Abstract
A single colony of the non-native grass Poa pratensis L., which was introduced inadvertently to Cierva Point, Antarctic Peninsula, during the 1954–1955 season, was still present during a survey in February 2012, making it the longest surviving non-native vascular plant colony known in Antarctica. Since 1991, the grass cover has roughly tripled in size, with an annual increase in area of approximately 0.016 m2, and an estimated maximum radial growth rate of 1.43 cm y−1. However, it remains restricted to the original site of introduction and its immediate surroundings (c. 1 m2). Annual flowering of the plants occurred during the 2010/2011 and 2011/2012 seasons; however, there has been no seed production and only incomplete development of the sexual structures. Current environmental conditions, including low temperatures, may inhibit sexual reproduction. Lack of effective vegetative dispersal may be influenced by the low level of human activity at the site, which limits opportunities for human-mediated dispersal. Although P. pratensis has existed at Cierva Point for almost 60 years, it has not yet become invasive. Scenarios for the potential future development of the species in Antarctica and the associated negative impacts upon the native vegetation from competition are discussed in the context of regional climate change. Finally, we describe the environmental risk presented by P. pratensis and argue that this non-native species should be eradicated as soon as possible in accordance with the Protocol on Environmental Protection to the Antarctic Treaty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change in the Antarctic Peninsula region may present new threats to terrestrial ecosystems particularly by increasing the likelihood of successful establishment of non-native species (Convey et al. 2006; Hughes and Convey 2010). Once established, non-native species can become invasive and out-compete native organisms and spread beyond their original introduction site (Frenot et al. 2005; Olech and Chwedorzewska 2011; Molina-Montenegro et al. 2012). Introductions of non-native species, from a wide variety of biological groups, have been reported extensively within the sub-Antarctic islands and increasingly in Maritime Antarctica (Gremmen and Smith 1999; Frenot et al. 2005; Hughes and Worland 2010; Shaw et al. 2010). Their spatial distribution is correlated strongly with the location of human activities within the biome, with most of Antarctica’s non-native species reported from the western Antarctic Peninsula which also host almost 50 % of the continent’s research stations (Tin et al. 2009; Lee and Chown 2009; Hughes and Worland 2010; Hughes et al. 2010; Olech and Chwedorzewska 2011; Greenslade et al. 2012; Chown et al. 2012a; Molina-Montenegro et al. 2012). As climate warming continues within the Antarctic Peninsula region and further extends to the rest of Antarctica over the coming decades, biological invasions may present an increasing threat to terrestrial biodiversity and a major challenge for Antarctic conservation (Hughes and Convey 2010; Chown et al. 2012a, b; Hughes et al. 2013).
Non-native vascular plants in Antarctica
Non-native plants that are known to have established in the maritime Antarctica include Poa pratensis L. (commonly known as smooth meadow grass, Kentucky bluegrass or common meadow grass; Corte 1961) and Poa annua L. (annual meadow grass or annual bluegrass; Olech 1996; Chwedorzewska 2008; Olech and Chwedorzewska 2011; Molina-Montenegro et al. 2012), but recently, propagules and pollen of the rush Juncus bufonius L. var. bufonius have also been recorded (Cuba-Diaz et al. 2013). The Poaceae, in particular, contains several species that have become invasive both globally and in the sub-Antarctica (Shaw et al. 2010) and may present a particular threat to Antarctic ecosystems. P. pratensis was among the first recorded vascular plants introduced inadvertently to Antarctica that survived over-wintering, having tolerated conditions on Deception Island (South Shetland Islands) between 1931 and 1948 (Longton 1966). P. annua was also recorded at the whaling station on Deception Island and in separate events, was introduced later to Signy Island (South Orkney Islands) and Galindez Island (Argentine Islands, Antarctic Peninsula) (Smith 1996). All of these populations died out, with the Deception Island population being destroyed during a volcanic eruption in 1970. Currently, P. annua is the most widespread non-native vascular plant in Antarctica and presents the greatest threat to Antarctic terrestrial ecosystems, particularly on King George Island, South Shetland Islands, where P. annua has spread ca. 1.5 km from its site of introduction at Arctowski Station, since 1985/1986 (Olech 1996; Chwedorzewska 2008; Olech and Chwedorzewska 2011).
