Abstract
We investigated current diets of the six most abundant benthic fish in the northern Bering Sea. Our objective was to explore feeding strategies and potential competition with other top predators as ecosystem changes occur in the northern Bering Sea ecosystem. Our approach used stomach content data collected from field sampling during spring 2006 and 2007. Calanoid copepods and ampeliscid amphipods were important prey of Arctic cod (Boreogadus saida) but in different proportions depending upon fish size, feeding location, and local environmental conditions. Snailfish (Liparidae) occupied a broad niche and fed on a variety of benthic amphipods. Arctic alligatorfish (Ulcina olrikii) and Arctic staghorn sculpin (Gymnocanthus tricuspis) consumed ampeliscid amphipods predominantly. Shorthorn sculpin (Myoxocephalus scorpius) had a less-diverse diet, with snow crab (Chionoecetes opilio) most important by weight. Finally, all Bering flounder (Hippoglossoides robustus) sampled had empty stomachs. Our results indicate that ampeliscid amphipods, which have high biomass in the central region of the northern Bering Sea, are the most important prey for the dominant groundfish in the Chirikov Basin. Generally, all dominant benthic fish in the northern Bering Sea had narrow feeding niches, except snailfish. High diet overlap was found among many of the fish species, including Arctic cod and snailfish, snailfish and Arctic alligatorfish, and Arctic alligatorfish and Arctic staghorn sculpin. These findings are consistent with a relatively short food chain for benthic fish that are for the most part specialized feeders with narrow preferences for food and may be affected by changes in benthic prey distributions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Bering Sea is one of the most productive marginal seas in the sub-Arctic. It includes commercial fisheries in the southeastern Bering Sea, and it is also an important foraging area for seabirds and mammals (Loughlin et al. 1999; Aydin and Mueter 2007). The northern portion of the Bering Sea shelf is seasonally covered by ice and is rich in benthic infauna supporting marine mammal, seabird, and fish predators. For example, gray whales (Eschrichtius robustus) are seasonally found in the Chirikov Basin (north of St. Lawrence Island, hereafter SLI), which they use as an important feeding ground (Moore et al. 2003). The entire world population of spectacled eiders (Somateria fischeri) also winters among leads in the pack ice south of SLI (Lovvorn et al. 2003) consuming clams on the sea floor. However, the Bering Sea is also undergoing a northward biogeographical shift that may be climate related (Overland and Stabeno 2004; Grebmeier et al. 2006; Bluhm and Gradinger 2008; Grebmeier 2012). Better knowledge of the fish community, including general trophic relationships in this area, is therefore important for understanding the potential ecological impact of climate change. This information may also inform future decision making about possible commercial exploitation of fish resources.
Near-freezing (<0 °C) bottom water that forms in the winter as a result of sea ice formation in the seasonal polynya south of SLI, hereafter referred to as cold pool water, is present throughout the year and limits the number of benthic fish (Wyllie-Echeverria and Wooster 1998; Sigler et al. 2011). Although cold bottom water temperatures limit the northward migration of fish populations, some fish species, such as Arctic cod (B. saida), Bering flounder (H. robustus), and snailfish (Liparidae) forage in this area and are an essential link in the food web (Cui et al. 2009). With the retreat of seasonal sea ice and depending upon the spatial extent of the cold pool south of SLI, commercial fish and sub-Arctic species in the southeastern Bering Sea are expected to expand northward and thus might affect food availability for top predators, including walrus (Odobenus rosmarus), bearded seals (Erignathus barbatus), belugas (Delphinapterus leucas), gray whales, and spectacled eiders (Tynan and DeMaster 1997; Grebmeier and Dunton 2000; Grebmeier et al. 2006). With earlier seasonal ice retreat, a more pelagic food web is expected instead of the current system that provides a high proportion of organic carbon directly to the benthos (Stabeno and Overland 2001). During years of light sea ice, several fish surveys have also shown an increase in the abundance of age-0 pollock, while simultaneously a decrease in large species of high-lipid crustacean zooplankton from the southeastern Bering Sea middle shelf (Hunt et al. 2011). In addition, several studies have found evidence for a decrease in benthic productivity in the northern Bering Sea over the last two decades (e.g., Moore et al. 2003; Grebmeier et al. 2006; Coyle et al. 2007; Grebmeier 2012). Reorganization of fish ranges in the Bering Sea could have significant ecological impacts upon predator–prey relationships if the available food resources change. Since species distributions also reflect interspecific interactions between prey and predator populations, these potential changes in predator–prey relationships can be relevant for understanding species distribution shifts that may be observed with climate change (Davis et al. 1998). We used these considerations in our study design with the intent of improving understanding of the trophic linkages among different organisms in the community and evaluation of foraging.
