Abstract
Information on the sequences of generations and reproductive states of salps in the Southern Ocean is essential for an improved understanding of salp population growth, although changes in distribution patterns of two species of salps, Salpa thompsoni and Ihlea racovitzai, in the Southern Ocean have the potential to alter the Southern Ocean ecosystem. We used stratified, quantitative sampling from the surface to 2,000 m depth with an RMT 8 net in January of 2005 and 2006 to determine the distribution and population structure of salps in the north of Lützow-Holm Bay, East Antarctica. Ihlea racovitzai occurred in both 2005 and 2006, but S. thompsoni was found only in 2005. Ihlea racovitzai occurred abundantly along the ice edge where Antarctic Winter Water was well developed, whereas S. thompsoni was more abundant at northern stations affected by warm Modified Circumpolar Deep Water. Small solitary stages of I. racovitzai dominated in 2005, but they had declined significantly by 2006. The S. thompsoni population was composed of small immature aggregates and mature solitary stages, suggesting that the solitary stages were reproducing. We did not find mature aggregate and immature solitary stages in the present study and thus suggested that S. thompsoni was unable to complete its life cycle in the north of Lützow-Holm Bay because of failure of sexual reproduction in the aggregate stage. The S. thompsoni population was therefore probably transported to our study area by advection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salps are ubiquitous species in the oceans, where they occur periodically in dense swarms in waters over continental shelves and slopes (Andersen 1998). Only two species are known to occur in the Southern Ocean, Salpa thompsoni and Ihlea racovitzai (Foxton 1966, 1971; Casareto and Nemoto 1986). Salpa thompsoni is the more abundant in the Southern Ocean overall, serving as a major herbivorous zooplankton (Voronina 1998). In the classic work of Foxton (1966), S. thompsoni is described as adapted to oceanic conditions of the Antarctic Circumpolar Current (ACC), and it is seldom found in the neritic area across the high Antarctic zone. However, recent studies have reported that the distribution of S. thompsoni is shifting southward as ocean warming proceeds (Chiba et al. 1999; Pakhomov et al. 2002; Atkinson et al. 2004). Salpa thompsoni has high reproductive ability (Daponte et al. 2001) and occasionally forms dense swarms that dominate macrozooplankton assemblages (e.g. Hosie 1994; Nishikawa et al. 1995; Dubischar and Bathmann 1997; Chiba et al. 1998; Hosie et al. 2000). Salp swarms may have negative effects on the reproduction and survival of larval Euphausia superba (Huntley et al. 1989; Loeb et al. 1997), and thus the southward shift of S. thompsoni has the potential to alter the Southern Ocean food web (Atkinson et al. 2004).
Unlike S. thompsoni, I. racovitzai occurs in the high Antarctic cold-water zone (Foxton 1971; Hunt et al. 2007; Pakhomov et al. 2011a). Basic biological knowledge on this species was provided by Foxton (1971). Subsequent literature has mainly focused on the (1) spatial distribution (Casareto and Nemoto 1986; Pakhomov 1994; Pakhomov et al. 1994; Nishikawa et al. 1995; Hunt et al. 2007; Ono et al. 2010; Pakhomov et al. 2011a) and (2) embryo and stolon development (Esnal and Daponte 1990; Daponte and Esnal 1994). Recent work has also demonstrated that the life cycle is basically similar to that of S. thompsoni (Pakhomov et al. 2011a). The paucity of information on I. racovitzai likely stems from the apparent rarity of this species in comparison with S. thompsoni, which has received more attention in recent decades (e.g. Huntley et al. 1989; Kawaguchi et al. 2004; Pakhomov et al. 2006; Ono et al. 2010; Pakhomov et al. 2011b). Nevertheless, there are reports that I. racovitzai occurs abundantly in some locations and times (Nishikawa et al. 1995; Pakhomov et al. 2011a).