Transplantation experiments
With a few exceptions, the intentional introduction of non-native species and non-sterile soil to the Antarctic Treaty area is prohibited by the Protocol on Environmental Protection to the Antarctic Treaty (signed in 1991, entered into force 1998; Hughes and Convey 2010). However, during the 1930–1970s, scientist from several nations undertook transplantation experiments, predominantly in the Antarctic Peninsula region, South Shetland Islands and South Orkney Islands, to assess the ability of non-native plants to survive under Antarctic conditions (for a review see Smith 1996). In general, few of the non-native plants that were introduced intentionally survived longer than 2–3 years. Nevertheless, these transplantation experiments involved the importation of non-sterile soils (e.g., from the sub-Antarctic islands or Patagonia) that contained propagules, such as seeds, insect eggs and larvae, plant fragments, or even mature plants, which provided the opportunity for unintentional non-native species introductions (Smith 1996; Hughes and Worland 2010).
Transplantation experiments in 1954–1955 led to the inadvertent introduction to Cierva Point, Danco Coast, Antarctic Peninsula, of the only surviving colony of P. pratensis that is known currently in Antarctica (Corte 1961). Nothofagus antarctica (G. Forst.) Oerst. (Antarctic beech) and N. pumilio (Poepp. and Endl.) Krasser (lenga beech) trees of small size were transplanted, together with their original soil and associated plant species, from Tierra de Fuego to Cierva Point to assess their capacity for survival in Antarctica. The trees did not survive wintering. However, the accompanying grass became established within the original experimental plot and persists to the present day, representing the longest survival of a non-native plant in the Antarctic. After Corte’s report of 1961, the next available information on the colonization status of P. pratensis was from the summer of 1990–1991, when N. Scutari informed Smith (1996) that the grass was limited to a single circular colony of approximately 40 cm in diameter. In 1995, O. Benitez observed immature inflorescences and reported that the colony was still restricted to within the original experimental plot (Smith 1996).
In February 2012, we visited the P. pratensis colony on Cierva Point and evaluated the distribution and characteristics of the introduced grass population. Here, we present the results of this survey, describe the reproductive strategies available to the plant and discuss the potential future of P. pratensis in Antarctica.
Methods
Study site
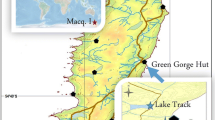
Cierva Point (Punta Cierva), Danco Coast, northwest Antarctic Peninsula (64º10′S, 60º57′W), is an ice-free area of ca. 3 km2 where the Argentine Base Primavera is located. The site contains several small rock outcrops with predominantly north-facing slopes, which produces climatic conditions favorable for plant growth (Mataloni et al. 1998). Closed vegetation is extensive between outcrops with extensive moss carpets and more open moss and lichen communities and also swards of the native grass Deschampsia antarctica Desv. Breeding colonies of several marine bird species are also present, including a gentoo penguin (Pygoscelis papua) colony. A single report exists of a non-native insect of the order Mecoptera (a snow scorpion fly; possibly a Boreus sp.) at the site, but its current colonization status and distribution are unknown (Convey and Quintana 1997).
Growth measurements
All research was performed in February 2012. To check the distribution of P. pratensis at the location an area of approximately 1 km2 around the original transplantation site was surveyed by five people over a 4-h period. Particular care was taken to differentiate between the indigenous grass Deschampsia antarctica and the non-native P. pratensis. No P. pratensis plants, other than the colony at the original introduction site, were found. The colony was geo-positioned and photographed. The extent of the colony’s perimeter was measured. The mean growth rate between 1991 and 2012 was calculated based upon the report of N. Scutari, contained within Smith (1996), which stated that the plant was restricted to a circle of c. 40 cm in diameter within the original plot surrounded by boulders. Samples of flowering grass plants from the current and dead plant material from the previous year were collected for later examination in the laboratory. Samples of the associated indigenous flora (mosses and phanerogams) were also collected for identification in the laboratory. Specimens were kept in herbaria at the Universidad Autónoma de Madrid and the British Antarctic Survey (international codes MAUAM and AAS, respectively).