Despite the importance of potential ecological changes, studies of predator–prey relationships in groundfish are limited in the northern Bering Sea. In order to fill this gap and to better understand trophic relationships in this area, we undertook this study with the following objectives: (1) to examine the diet of dominant fish communities in the northern Bering Sea, (2) to identify prey strategies, find important prey and characterize niche width of these dominant fish species, and (3) to explore the potential competition for food resources.
Materials and methods
Fish sampling
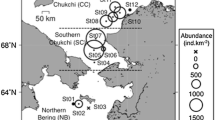
Fish were collected in the northern Bering Sea around SLI (Fig. 1) during two cruises on the USCGC Healy from May 7 to June 5, 2006 (hereafter referred to as HLY0601) and May 16 to June 18, 2007 (hereafter referred to as HLY0702). We sampled groundfish using both an otter trawl (4.3-m-long, 1.9-cm-stretched mesh, opening 3.43 m wide) and a beam trawl (4.3-m-long, 1.9-cm-stretched mesh, opening 4 m wide). Fish samples were caught at 43 stations (60 hauls) by otter trawl in 2006 and at total 52 stations (77 hauls) by both otter and beam trawls in 2007. We added a beam trawl in the second year for better quantitative analysis (for details see Cui et al. 2009). Depths of the sampled stations ranged from 35 to 96 m.
Diet analysis
Fish samples were returned to the shore-based laboratory frozen and were sorted and identified to species or to the lowest possible taxonomic level using published keys (Mecklenburg et al. 2002) and measured for total length (TL, ±1 mm). Prey found in fish stomachs were identified to the lowest possible taxonomic level, which varied with the digestion stage, using a dissecting microscope in the laboratory. In addition, the abundance and total wet mass of each recognizable organism was determined for each stomach. For further analysis, prey items were pooled into major prey categories based on taxonomic similarity. The abundance of the different prey items in the diet of the dominant demersal fish species was expressed as a numeric frequency (N i : percentage of the number of individuals of prey i in relation to the sum of all prey individuals in all the stomachs), frequency of occurrence (F i : percentage of stomachs which contained a particular prey i), weight frequency (W i : percentage of weight of prey i in total stomach content weight), and the index of relative importance adjusted to 100 % (% IRI = F i × (N i + W i )) (Cortés 1997).
We used the graphical approach of Amundsen et al. (1996) to explore feeding strategy, prey importance, and niche width for each fish species. This representational method is based on a two-dimensional plot of the prey-specific abundance (P i , which is the number of individuals of prey i divided by the total number of prey individuals within the stomachs containing prey i) against F i (Fig. 2). The new parameter, P i , is used instead of prey abundance since the abundance of prey in each stomach is standardized (i.e., to 100 %) in this methodology (Amundsen et al. 1996). Therefore, the average abundance of the stomach content is independent of the prey items consumed. We used the graphical representations of feeding strategies, prey importance, and niche width that were defined by Amundsen et al. (1996; Fig. 2). Prey in the upper part of the graph are consumed by specialized predators, while those located in the lower portion of the plot are consumed on a more general basis (generalists). The diagonal line from the lower left to upper right indicates prey importance for the whole population, prey plotting to the upper right are considered dominant prey, and if there are only a few data points (or even just one), it indicates a predator population with a narrow niche width. In contrast, if prey points are only located along or below the diagonal from the upper left to the lower right, the predator population is classified as having a broad niche width. Prey points occurring to the upper left of the diagram indicate individual predators that specialize on different prey items, while prey points occurring in the lower right region reflect those prey that are only been eaten occasionally (i.e., not dominant prey) by the majority of individuals.