Salps have a unique life cycle in which an aggregated sexual blastozooid generation (aggregate stage) alternates with a solitary asexual oozooid generation (solitary stage) (Godeaux et al. 1998). Hereafter, we refer to the aggregate and solitary stages as aggregates and solitaries, respectively. Detailed investigations of the sequences of generations and reproductive states are required for an improved understanding of salp population growth.
Previous studies have reported (1) the occurrence of S. thompsoni below 200 m and (2) differences in the frequencies of maturity stages by depth (Caldwell 1966; Foxton 1966; Casareto and Nemoto 1986; Ono et al. 2010; Pakhomov et al. 2011b). Hosie et al. (2000) recommended reevaluation of the status of S. thompsoni as a warm-water species since its distribution is shifting southward. However, Ono et al. (2010) demonstrated that the species does not complete its life cycle in waters south of the Southern Boundary of the Antarctic Circumpolar Current (SB) off Adélie Land, as also found in the Lazarev Sea by Pakhomov et al. (2011a). Hence, details of population structure are required to determine whether the species is self-sustaining in waters it has occupied during its southward distribution shift. However, most studies, other than those of Ono et al. (2010) and Pakhomov et al. (2011a), have focused on waters shallower than 200 m. Reproductive information on S. thompsoni at high southern latitudes is essential for projection of Southern Ocean ecosystem responses to upcoming climate changes.
Accordingly, we examined the spatial distributions and population structures of the salps I. racovitzai and S. thompsoni through a stratified, quantitative sampling program in waters from the surface to 2000 m depth in the north of Lützow-Holm Bay, East Antarctica, in the austral summers of 2005 and 2006.
Materials methods
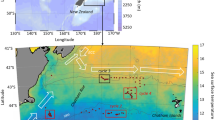
Samples were taken from the training and research vessel (TRV) Umitaka Maru of the Tokyo University of Marine Science and Technology (TUMSAT) from 6 to 13 January in 2005 and from 12 to 17 January 2006, in the northern portion of Lützow-Holm Bay (Fig. 1). The westward coastal current dominated during the present study; a cyclonic gyre formed by its interaction with Gunnerus Bank was located in the westernmost part (Fig. 1) (Hunt et al. 2007). Samples were collected using a rectangular midwater trawl (RMT) 1+8 equipped with three sets of open/close net system (Ocean Scientific International Ltd., Baker et al. 1973), which was towed obliquely in six different strata between the surface and 2,000 m (0–50, 50–100, 100–200 and 200–500, 500–1,000, 1,000–2,000 m; Table 1). The ship’s speed during the net tows was 1 m s−1. Samples were immediately preserved on board in buffered 5 % formalin-seawater solution. The filtered volume was calculated from the mouth area of the net and the flow meter counts. The sampling stations in 2006 were located about one degree north of those in 2005 because of delayed ice edge retreat in the second year (Fig. 1). This study only examined the samples from the RMT 8 (mouth area: 8 m2, mesh size: 4.5 mm), because the filtered water volumes were less reliable in the 1 m2 net, as the 330 μm mesh was often lodged with phytoplankton. Small individuals were probably under-sampled due to somewhat large mesh size used, although the finer meshes in the end of the net and cod end retained sufficient small individuals to permit review of length-frequency distribution.
Sampling and observation stations (L1–L12; see Table 1 for details) in the waters north of Lützow-Holm Bay, East Antarctica, in 2005 (left) and 2006 (right). Contours indicate bottom depths (m). Dashed lines denote the pack-ice edge roughly estimated from SeaWiFS satellite images
When the initial catch was large, subsamples were taken and divided into half- to one-eighth aliquots using a Motoda plankton splitter (Motoda 1959). Species and generation stages of salps were identified and counted. We followed the nomenclature of Casareto and Nemoto (1987), who suggested that S. thompsoni is a senior synonym of S. gerlachei. The abundance of each salp species (ind. 1,000 m−3) was calculated from the number of individuals and associated filtered volumes for each of the sampling layers. Horizontal distributions were compared by total abundances (ind. m−2) in the water column calculated from total individuals, volumes filtered, and maximum sampling depths at the stations.