Meteorological information
Meteorological data was obtained from the SCAR READER project, hosted by the British Antarctic Survey, and available at: http://www.antarctica.ac.uk/met/READER/data.html. Air temperature data were obtained from the Racer Rocks remote meteorological station (64°04′S, 61°36′W) located ca. 30 km northwest of Cierva Point. Like the P. pratensis introduction site on Cierva Point, the Racer Rocks meteorological station is located on the coast c. 17 m a.s.l. Air temperature data for the local area spanned over the last two decades (1989–2003) but was not continuous. Complete records of average monthly temperatures for all austral summer months (November–March) were available for only 3 years (1990–1991, 1991–1992 and 1999–2000). Additionally mean monthly temperature data exist for nine other years where one or more monthly record contains data with less than 90 % of the daily data available (i.e., summer seasons 1989–1990, 1992–1993 to 1998–1999 and 2000–01). Mean monthly temperatures for seasons 2001–2002 and 2002–2003 were not calculated due to the poor quality of the dataset. Due to their close proximity (30 km), temperature data from Racer Rocks may reflect temperature conditions similar to those on Cierva Point. Temperature records were updated with additional seasonal data for 2012–2013 obtained from a similar meteorological station installed recently at Cierva Point (J. Bockheim pers. comm.). The maximum monthly mean temperatures for each month of the summer season (November–March), recorded during the period 1989 and 2013, were used to indicate the upper temperature limit during this period.
Results
Current colony extent
The field survey located a single colony of P. pratensis at 64º09′20.9″S, 060º57′20.5″W (Fig. 1). The colony was growing in shallow soil over bedrock, situated ca. 20 m a.s.l. and ca. 25 m from the coast. The dense grass mat covered a roughly rectangular area of 0.53 m2, with sides of ca. 0.7 and 1.0 m (0.64 m2) with boulders within it (0.11 m2). The mat size described in Smith (1996) corresponded to an maximum area of 0.18 m2. After 21 years the grass mat has extended just beyond the boundary of the original plot, which was delimited by small boulders. Compared with the previous record, P. pratensis has extended its area of coverage by at least 0.35 m2 since 1991, with an estimated mean expansion of 0.016 m2 y−1. In places, where grass growth was not restricted by boulders, linear growth has reached up to ca. 30 cm in 21 years, giving a maximum mean linear growth rate of 1.43 cm y−1. Within this period, available temperature data from Racer Rocks during 1990–2001 showed a maximum monthly mean temperature in February 1990 of 2.2 °C (Fig. 4). Temperature data from Cierva Point meteorological station during the 2012–2013 season were below the maximum monthly mean records detected at the Racer Rocks station.
Plant status and associated species
Poa pratensis plants in the colony were healthy (Figs. 2, 3) and numerous shoots were flowering (Fig. 2d). Intermixed with the colony were two terricolous mosses, Sanionia uncinata (Müll and Hall) Ochyra and Hedenäs, and Syntrichia magellanica (Mont.) R.H. Zander. Both mosses tolerate a wide range of conditions and are common throughout the Antarctic Peninsula (Ochyra et al. 2008). Also within the plot were found small well-developed tussocks of Deschampsia antarctica (Fig. 2e) alongside lichen species (e.g., Usnea spp.).
Poa pratensis at Cierva Point. a The immediate vicinity of the introduced plant colony. b The original plot colonized by P. pratensis. Total extent of the colony in February 2012, with the extent of the colony in 1991 has shown (plant c. 40 cm in diameter). c Adjacent growth of P. pratensis and native plants beyond the limits of the original plot. d P. pratensis flowering
Poa pratensis materials from Cierva Point. a Specimens from a sheet kept at MAUAM Herbarium (label measured 12.1 × 5.2 cm), b detail of an inflorescence, c spikelet, d open spikelet, showing in the center the anthers of two stamens corresponding to the basal floret, e dissected floret, showing its three stamens; the anthers do not contain developed pollen grains; the pistil is aborted
Examination of P. pratensis spikes that had developed in the present growing season and spike-remnants from the previous growing season revealed incomplete development of the reproductive structures inside the spikes (Fig. 3). Each spike contained one to three flowers. The reproductive structures (both anthers and pistil) were under-developed or aborted, and had not formed mature organs (Fig. 3d, e). In consequence, fecundation was not possible and seeds were absent.