Explanatory diagram for interpretation of feeding strategy, niche width contribution, and prey importance from prey information. BPC between-phenotype component; WPC within-phenotype component (from Amundsen et al. 1996)
In order to document the abundance of benthic invertebrates in relation to the prey collected in the fish stomachs, benthic invertebrate samples were collected throughout the study area by van Veen grab (0.1 m2) in 2006 (number of stations, n = 82). Four replicate samples were taken at each station, and the samples were washed over a 1-mm sieve, with retained animals preserved in 10 % buffered formalin, and later sorted and identified to family level in the laboratory.
Diet overlap (R o ) between size classes and species was evaluated using Schoener’s index: \( R_{o} = 1 - \frac{1}{2}\sum {\left| {p_{iA} - p_{iB} } \right|} \) (Linton et al. 1981), where P iA and P iB are the abundance of prey i in the diet of species A and B, respectively. Both N i (numeric frequency) and W i (weight frequency) were used to compute this index. The overlap index, R o , varies between 0 (no overlap) and 1 (complete overlap). Index values ≥0.6 are considered biologically significant (Zaret and Rand 1971; Warburton and Blaber 1992; Dolbeth et al. 2008). We used this overlap index as an indication of competition (Lawlor 1980), since a direct measurement of competition was outside the scope of this field study.
Statistical analyses
Differences in dietary composition among groups, such as fish species, sizes, locations, and years were analyzed using one-way analysis of similarity (ANOSIM). The analysis was conducted using a Bray–Curtis similarity matrix of biomasses with 999 permutations of standardized (100 %) stomach contents data that were square-root transformed. These pre-treatments are applied to decrease the influence of highly dominant prey in stomachs so that similarity calculations will be more sensitive to the occurrence of less common prey species. Among groups with significant differences in diet, a similarity percentages (SIMPER) analysis was conducted to further identify those prey items that contributed most of the dissimilarity. ANOSIM and SIMPER analysis were both performed with PRIMER software (v. 6, Plymouth Routines In Multivariate Ecological Research, Clarke and Warwick 2001).
Results
Dominant fish type and diet composition
Arctic cod, Bering flounder, snailfish, Arctic staghorn sculpin (G. tricuspis), shorthorn sculpin (M. scorpius), and Arctic alligatorfish (U. olrikii) were dominant groundfish sampled in the bottom trawl survey (Cui et al. 2009). Of the 297 Bering flounder stomachs examined, all of them were empty in both 2006 and 2007. All other fish species contained prey in their stomachs.
Arctic cod
In 2006, the total lengths (TL) of Arctic cod ranged between 80 and 90 mm (n = 4) and 100–220 mm (n = 180). All of the fish were treated as one group without respect to size. The dominant prey by weight were calanoid copepods (95.0 % N i , 42.5 % W i , 74.5 % F i , 74.0 % IRI) and amphipods (1.8 % N i , 42.9 % W i , 76.6 % F i , 24.7 % IRI), composed predominantly of benthic amphipods and some planktonic hyperiid amphipods (1.3 % W i ) (Table 1; Fig. 3). The most common amphipods were from the benthic family Ampeliscidae (12.5 % W i ). There were geographical differences in prey. Arctic cod from south of SLI (n = 147) preyed mainly on calanoid copepods (57.1 % W i ) followed by benthic amphipods (24.7 % W i ) and euphausiids (7.3 % W i ), while those from north of SLI (n = 23) consumed almost exclusively benthic amphipods (93.0 % W i ), which were primarily from the family Ampeliscidae in 2006 (Fig. 4). Sixty-five percent of Arctic cod caught from south of SLI consumed both amphipods and copepods, with only 25 % feeding on copepods but no amphipods. By comparison, 86 % of Arctic cod collected from north of SLI consumed amphipods but no copepods.
In 2007, we grouped Arctic cod into two groups based upon size (small: TL = 70–110 mm, n = 20 and large: TL = 140–220 mm, n = 13) rather than geographically as in 2006 (south and north of SLI) because of sample size constraints. Small Arctic cod mainly consumed calanoid copepods (94.2 % N i , 42.3 % W i , 55.0 % F i , 75.4 % IRI), followed by euphausiids (40.2 % W i ) and oedicerotid amphipods (15.7 % W i , Fig. 4). Large Arctic cod consumed amphipods (53.3 % W i ) mainly including Ampeliscidae (15.3 % W i , benthic), Hyperiidae (9.2 % W i , pelagic), and Lysianassidae (6.4 % W i , benthic). Large Arctic cod also consumed fish (30.2 % W i ) which was only 2 % of total number of prey, tended to bias results based on prey weight. By weight, euphausiids were also secondarily important (12.7 % W i ) behind amphipods and fish.