Population structure data were assembled by examination of all individuals in either original samples or subsamples. Following Foxton (1966), we measured body length (BL; oral–atrial distance) to the nearest 1 mm using callipers. Nishikawa and Terazaki (1996) showed that BL of the salp Thalia democratica shrinks during the first ~5 months in preservative and becomes almost constant thereafter. Reinke (1987) reported that the BL of S. thompsoni aggregates shrinks up to 14.9 % of the original live length after 15 months of preservation. Our measurements were taken more than 6 months after sampling. There are no usable reports of BL shrinkage in S. thompsoni solitaries or any stage of I. racovitzai. Therefore, we assumed that BLs had shrunk to constant values in our samples and we did not include a shrinkage factor in our calculations. Maturity stages of S. thompsoni have been described in several previous reports (Foxton 1966; Casareto and Nemoto 1986; Chiba et al. 1999; Daponte et al. 2001). Following these earlier works, we determined the maturity stages of aggregates by the morphological characteristics of embryos inside the aggregate bodies. Individuals with empty or no embryos were classified into stage X following Chiba et al. (1999). The maturity stages of the solitaries described by Foxton (1966), Casareto and Nemoto (1986), and Daponte et al. (2001) were determined by the morphologies of the stolons. Stages 4 and spent of the aggregates and higher than stage 4a solitaries were classified as mature individuals following Casareto and Nemoto (1986). No description of maturity stages for I. racovitzai existed at the time of this study, and thus we measured only BL without classification into stages. Unidentified and/or damaged specimens of both species were excluded from the analyses of population structures. In the present study, they occupied 10.1 % in 2005 and 73.0 % in 2006 of total measured salps.
Vertical profiles of water temperature and salinity at each station were obtained by conductivity, temperature, depth (CTD, SBE911, Sea-Bird Electronics) casts except for at Stn. 05-L1 in 2005 and Stn. 06-L12 in 2006, when measurements were taken using an Integrated CTD (ICTD, Falmouth Scientific Inc.) and Smart CTD (Ocean Scientific Instrument Ltd.) equipped with an RMT controller, respectively. Water masses were classified using potential temperature (θ) and salinity based on the reports of Bindoff et al. (2000) and Tomczak and Liefrink (2005). Except for Stn. 06-L12 in 2006, seawater for measurement of chlorophyll a concentration (Chl a) was sampled with Niskin bottles at each station from 8 to 12 layers between the surface and 200 m; 200 mL of each water sample was filtered through a Whatman GF/F filter, and the filter was then soaked in 6 mL of N, N-dimethylformamide to extract chlorophyll a (Suzuki and Ishimaru 1990). The Chl a was then determined fluorometrically (Strickland and Parsons 1972) using a fluorometer (Turner Designs 10R).
Results
Environmental conditions
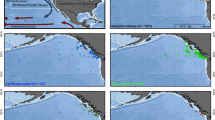
Antarctic Winter Water (WW: θ between −1.9 and −1.5 °C and salinity between 34.2 and 34.5; Tomczak and Liefrink 2005) occurred in the upper 200 m and was widely distributed near the ice edge (Stns. 05-L4, 05-L8, and 05-L12) in 2005 (Fig. 2). Modified Circumpolar Deep Water (MCDW: θ < 1.8 °C and salinity <34.7; Bindoff et al. 2000) occurred below the WW. The WW was covered by warm Summer Surface Water (SSW). The depth of water at 0 °C ranged from 78 to 230 m and was deeper at stations near the ice edge (Stns. 05-L4, 05-L8, and 05-L12). In 2006, the depth range of the WW was narrower than in 2005, and the depth of 0 °C water ranged from 76 to 105 m. Surface temperature was colder in 2006 than in 2005. Low-salinity water occurred at Stn. 06-L4. All stations were located far south of the SB (Hunt et al. 2007).