Discussion
Physiological performance
While many species newly introduced to Antarctica by either natural or anthropogenic means, may die out soon after arrival, our survey has shown that the non-native grass P. pratensis has survived 58 Antarctic winters at Cierva Point and, furthermore, in the last two decades the small population has started to expanded its area of colonization.
Some studies exist that help to explain the Antarctic winter hardiness of the P. pratensis. During physiological studies in the laboratory, Beard (1969) showed P. pratensis to be tolerant of low temperatures, and more so than other turf grasses including P. annua, while Gudleifsson et al. (1986) found that P. pratensis plants showed progressive tolerance both to cold hardiness in the laboratory and wintering stress in the field. While P. pratensis at Cierva Point is capable of tolerating Antarctic winter conditions the colony was not able to produce mature flowers in the austral summer growing season, and therefore, plants were unlikely to be capable of successful sexual reproduction. Environmental conditions, while not preventing vegetative growth, may not be suitable for the completion of sexual reproduction, which is supported by the report of immature inflorescences in the grass at Cierva Point in 1995 (Smith 1996) and by Corte (1961) at the end of the 1961 season, 6 years after its introduction. Studies have shown that P. pratensis has dual floral induction requirements to produce seeds: (1) a period of low temperatures (below 5 °C) and short days that stimulate tiller emergence and primary floral induction, which is followed by over-wintering and vernalization, and (2) a period of long days and temperatures above 10 °C to allow a second stage of tiller elongation and floral development (Heide et al. 1987; Holman and Thill 2005). Meteorological records for the general area showed a cold summer temperature regime with mean temperatures peaking in January–February at slightly over 0 °C (Fig. 4), which would prevent the second stage of floral development. The short length and low temperature of the summer season has been identified previously as the primary factor preventing the establishment of non-indigenous plants after reaching the Antarctic (Convey 1996). Even if viable seeds were produced, summer temperatures in the area, and throughout the western Antarctic Peninsula in general, are still far below the optimum for P. pratensis seed germination (c. 10–15 °C) (Tarasoff et al. 2007; Turner et al. 2009).
Mean monthly temperature records from Racer Rocks Station (64° 04′ S, 61° 36′ W) and Cierva Point station (64º10′S, 60º57′W). Summer seasons’ data (November–March), where values are available for all 5 months, are shown either by black rhombuses for Racer Rocks or by black squares for Cierva Point, while seasons where data are not available for all five summer months are shown by black slashes. For each month during the summer season, maximum monthly mean temperatures, recorded between 1989 and 2013, were used to indicate the upper temperature limit during this period
At Cierva Point the lateral vegetative growth of P. pratensis has roughly tripled the extent of the colony in a little more than two decades, albeit on a small spatial scale. Malyshev and Henry (2012) found a switch from reproductive to vegetative growth with increasing stress, induced, for example, by severe freezing conditions or scarcity of nutrients. In Antarctica both the general scarcity of nitrogen and severe winter conditions may be responsible for promoting vegetative growth during the austral summer, allowing it to survive in the prevailing conditions. Recent climatic warming in the Antarctic Peninsula may enhance growth and biomass production in both indigenous and non-native vascular plant species in the region (Convey et al. 2006; Day et al. 1999, 2008; Turner et al. 2009). However, the photosynthetic temperature optimum for Deschampsia antarctica from Anvers Island, Danco Coast, was c. 10 °C (Krna et al. 2009) while the value for P. pratensis was 22 °C (laboratory measurement; Abraham et al. 2008) indicating that any beneficial effects may not be equal across all vascular plant species.
Plant dispersion
Despite an increase in the size of the P. pratensis colony through vegetative growth, propagules (such as dispersed plant fragments) have not established successfully in the wider local area. The lack of effective dispersion we observed can be attributed to (1) the low occurrence of dispersion events and (2) propagules not being deposited in ground with the appropriate ecological conditions for establishment.