Using ANOSIM, the diet composition by biomass in Arctic cod was significantly different between years (2006 vs. 2007, R = 0.268, p = 0.001), between regions (south vs. north of SLI for two groups in 2006, R = 0.512, p = 0.001), and by fish size (small vs. large within the two size class groups in 2007, R = 0.35, p = 0.001). SIMPER analysis showed that differences in the biomass of copepods (29, 33, and 23 % dissimilarity, respectively) explain most of the differences. Euphausiids (19 and 21 % of dissimilarity, respectively) contributed differences secondarily between 2006 and 2007, and between the two size class groups of Arctic cod. Ampeliscid amphipods contributed secondarily to the difference between groupings south and north of SLI with 21 % dissimilarity.
Snailfish
In snailfish stomachs examined in 2006 (n = 44), benthic amphipods were the most important food (88.8 % N i , 75.0 % W i , 100.0 % F i , 91.6 % IRI) (Table 1; Fig. 3); most common by weight were the families Ampeliscidae (24.4 % W i ), followed by Lysianassidae (16.2 % W i ) and Melitidae (10.8 % W i ). In the 144 stomachs sampled in 2007, benthic amphipods again were dominant (87.9 % N i , 86.6 % W i , 94.4 % F i , 98.5 % IRI). Families Lysianassidae (40.5 % W i ), Melitidae (13.2 % W i ), and Ampeliscidae (11.1 % W i ) were most common by weight (Table 2; Fig. 3). There was no significant difference in prey composition by biomass (R = 0.064, p = 0.052) between the 2 years using ANOSIM.
Arctic staghorn sculpin
In Arctic staghorn sculpin stomachs examined in 2006 (n = 76), benthic amphipods were the most important prey (22.0 % N i , 92.5 % W i , 100 % F i , 91.5 % IRI), and the family Ampeliscidae (51.6 % W i ) was most common by weight (Table 1; Fig. 3). Polychaetes (31.3 % W i ) were secondarily important prey by biomass, followed by significant numbers of bivalve siphons (12.8 % N i ). Small sample sizes either in 2006 or 2007 precluded a similar analysis of food preferences for some fish species.
Shorthorn sculpin
In shorthorn sculpin stomachs examined in 2007 (n = 42), crabs (14.3 % N i , 69.1 % W i , 81.0 % F i , 41.0 % IRI), benthic amphipods (46.5 % N i , 28.4 % W i , 83.3 % F i , 37.8 % IRI), and polychaetes (21.0 % N i , 24.0 % W i , 71.4 % F i , 19.5 % IRI) were the most common prey categories (Table 2; Fig. 3). Snow crab (C. opilio) (61.5 % W i ) was the dominant prey species within the crab category; Melitidae (3.7 % W i ) was the most common prey within the amphipod families; Ampharetidae (23.9 % W i ) was the most common polychaete family consumed by weight and in some specimens was consumed in large numbers (Fig. 5).
Photos of shorthorn sculpin prey in two stomachs. Above: Total length (TL) of sculpin = 352 mm, station: RUS2, Trawl: beam, included 50 ampharetid polychaetes, one shrimp; Below: TL of sculpin = 352 mm, station: KIV3, trawl: beam, included two C. opilio, one Hyas coarctatus, and four ampharetid polychaetes
Arctic alligatorfish
Most of the stomachs examined were either empty or filled with small amounts of sand in 2006 and 2007. In six stomachs examined in 2007, benthic amphipods (82.8 % N i , 83.3 % W i , 100 % F i , 95.4 % IRI) were the most important prey, with Ampeliscidae (44.1 % W i ) the most common amphipod family (Table 2; Fig. 3). Arctic alligatorfish also consumed large numbers of bivalve siphons (13.8 % N i ), and crabs (16.6 %) by weight.