Vertical profiles of potential temperature (°C; upper panels) and salinity (lower panels) in the waters north of Lützow-Holm Bay, East Antarctica, in 2005 (left) and 2006 (right). See Table 1 for details of stations
In 2005, high Chl a distributed in the upper 100 m, and Chl a max occurred between 0 and 50 m (Fig. 3). Mean Chl a in the upper 200 m ranged between 0.44 and 1.05 μg L−1. In 2006, Chl a values were totally smaller than in 2005. Chl a max ranged 30–100 m (Fig. 3), and mean Chl a in the upper 200 m was lower (0.12–0.22 μg L−1) than in 2005.
Vertical profiles of chlorophyll a concentration (μg L−1) in the waters north of Lützow-Holm Bay, East Antarctica, in 2005 (left) and 2006 (right). See Table 1 for details of stations
Spatial distributions and population structure of I. racovitzai
Ihlea racovitzai was the numerically dominant salp in 2005 and 2006 (Table 2). In 2005, I. racovitzai occurred at all stations (Fig. 4). Abundances of this species (8.85–9.65 ind. m−2) near the ice edge, where the WW was well developed, were higher than those at Stns. 05-L1 and 05-L9 (1.52 and 4.26 ind. m−2, respectively). Solitaries comprised 94.6–100 % of the I. racovitzai populations (Table 2). Aggregates were found at Stns. 05-L4 (0.03 ind. m−2) and 05-L9 (0.23 ind. m−2). In 2006, I. racovitzai abundance was lower than in 2005 (Fig. 4; Table 2). Solitaries occurred across stations, whereas aggregates were only found at Stn. 06-L1 (Fig. 4).
In 2005, the BL of I. racovitzai solitaries ranged between 12 and 44 mm, with a peak at 23 mm; 98.5 % of all solitaries were small individuals (<30 mm) (Fig. 5). The aggregates had BL values between 7 and 10 mm (figure not shown). In 2006, the solitaries ranged from 22 to 37 mm BL (Fig. 5). No measurable aggregates were collected.
In 2005, I. racovitzai solitaries occurred between the surface and 2,000 m depth (Fig. 6). The solitaries mainly distributed in the upper 500 m depth and densely in the 100–200 m layer (mean ± SD: 14.31 ± 9.39 ind. 1,000 m−3). The aggregates occurred in the 0–50 and 100–200 m layers (0.09 ± 0.19 and 0.10 ± 0.16 ind. 1,000 m−3, respectively). In 2006, I. racovitzai occurred between the surface and 500 m depth (Fig. 6). The solitaries were mainly found in the 200–500 m layer (0.64 ± 0.40 ind. 1,000 m−3), while the aggregates were present only in the 0–50 m layer (0.04 ± 0.10 ind. 1,000 m−3). In the temperature–salinity diagram (Fig. 7), large abundances of I. racovitzai were recorded around the WW less than 0 °C in 2005, while plots in 2006 were located in the upper-right corner, the MCDW, and the surface waters characterized by low salinity.
Spatial distributions and population structures of S. thompsoni
In 2005, S. thompsoni occurred at four stations, but not at Stn. 05-L1 (Fig. 8). Abundances of S. thompsoni near the ice edge (1.6–3.7 ind. m−2) were lower than that at Stn. 05-L9, where warm SSW occurred (9.1 ind. m−2). Aggregates dominated throughout the survey area, with solitaries comprising just 2.2–7.9 % of total abundances (Table 2). In 2006, S. thompsoni was not collected in northern Lützow-Holm Bay.
In 2005, the BL of S. thompsoni aggregates ranged between 3 and 11 mm, with a single frequency peak at 7 mm (Fig. 9). The youngest-stage individuals (stage 0) predominated, accounting for 65.6 %, and stage X made up 8.6 % of all aggregates. Among solitaries, BL ranged from 46 to 83 mm, and maturity stages were all above stage 4a. Mature aggregates and immature solitaries were not collected.