Dispersion events of P. pratensis propagules at Cierva Point are likely to be infrequent. Non-native plant species in the sub-Antarctic and Antarctic may be dispersed by anthropogenic and natural means (Frenot et al. 2005; Whinam et al. 2007; Chown et al. 2012a; Molina-Montenegro et al. 2012). The location of P. pratensis at Cierva Point on an isolated rocky peninsula with no vehicle traffic and limited foot access, suggests that the risk of further propagule dispersal by humans may be low. Also the production of propagules by fragmentation and their later dispersion may not be favored by the small size of the colony, and its location outside of the areas of frequent human and bird activity (Vera 2011; Parnikoza et al. 2012).
In the case of P. pratensis propagule dispersion at Cierva Point, the observed absence of establishments at new locations locally may be due to (1) the harsh weather conditions, although these may have been ameliorated by climate change (see below), (2) the soil characteristics in the area, which may be less fertile than the imported soil present at the introduction site, but are still allowing stoloniferous growth (shown by the colony expansion beyond the original site) and (3) the exclusion of propagules from favorable colonization sites by existing communities of native lichens, bryophytes and grasses (see next section).
The environmental barriers for colonization in Antarctica are likely to be progressively weakened with on-going climate change (Hughes and Convey 2010; Chown et al. 2012a, b). While it may be difficult to determine the effect of climate change on non-native plant distribution and abundance in Antarctica, several studies report increases in native plant distribution (Fowbert and Smith 1994; Smith 1994) and since the mid 1980s, the non-native P. annua at Arctowski Station has expanded its range substantially (Olech and Chwedorzewska 2011). Native Antarctic plants have also shown improved sexual performance under warmer conditions (Day et al. 1999), but failed to produce viable seeds during unfavorable growing seasons (Convey 1996, 2006) suggesting increasingly favorable conditions for general vascular plants growth, competition and dispersion in the future.
Competition effects
Non-native plants may compete with native organisms and, if they become invasive, may cause changes in biodiversity and native species abundance over a range of spatial scales (Frenot et al. 2005; Shaw et al. 2010). P. pratensis can also undergo vigorous vegetative reproduction from rhizomes, which can prevent other plants from establishing nearby (Holman and Thill 2005). Within the P. pratensis colony at Cierva Point, a single plant of D. antarctica was absorbed by P. pratensis expansion (Fig. 2b). Furthermore, the expansion of the P. pratensis colony into moss carpet, adjacent to the introduction site, showed that under the correct conditions, the grass can out-compete native cryptogam communities. Molina-Montenegro et al. (2012) showed that P. annua caused reduced biomass and photosynthetic performance in Antarctica’s two native vascular plants, the grass Deschampsia antarctica and the pearlwort Colobanthus quitensis, while Krna et al. (2009) showed that vegetative growth of D. antarctica was reduced when grown in close proximity to neighboring plants. Further expansion of P. pratensis may affect the establishment of other plant species (Bosy and Reader, 1995) and the colonization success of other non-native species (e.g., the possible Boreus sp., found on Cierva Point by Convey and Quintana 1997), as well as altering nutrient cycling and storage processes (Hendrickson and Lund 2010).
Management of non-native plants in Antarctica
Management of non-native plants within Antarctica has a patchy record. A single vascular plant, possibly Puccinellia svalbardensis Ronning (formerly identified as Poa trivialis L.), thought to have originated from the Arctic was found near a field hut near Syowa station without flowering and eradicated in 2007 (M. Tsujimoto, pers. comm). In January 2010, the dicotyledonous plants Nassauvia magellanica J.F. Gmel. and Gamochaeta nivalis Cabrera were reported at Whalers Bay, Deception Island (Smith and Richardson 2011). G. nivalis was washed away by a melt stream, but N. magellanica persisted in the area for 1 year after its discovery until subsequently eradicated (Hughes and Convey 2012). P. annua was also found recently near General Bernardo O’Higgins, Gabriel González Videla and Almirante Brown research stations on the Antarctic Peninsula (Molina-Montenegro et al. 2012). The grass has been eradicated from General Bernardo O’Higgins and Gabriel González Videla stations, but its status at Almirante Brown station is unknown. Lack of early action to remove the first specimens of P. annua from Arctowski Station in the mid-1980s has allowed the plant to spread over 1.5 km into ASPA 128 Western Shore of Admiralty Bay, King George Island (Chwedorzewska 2008). Management of non-native species depends upon (1) the availability of monitoring data which report the their presence and (2) their fast removal where feasible (Convey 2011; Hughes and Convey 2010). Thus, if effective control or eradication of a non-native plant is to be achieved, it is essential that management action is taken at the earliest opportunity, as recommended in the Antarctic Treaty Consultative Meeting (ATCM) Committee for Environment Protection (CEP) Non-native species manual (CEP 2011).