Feeding strategies
Prey importance and feeding strategy (e.g., generalists vs. specialists) were obtained by evaluating diagrams of prey-specific abundance (P i ) against the frequency of occurrence (F i ) (Fig. 6). All of the fish species studied generally had narrow niche widths, except snailfish and large Arctic cod. These evaluations indicated that calanoid copepods were important prey for Arctic cod south of SLI. All prey types of Arctic cod north of SLI plotted in the upper part of the diagram, which indicates that Arctic cod in this area are specialized feeders, with one prey point (ampeliscid amphipods) located toward the upper right corner, indicating a narrow feeding niche. Where size differentiation was possible in 2007, small Arctic cod show dominant prey points associated with calanoid copepods positioned toward the upper right of the plot, although a few individual Arctic cods occasionally consumed small amounts of other prey. The large Arctic cod class specialized on relatively few prey items such as Lysianassid amphipods, ampeliscid amphipods, and calanoid copepods at the individual level. Prey points of snailfish were skewed toward the lower half of the plot, and only two prey species were above 50 % prey-specific abundance in 2006 and 2007. Snailfish are generalists and do not have any specific dominant prey, implying a wide niche width. On the other hand, Arctic alligatorfish and Arctic staghorn sculpin specialized on a single dominant prey—ampeliscid amphipods. Shorthorn sculpin specialized on melitid amphipods in some individuals and ampharetid polychaetes more generally, with opportunistic feeding on snow crabs. The prey point representing snow crabs is located in the lower right part of the diagram because the relatively high mass of crabs were low in abundance in the diet (see Table 2; Fig. 6). Thus, any evaluation that considers biomass instead of abundance would move this conceptual prey point upwards, and from this standpoint, snow crabs were dominant prey for shorthorn sculpin.
Diet overlap
Diet overlap for all combinations of fish species are presented in Table 3. The prey of Arctic cod (grouped together) in 2006 significantly overlapped with prey of small-sized Arctic cod in 2007 by both abundance (R o = 1.0) and biomass (R o = 0.6). It should be pointed out that smaller prey, such as copepods that contribute relatively little to overall food biomass, bias the overlap index by abundance, in contrast to larger prey items such as crabs or fish. Snailfish showed low overlap with other species by abundance in both years, but snailfish in 2006 had high dietary overlap by biomass with Arctic cod in 2006, large Arctic cod in 2007, snailfish in 2007, and Arctic alligatorfish in 2007 (all ≥0.6). Arctic staghorn sculpin had high dietary overlap with Arctic alligatorfish in both prey abundance and biomass (both ≥0.6). Shorthorn sculpin did not demonstrate biologically significant dietary overlap with the other species studied.
Discussion
Arctic cod
We observed that benthic amphipods, especially benthic ampeliscid amphipods and pelagic calanoid copepods were the most common prey of Arctic cod but in different proportions depending upon fish size, geographical occurrence, and local environmental conditions. Lowry and Frost (1981) reported that Arctic cod fed predominantly on the benthos in the northern Bering Sea including ampeliscid amphipods, shrimps, and mysids (sampled 27 May–10 June 1978). By comparison, calanoid copepods and pelagic amphipods are the predominant prey of Arctic cod sampled in offshore waters of the Beaufort and Chukchi Seas both near the surface and underneath the ice in winter (mean size ~92 mm, Craig et al. 1982) and from the bottom waters in summer (Lowry and Frost 1981; Coyle et al. 1997). In our study in the northern Bering Sea, small Arctic cod mainly fed on calanoid copepods, and large Arctic cod primarily fed on benthic amphipods in ice-free areas or under low-ice conditions. This finding may be related to size limitations of younger Arctic cod preying on benthic amphipods relative to small copepods, or a lower capability for younger Arctic cod to migrate vertically as adult predators can. From our graphical analyses, small Arctic cod were identified as specialized feeders occupying a narrow niche, while large Arctic cod occupied a broader niche. This finding is consistent with those of Woodward and Hildrew (2002), who pointed out that small predators tend to have narrower diets since they are limited to feeding on a sub-set of the total prey size spectrum of larger predators. Many other studies have also reported that small Arctic cod (<100 mm) eat mainly copepods, with pelagic amphipods increasing in importance as prey for large Arctic cod (>100 mm, Bohn and McElroy 1976; Bain and Sekerak 1978; Hop et al. 1997). Arctic cod are thought to be associated with ice for protection from predators and for feeding habitat (Crawford and Jorgenson 1993; Hop et al. 1997; Gradinger and Bluhm 2004). This species also obtains significant energy from primary consumers that feed on ice algal blooms (Lonne and Gulliksen 1989). In our sampling, under heavier ice observed in 2006 (Cui et al. 2009), Arctic cod consumed more calanoid copepods compared with those sampled in 2007 under reduced sea ice conditions. Arctic cod also fed differently south (pelagic feeding) and north (primarily benthic feeding) of SLI. This finding likely occurred because the dominant prey of Arctic cod, such as, benthic ampeliscid amphipods have high values of biomass at some locations north of SLI (>200 g wet wt m−2, n = 4, Fig. 7; also see ampeliscid data presented in Fig. 1 and Table 4 in Heide-Jørgensen et al. 2012), while the total biomass of all benthic amphipods is much lower to the south of SLI (<20 g wet wt m−2, Fig. 7). Similarly, bivalves and polychaetes that are not typical prey for Arctic cod are the predominant benthic invertebrates in the area offshore south of SLI as well as the Chukchi Sea (see Grebmeier 2012). As pointed out earlier, these regions in the Pacific Arctic are where Arctic cod are considered primarily to be pelagic feeders. Our study indicates that there are continental shelf locations when large Arctic cod prefer benthic amphipods rather than copepods, perhaps due to a prey preference for high-lipid content ampeliscid amphipods (Highsmith and Coyle 1990) when available.