We found S. thompsoni in all discrete depth layers between the surface and 2,000 m (Fig. 10), with larger abundances of both generations occurring in 0–50 and/or 100–500 m. In the temperature–salinity diagram (Fig. 7), S. thompsoni was widely distributed in the surface waters (0–50 m) characterized by low salinity (three plots on the left half) to the MCDW located in the upper-right corner, but larger abundance was found in the MCDW (Fig. 7). The aggregates occurred in the all sampling layers and densely distributed in the 100–200 m layer (mean ± SD: 4.41 ± 6.28 ind. 1,000 m−3). The solitaries occurred in the upper 500 m and abundantly in the 0–50 m layer (0.22 ± 0.23 ind. 1,000 m−3) (Fig. 10).
Discussion
Ihlea racovitzai
We rarely found I. racovitzai aggregates, in contrast to Casareto and Nemoto (1986) who found that aggregates were dominant in the upper 600 m of waters south of Australia during midsummer. Pakhomov et al. (2011a) collected rare aggregates in summer from the top 200 m in the Lazarev Sea, with numbers increasing in autumn. Our sampling protocol likely covered the depth range of I. racovitzai aggregates, and thus I. racovitzai aggregates may be not numerous in the waters north of Lützow-Holm Bay in the early summer, although we still need investigations over longer sampling period of time tha in the present study.
The diel vertical migration (DVM) of salps has been observed in the Southern Ocean (e. g. Foxton 1966, 1971; Casareto and Nemoto 1986; Nishikawa and Tsuda 2001), although we did not examine DVM in the present study because we conducted all samplings during daylight except for at Stns. 05-L4 and 05-L8 in 2005 (Toda et al. 2010). Furthermore, the sample sizes were too small to examine DVM. For I. racovitzai, Foxton (1971) reported this species mainly distributed in the top 100-m layer with absence in the deeper layers during summer. Casareto and Nemoto (1986) found a largely uniform vertical distribution down to 600 m between day and night during summer. However, Pakhomov et al. (2011a) reported a weak vertical movement during summer and a strong diel migration during fall. A seasonal data of DVM are necessary to understand the biology of I. racovitzai.
Ihlea racovitzai occurs preferentially in waters colder than 0 °C (Foxton 1971; Pakhomov et al. 1994; present study). Pakhomov et al. (2011a) reported that the species avoids areas with warmer water influence. We found I. racovitzai abundantly in 2005 near the ice edge, where the WW and low-temperature water (<0 °C) were widely distributed. In fact, the species was distributed vertically in and around the WW. However, in 2006, they seemed to avoid cold-water mass, being found in the MCDW, although abundance was very low. This distribution pattern in 2006 may be attributable to result of the DVM, because all samplings were made during daylight time in 2006.
According to Pakhomov et al. (2011a), I. racovitzai solitaries grow from 18 to 28 mm during summer, and the life expectancy is likely 1 year. Therefore, I. racovitzai abundance and population structure are affected by the survival rates of early recruits. Ihlea racovitzai reproduces sexually during fall, and young solitaries dominate during winter (Pakhomov et al. 2011a). The high frequency of small solitaries in 2005 was likely a function of high survival rates among new solitaries recruited in the preceding year. Conversely, the decline in young solitaries in 2006 suggests the failure of sexual reproduction by aggregates and/or high mortality of young solitaries during winter in northern Lützow-Holm Bay. Otherwise, they may be distributed further north of our study area due to delayed sea-ice retreat in 2006.
Salpa thompsoni
Salpa thompsoni occurs mainly in warm waters and is found north of the SB (e.g. Foxton 1966; Nicol et al. 2000; Pakhomov et al. 2006; Tanimura et al. 2008; Ono et al. 2010). However, in some cases, intrusions of warm-water masses transport the species to high latitudes where cold waters are predominant (Chiba et al. 1999; Ono et al. 2010; Pakhomov et al. 2011a). In 2005, warmer MCDW intruding in our study area may have been a pathway to the northern parts of Lützow-Holm Bay in the early summer.