Given the long persistence of the P. pratensis at Cierva Point, continuing climate warming in the region and on-going human activity in the area, a major expansion in non-native grass distribution at the site is likely at some point and may have begun already. Wider expansion of P. pratensis may have negative consequences for local indigenous biological communities, and in particular, for those found within the adjacent ASPA 134 Cierva Point and offshore islands, Danco Coast, Antarctic Peninsula, which was designated primarily to protect well-developed vegetation (ATCM 2006). The CEP has discussed the issue of P. pratensis at Cierva Point, and it is hoped that an eradication of the colony will be attempted soon.
References
Abraham EM, Meyer WA, Bonos SA, Huang B (2008) Differential response of hybrid bluegrass and Kentucky bluegrass to drought and heat stress. Hort Science 43:2191–2195
Antarctic Treaty Consultative Meeting (2006) Management Plan for Antarctic Specially Protected Area No. 134 Cierva Cove, Danco Coast. Measure 1 Annex D, ATCM XXIX Edinburgh, 12–23 June
Beard JB (1969) Winter injury of turfgrasses. In: Proceedings of the First International Turfgrass Research Conference, Sports Turf Research Institute, Bingley Yorkshire, pp 226–246
Bosy JL, Reader RJ (1995) Mechanisms underlying the suppression of forb seedling emergence by grass (Poa pratensis) litter. Funct Ecol 9:635–639
Chown SL, Huiskes AHL, Gremmen NJM, Lee JE, Terauds A, Crosbie K, Frenot Y, Hughes KA, Imura S, Kiefer K, Lebouvier M, Raymond B, Tsujimoto M, Ware C, Van de Vijver B, Bergstrom DM (2012a) Continent-wide risk assessment for the establishment of non indigenous species in Antarctica. P Natl Acad Sci USA 109:4938–4943
Chown SL, Lee JE, Hughes KA, Barnes J, Barrett PJ, Bergstrom DM, Convey P, Cowan DA, Crosbie K, Dyer G, Frenot Y, Grant SM, Herr D, Kennicutt MC, Lamers M, Murray A, Possingham HP, Reid K, Riddle MJ, Ryan PG, Sanson L, Shaw JD, Sparrow MD, Summerhayes C, Terauds A, Wall DH (2012b) Challenges to the future conservation of the Antarctic. Science 337:158–159
Chwedorzewska KJ (2008) Poa annua L. in Antarctic: searching for the source of introduction. Polar Biol 31:263–268
Committee for Environmental Protection (2011) CEP Non-native Species Manual. Available at: http://www.ats.aq/documents/atcm34/ww/atcm34_ww004_e.pdf
Convey P (1996) Reproduction of Antarctic flowering plants. Antarct Sci 8:127–134
Convey P (2006) Antarctic climate change and its influence on terrestrial ecosystems. In: Bergstrom DM, Convey P, Huiskes AHL (eds) Trends in Antarctic Terrestrial and Limnetic Ecosystems: Antarctica as a Global Indicator. Springer, Dordrecht, pp 253–272
Convey P (2011) Antarctic terrestrial biodiversity in a changing world. Polar Biol 34:1629–1641
Convey P, Quintana RD (1997) The terrestrial arthropod fauna of Cierva Point SSSI, Danco Coast, Northern Antarctic Peninsula. Eur J Soil Biol 33:19–29
Convey P, Frenot Y, Gremmen N, Bergstrom DM (2006) Biological invasions. In: Bergstrom DM, Convey P, Huiskes AHL (eds) Trends in Antarctic Terrestrial and Limnetic Ecosystems: Antarctica as a Global Indicator. Springer, Dordrecht, pp 193–220
Corte A (1961) La primera fanerógama adventicia hallada en el continente antártico. Contribución del Instituto Antártico Argentino 62:3–16
Cuba-Diaz M, Troncoso JM, Cordero C, Finot VL, Rondanelli-Reyes M (2013) Juncus bufonius, a new non-native vascular plant in King George Island, South Shetland Islands. Antarct Sci 25:385–386