Other species
In our study, the main prey of Arctic staghorn sculpin where they primarily occur north of SLI (Cui et al. 2009) were ampeliscid amphipods (Fig. 7), which again are the most abundant benthic invertebrates in that area. By comparison, in the northeastern Chukchi Sea in summer (August–September), these fish have been observed to consume polychaetes observed in our study area (Ampharetidae, Flabelligeridae, Nephtys sp., Opheliidae and Pectinaria sp.) or euphausiids (Thysanoessa sp.) (Coyle et al. 1997).
Although Bering flounder in our study were not found to have any food in their stomachs, fish caught in summer in the northeastern Chukchi Sea contained fish (mainly Lumpenus sp.) and crustaceans (Byblis spp. shrimps, crabs) (Coyle et al. 1997). In that same study, stomach emptiness was more prevalent in Bering flounder (25 %) than in other demersal fish (0–7 %). Another factor influencing our findings may be the presence of the persistent cold pool (−1.8 to −1.4 °C) of bottom water south of SLI where the Bering flounder were primarily collected during our sampling (Cui et al. 2009). These near-freezing temperatures on the bottom might seasonally depress feeding for species such as Bering flounder, although the other species we collected were feeding. Other work has found fish consume less prey or have more empty stomachs in winter than summer (e.g., Woodward and Hildrew 2002), thereby potentially supporting the hypothesis that low temperatures suppressed feeding in Bering flounder. Starving fish are usually associated with larval stages (O’Connell 1980), but the Bering flounder in our study were mainly over 200 mm in size with a few juveniles (<50 mm). The reasons for the number of Bering flounder without stomach food contents are not clear although starvation can cause significant mortality in juveniles and recruits during overwintering (review by Sogard 1997).
Potential competition for food resources
Arctic cod and snailfish share the same habitat southwest of SLI (Cui et al. 2009). These fish had high prey overlap by biomass in both years of sampling on their most common prey, benthic amphipods, such as Ampeliscidae and Melitidae. This finding suggests potential competition between those two fishes for similar food resources (Fig. 7). Snailfish, which usually have a sucking disk modified from pelvic fins, are morphologically adapted to live on the bottom and are strict obligate benthic feeders. Snailfish in our study area prey upon a diverse set of benthic amphipods and have no particularly important food category. Based upon the assessment of feeding strategy using the modified Costello method (Amundsen et al. 1996), snailfish are opportunistic feeders that will consume any prey of an appropriate size that is encountered. Arctic cod, in contrast, forage in the water column and feed predominantly on pelagic copepods south of SLI, where those two fish species co-occur. Thus, even with a high degree of similar potential prey, Arctic cod and snailfish are not likely to directly compete for prey.
Arctic staghorn sculpin and Arctic alligatorfish, both coexist north of SLI (Cui et al. 2009) and have significantly high prey overlap by both prey biomass and abundance (R 0 > 0.6). The two fish species share the same dominant prey, ampeliscid amphipods, and both occupy narrow niches. However, the relatively high biomass of ampeliscid amphipods (50–200 g wet wt m−2, Fig. 7, Heide-Jørgensen et al. 2012) in the same area where these two fish species are most common indicates that food limitation may not be an issue.