We collected no mature aggregates or immature solitaries of this species in the north of Lützow-Holm Bay. Salps are protogynous hermaphrodites of which more advanced individuals become males (Godeaux et al. 1998). Accordingly, eggs in female aggregates require fertilization by sperm released into the water by male (advanced stage) aggregates. The occurrence of mature solitaries indicates that the asexual reproduction is activated. The S. thompsoni aggregates in the present study were composed of young immature individuals (stages 0–2), and young solitaries were absent, indicating that the aggregates likely did not produce new solitaries sexually in the north of Lützow-Holm Bay at least in the early summer. Generally, salps have a life cycle in which the sexual generation alternates with an asexual generation (Godeaux et al. 1998). Therefore, the life cycle of salps cannot be completed when aggregates and/or solitaries fail to reproduce. Salpa thompsoni overwinters as young solitary stages in deep waters, and they rise to shallow waters to grow and reproduce the new aggregates during the spring and summer (Foxton 1966). We did not examine the population structures of S. thompsoni in the fall and winter of 2005. However, if S. thompsoni reproduce new solitaries sexually in the north of Lützow-Holm Bay in 2006, S. thompsoni would be transported southward by advection only in summer, as indicated by Hunt et al. (2007). The population is likely not self-sustaining in the north of Lützow-Holm Bay, as suggested also south of the SB off Adélie Land (Ono et al. 2010).
Concluding notes
The cold-water species I. racovitzai occurs mainly in slope waters, although its abundance is highly variable among years (present study; Hunt et al. 2007). In contrast, S. thompsoni is a typical ACC species whose distribution is likely to be shifted southward by warm ACC intrusions during summer. We found S. thompsoni south of the SB, but suggested that the species was unable to complete its life cycle there, probably due to low temperatures. As indicated by recent studies (Hosie et al. 2000; Atkinson et al. 2004), the southward shift of the species should be monitored, especially since it has a competitive relation with E. superba. Monitoring will require more than simple presence/absence data since information on sexual performance (and hence population sustainability) can be obtained only from studies of population structure over longer periods of time than in the present study. The southward shift of S. thompsoni may influence not only the distribution and survival of E. superba, but also those of I. racovitzai (Perissinotto and Pakhomov 1998; Hunt et al. 2007) through competition for phytoplanktonic food items.
References
Andersen V (1998) Salp and pyrosomid blooms and their importance in biogeochemical cycles. In: Bone Q (ed) The biology of pelagic tunicates. Oxford University Press, Oxford, pp 125–138
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Baker AdeC, Clarke MR, Harris MJ (1973) The N. I. O. combination net (RMT 1+8) and further developments of rectangular midwater trawls. J Mar Biol Assoc UK 53:167–184
Bindoff NL, Rosenberg MA, Warner MJ (2000) On the circulation and water masses over the Antarctic continental slope and rise between 80 and 150°E. Deep Sea Res II 47:2299–2326
Caldwell MC (1966) The distribution of pelagic tunicates, family Salpidae in Antarctic and Subantarctic waters. Bull South Calif Acad Sci 65:1–16
Casareto BE, Nemoto T (1986) Salps of the Southern Ocean (Australian sector) during the 1983–84 summer, with special reference to the species Salpa thompsoni Foxton 1961. Mem Natl Inst Polar Res Spec Issue 40:221–239
