish Meterological Institute (DMI) for providing tide model data. The study was funded by the Danish Environmental Protection Agency (grant no. 112-00115), the Bureau of Minerals and Petroleum, Greenland Government, the Greenland Climate Research Center, the Danish Ministry of the Environment (DANCEA), the Ministry of Education, Research and Nordic Cooperation (IIN), Aage V. Jensens Charity Foundation, and is a part of the Arctic Science Partnership (http://asp-net.org).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Høgslund, S., Sejr, M.K., Wiktor, J. et al. Intertidal community composition along rocky shores in South-west Greenland: a quantitative approach. Polar Biol 37, 1549–1561 (2014). https://doi.org/10.1007/s00300-014-1541-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1541-7