Day TA, Ruhland CT, Grobe CW, Xiong F (1999) Growth and reproduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia 119:24–35
Day TA, Ruhland CT, Xiong FS (2008) Warming increases aboveground biomass and C stocks in vascular-plant-dominated Antarctic tundra. Glob Change Biol 14:1827–1843
Fowbert JA, Smith RIL (1994) Rapid population increase in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arctic Alpine Res 26:290–296
Frenot Y, Chown SL, Whinam J, Selkirk P, Convey P, Kotnicki M, Bergstrom D (2005) Biological invasions in the Antarctic: extent, impacts and implications. Biol Rev 80:45–72
Greenslade P, Potapov M, Russell D, Convey P (2012) Global collembola on Deception Island. J Insect Sci 12:111. Available at: http://www.insectscience.org/12.111
Gremmen NJM, Smith RIL (1999) New records of alien vascular plants from Marion and Prince Edward Islands, sub-Antarctic. Polar Biol 21:401–409
Gudleifsson BE, Andrews CJ, Bjornsson H (1986) Cold hardiness and ice tolerance of pasture grasses grown and tested in controlled environments. Can J Plant Sci 66:601–608
Heide OM, Bush MG, Evans L (1987) Inhibitory and promotive effects of gibberellic acid on floral initiation and development in Poa pratensis and Bromus inermis. Physiol Plantarum 69:342–350
Hendrickson JR, Lund C (2010) Plant community and target species affect responses to restoration strategies. Rangeland Ecol Manag 63:435–442
Holman JD, Thill D (2005) Kentucky Bluegrass: growth, development and seed production. Univ Idaho Ext Bull 843:12
Hughes KA, Convey P (2010) The protection of Antarctic terrestrial ecosystems from inter- and intra-continental transfer of non-indigenous species by human activities: a review of current systems and practices. Global Environ Change 20:96–112
Hughes KA, Convey P (2012) Determining the native/non-native status of newly discovered terrestrial and freshwater species in Antarctica—current knowledge, methodology and management action. J Environ Manage 93:52–66
Hughes KA, Worland MR (2010) Spatial distribution, habitat preference and colonization status of two alien terrestrial invertebrate species in Antarctica. Antarct Sci 22:221–231
Hughes KA, Lee JE, Ware C, Kiefer K, Bergstrom DM (2010) Impact of anthropogenic transportation to Antarctica on alien seed viability. Polar Biol 33:1125–1130
Hughes KA, Worland MR, Thorne M, Convey P (2013) The non-native chironomid Eretmoptera murphyi in Antarctica: erosion of the barriers to invasion. Biol Invasions 15:269–281
Krna MA, Day TA, Ruhland CT (2009) Effects of neighboring plants on the growth and reproduction of Deschampsia antarctica in Antarctic tundra. Polar Biol 32:1487–1494
Lee JE, Chown SL (2009) Breaching the dispersal barrier to invasion: quantification and management. Ecol Appl 19:1944–1959
Longton RE (1966) Alien vascular plants on Deception Island, South Shetland Islands. Brit Antarct Surv B 9:55–60
Malyshev AV, Henry HA (2012) Frost damage and winter nitrogen uptake by the grass Poa pratensis L.: consequences for vegetative versus reproductive growth. Plant Ecol 213:1739–1747
Mataloni G, Tesolín G, Tell G (1998) Characterization of a small eutrophic Antarctic lake (Otero Lake, Cierva Point) on the basis of algal assemblages and water chemistry. Polar Biol 19:107–114
Molina-Montenegro MA, Carrasco-Urra F, Rodrigo C, Convey P, Valladares F, Gianoli E (2012) Occurrence of the non-native annual bluegrass on the Antarctic mainland and its negative effects on native plants. Conserv Biol 26:717–723