Casareto BE, Nemoto T (1987) Latitudinal variation of the number of muscle fibres in Salpa thompsoni (Tunicata, Thaliacea) in the Southern Ocean: implications for the validity of the species Salpa gerlachei. Proc NIPR Symp Polar Biol 1:90–104
Chiba S, Horimoto N, Satoh R, Yamaguchi Y, Ishimaru T (1998) Macrozooplankton distribution around the Antarctic Divergence off Wilkes Land in 1996 austral summer, with reference to high abundance of Salpa thompsoni. Proc NIPR Symp Polar Biol 11:33–50
Chiba S, Ishimaru T, Hosie GW, Wright SW (1999) Population structure change of Salpa thompsoni from austral mid-summer to autumn. Polar Biol 22:341–349
Daponte MC, Esnal GB (1994) Differences in embryological development in two closely related species: Ihlea racovitzai and Ihlea magalhanica (Tunicata, Thaliacea). Polar Biol 14:455–458
Daponte MC, Capitanio FL, Esnal GB (2001) A mechanism for swarming in the tunicate Salpa thompsoni (Foxton, 1961). Antarct Sci 13:240–245
Dubischar CD, Bathmann UV (1997) Grazing impact of copepods and salps on phytoplankton in the Atlantic sector of the Southern Ocean. Deep Sea Res II 44:415–433
Esnal GB, Daponte MC (1990) Stolon development and some aspects of musculature in the characterization of Ihlea racovitzai (van Beneden, 1913) and Ihlea magalhanica (Apstein, 1894) (Tunicata, Thaliacea). Polar Biol 10:265–268
Foxton P (1966) The distribution and life-history of Salpa thompsoni Foxton with observations on a related species, Salpa gerlachei Foxton. Discov Rep 34:1–116
Foxton P (1971) On Ihlea magalhanica (Apstein) (Tunicata: Salpidae) and Ihlea racovitzai (van Beneden). Discov Rep 35:179–198
Godeaux J, Bone Q, Braconnot JC (1998) Anatomy of Thaliacea. In: Bone Q (ed) The biology of pelagic tunicates. Oxford University Press, Oxford, pp 1–24
Hosie GW (1994) The macrozooplankton communities in the Prydz Bay region, Antarctica. In: El-Sayed SZ (ed) Southern Ocean ecology: the BIOMASS perspective. Cambridge University Press, Cambridge, pp 93–123
Hosie GW, Schultz MB, Kitchener JA, Cochran TG, Richards K (2000) Macrozooplankton community structure off East Antarctica (80–150°E) during the austral summer of 1995/1996. Deep Sea Res II 47:2437–2463
Hunt BPV, Pakhomov EA, Trotsenko BG (2007) The macrozooplankton of the Cosmonaut Sea, east Antarctica (30°E–60°E), 1987–1990. Deep Sea Res I 54:1042–1069
Huntley ME, Sykes PF, Marin V (1989) Biometry and trophodynamics of Salpa thompsoni Foxton (Tunicata: Thaliacea) near the Antarctic Peninsula in austral summer, 1983–1984. Polar Biol 10:59–70
Kawaguchi S, Siegel V, Litvinov F, Loeb V, Watkins J (2004) Salp distribution and size composition in the Atlantic sector of the Southern Ocean. Deep Sea Res II 51:1369–1381
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900
Motoda S (1959) Devices of simple plankton apparatus. Mem Fac Fish Hokkaido Univ 7:73–94
Nicol S, Pauly T, Bindoff NL, Wright S, Thiele D, Hosie GW, Strutton PG, Woehler E (2000) Oceanic circulation off east Antarctica affects ecosystem structure and sea-ice extent. Nature 406:504–507
Nishikawa J, Terazaki M (1996) Tissue shrinkage of two gelatinous zooplankton, Thalia democratica and Dolioletta gegenbauri (Tunicata: Thaliacea) in preservative. Bull Plankton Soc Jpn 43:1–7
Nishikawa J, Tsuda A (2001) Diel vertical migration of the tunicate Salpa thompsoni in the Southern Ocean during summer. Polar Biol 24:299–302
Nishikawa J, Naganobu M, Ichii T, Ishii H, Terazaki M, Kawaguchi K (1995) Distribution of salps near the South Shetland Islands during austral summer, 1990–1991 with special reference to krill distribution. Polar Biol 15:31–39
Ono A, Ishimaru T, Tanaka Y (2010) Distribution and population structure of salps off Adelie Land in the Southern Ocean during austral summer, 2003 and 2005. La mer 48:55–70