Ochyra R, Smith RIL, Bednarek-Ochyra H (2008) The illustrated moss flora of Antarctica. Cambridge University Press, Cambridge
Olech M (1996) Human impact on terrestrial ecosystems in west Antarctica. Proc NIPR Symp Polar Biol 9:299–306
Olech M, Chwedorzewska KJ (2011) The first appearance and establishment of an alien vascular plant in natural habitats on the forefield of a retreating glacier in Antarctica. Antarct Sci 23:153–154
Parnikoza I, Dykyy I, Ivanets V, Kozeretska I, Kunakh V, Rozhok A, Ochyra R, Convey P (2012) Use of Deschampsia antarctica for nest building by the kelp gull in the Argentine Islands area (maritime Antarctica) and its possible role in plant dispersal. Polar Biol 35:1753–1758
Shaw J, Spear D, Greve M, Chown SL (2010) Taxonomic homogenization and differentiation across Southern Ocean Islands differ among insects and vascular plants. J Biogeogr 37:217–228
Smith RIL (1994) Vascular plants as indicators of regional warming in Antarctica. Oecologia 99:322–328
Smith RIL (1996) Introduced plants in Antarctica: potential impacts and conservation issues. Conserv Biol 76:135–146
Smith RIL, Richardson M (2011) Fuegian plants in Antarctica: natural or anthropogenically assisted immigrants? Biol Invasions 13:1–5
Tarasoff CS, Ball DA, Mallory-Smith CA (2007) Extreme ionic and temperature effects on germination of weeping alkaligrass (Puccinellia distans), Nuttall’s Alkaligrass (Puccinellia nuttalliana) and Kentucky bluegrass (Poa pratensis). Weed Sci 55:305–310
Tin T, Fleming ZL, Hughes KA, Ainley DG, Convey P, Moreno CA, Pfeiffer S, Scott J, Snape I (2009) Impacts of local human activities on the Antarctic environment. Antarct Sci 21:3–33
Turner J, Bindschadler RA, Convey P, Di Prisco G, Fahrbach E, Gutt J, Hodgson DA, Mayewski PA, Summerhayes C (eds) (2009) Antarctic Climate Change and the Environment. Scientific Committee on Antarctic Research, Cambridge
Vera ML (2011) Colonization and demographic structure of Deschampsia antarctica and Colobanthus quitensis along an altitudinal gradient on Livingston Island, South Shetland Islands Antarctica. Polar Res 30:7146. doi:10.3402/polar.v30i0.7146
Whinam J, Chilcott N, Bergstrom DM (2007) Subantarctic hitchhikers: expeditioners as vectors for the introduction of alien organisms. Biol Conserv 121:207–219
Acknowledgments
This survey was part of MIDAH CTM2010-11013 project financed by the Spanish National Polar Programme. We thank the BIO Las Palmas crew for safe transport to the field site under poor weather conditions, the staff of the Argentine Base Primavera for their support and hospitality and Miguel Angel de Pablo and Antonio Molina for helping with the field survey. We also thank Steve Colwell and Magdalena Biszczuk for the provision of meteorological data and mapping support, respectively. James Bockheim is also thanked for providing temperature records from Cierva Point. Finally we thank the anonymous reviewers for their useful and thought-provoking comments. This paper is a contribution to the SCAR EBA (Evolution and Biodiversity in Antarctica) research programme and the British Antarctic Survey’s Polar Science for Planet Earth core programme EO-LTMS (Environment Office—Long-Term Monitoring and Survey).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pertierra, L.R., Lara, F., Benayas, J. et al. Poa pratensis L., current status of the longest-established non-native vascular plant in the Antarctic. Polar Biol 36, 1473–1481 (2013). https://doi.org/10.1007/s00300-013-1367-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-013-1367-8