Pakhomov EA (1994) Diurnal vertical migrations of Antarctic macroplankton: Salpidae, Ctenophora, Siphonophora, Chaetognatha, Polychaeta, Pteropoda. Oceanology 33:510–511
Pakhomov EA, Grachev DG, Trotsenko BG (1994) Distribution and composition of macroplankton communities in the Lazarev Sea (Antarctic). Oceanology 33:635–642
Pakhomov EA, Froneman PW, Perissinotto R (2002) Salp/krill interactions in the Southern Ocean: spatial segregation and implications for the carbon flux. Deep Sea Res II 49:1881–1907
Pakhomov EA, Dubischar CD, Strass V, Brichta M, Bathmann UV (2006) The tunicate Salpa thompsoni ecology in the Southern Ocean. I. Distribution, biomass, demography and feeding ecophysiology. Mar Biol 149:609–623
Pakhomov EA, Dubischar CD, Hunt BPV, Strass V, Cisewski B, Siegel V, von Harbou L, Gurney L, Kitchener J, Bathmann U (2011a) Biology and life cycles of pelagic tunicates in the Lazarev Sea, Southern Ocean. Deep Sea Res II 58:1677–1689
Pakhomov EA, Hall J, Williams MJM, Hunt BPV, Stevens CJ (2011b) Biology of Salpa thompsoni in waters adjacent to the Ross Sea, Southern Ocean, during austral summer 2008. Polar Biol 34:257–271
Perissinotto R, Pakhomov EA (1998) The trophic role of the tunicate Salpa thompsoni in the Antarctic marine ecosystem. J Mar Syst 17:361–374
Reinke M (1987) On the feeding locomotory physiology of Salpa thompsoni and Salpa fusiformis. Ber Polarforsch 36:1–89
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Bull Fish Res Board Can 167:1–310
Suzuki R, Ishimaru T (1990) An improved method for the determination of phytoplankton chlorophyll using N, N-dimethylformamide. J Oceanogr Soc Jpn 46:190–194
Tanimura A, Kawaguchi S, Oka N, Nishikawa J, Toczko S, Takahashi KT, Terazaki M, Odate T, Fukuchi M, Hosie G (2008) Abundance and grazing impacts of krill, salps and copepods along the 140°E meridian in the Southern Ocean during summer. Antarct Sci 20:365–379
Toda R, Moteki M, Ono A, Horimoto N, Tanaka Y, Ishimaru T (2010) Structure of the pelagic cnidarian community in Lützow-Holm Bay in the Indian sector of the Southern Ocean. Polar Sci 4:387–404
Tomczak M, Liefrink S (2005) Interannual variations of water mass volumes in the Southern Ocean. J Atmos Ocean Sci 10:31–42
Voronina NM (1998) Comparative abundance and distribution of major filter-feeders in the Antarctic pelagic zone. J Mar Syst 17:375–390
Acknowledgments
We thank the captain, officers, and crew members of the TRV Umitaka Maru and all of the on-board cadets in the Advanced Course for Marine Science and Technology for their cooperation in sample collection. We are grateful to Drs. T. Ishimaru, Y. Yamaguchi, Y. Tanaka, and N. Miyazaki (TUMSAT) for their collaboration and valuable comments. We also thank our scientific colleagues from the National Institute of Polar Research (NIPR), Hokkaido University, and other institutes for help with processing samples and with hydrographic ship-based observations. Mr. R. Toda (TUMSAT) kindly helped us with producing some of the figures and with sorting zooplankton in net samples. Dr. G. W. Hosie (Australian Antarctic Division) provided critical input on the early draft. The present study was supported primarily by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Nos. 14255102 and 19255014 to T. Ishimaru and 16101001 to M. Fukuchi of the NIPR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ono, A., Moteki, M. Spatial distributions and population dynamics of two salp species, Ihlea racovitzai and Salpa thompsoni, in the waters north of Lützow-Holm Bay (East Antarctica) during austral summers of 2005 and 2006. Polar Biol 36, 807–817 (2013). https://doi.org/10.1007/s00300-013-1305-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-013-1305-9