Similarly, Arctic staghorn sculpin and shorthorn sculpin generally occur north of SLI (Cui et al. 2009) with high overlap of prey by abundance. The most common shared prey item for these fishes, ampharetid polychaetes (almost 25 % of prey by weight), are also very high in biomass (538 g wet wt m−2) at station, RUS2 north of SLI (Fig. 7). Thus, competition between those two predators on common resources may actually be low. Wiens (1993) postulated that such high feeding overlap may just reflect opportunistic use of resources in areas of high prey abundance.
North of SLI, gray whales also feed on ampeliscid amphipods (Moore et al. 2003 and references cited therein). Moreover, ampeliscid amphipods in the Chirikov Basin have been decreasing in abundance and biomass (Moore et al. 2003; Coyle et al. 2007), as well as in spatial extent in recent years (Grebmeier Unpublished data, see figure DBO2 at http://www.arctic.noaa.gov/dbo/related_ts.html). One hypothesis that has been advanced is that top-down control by foraging whales is the primary reason for the observed amphipods decline, as these marine mammals consume the larger size fractions (e.g., 20–30 mm) (Coyle et al. 2007). By comparison, the dominant benthic fish in this area consume ampeliscid amphipods from the size of larvae (~1 mm) to 40 mm in length (mostly <25 mm). These findings indicate that there is some overlap in prey size between benthic fish and gray whales. Densely packed ampeliscid amphipods build and live in mucous tubes that penetrate several cm in the sediment (Highsmith and Coyle 1992). Although ampeliscid amphipods leave their burrows under some circumstances when disturbed (personal observation), those amphipods are probably not very susceptible to fish predators except when extending out of their tubes to feed. However, benthic fish could feed on ampeliscid amphipods made accessible by gray whales feeding activity, which remobilizes bottom sediments and brings some prey to the surface. Other studies also found that seabirds feed on benthic amphipods associated with gray whales feeding activity (Obst and Hunt 1990; Grebmeier and Harrison 1992). So while there is potential competition for food between benthic fish and gray whales, it is also possible that gray whale foraging could mobilize food resources for fish.
South of SLI, other predators are also present, such as the entire world population of spectacled eiders in the winter, which consume clams (Lovvorn et al. 2003, 2009). Our study indicates that a few benthic fish species, for example, Arctic staghorn sculpin and Arctic alligatorfish, also consumed clams, although only siphons were observed in the stomachs analyzed. However, those two fish species were primarily found north of SLI. Therefore, there is no evidence yet indicating that the dominant groundfish in the area compete for prey with these sea ducks.
Our study documents that ampeliscid amphipods are an important food resource for the dominant groundfish in the northern Bering Sea north of SLI. The indications that ampeliscid amphipods are declining in abundance in the northern Bering Sea (Moore et al. 2003; Coyle et al. 2007) suggests potential implications for fish feeding patterns as the benthic biological system changes (Grebmeier et al. 2012). Moreover, most of the fish species studied can be classified as specialist feeders and adaptations may be clearly necessary if changes to benthic biological community continue in the north Bering Sea. Some of these fish species share the same habitat and food prey resources, but there is no strong evidence of interspecific competition among benthic fish for food in this study. Further research is required to evaluate seasonal changes and trends in prey composition. Given rapid changes that have been observed in the ecosystem structure over recent decades, additional changes may impact the availability of prey to fish populations.
References
Amundsen PA, Gabler HM, Staldvik FJ (1996) A new approach to graphical analysis of feeding strategy from stomach contents data—modification of the Costello (1990) method. J Fish Biol 48:607–614
Aydin K, Mueter F (2007) The Bering Sea—A dynamic food web perspective. Deep-Sea Res II 54:2501–2525
Bain H, Sekerak AD (1978) Aspects of the biology of arctic cod, B. saida, in the central Canadian Arctic. Report by LGL Ltd., Toronto, for Polar Gas Project, Toronto. 104 p
Bluhm BA, Gradinger R (2008) Regional variability in food availability for Arctic marine mammals. Ecol Appl 18:S77–S96
Bohn A, McElroy RO (1976) Trace metals (As, Cd, Cu, Fe and Zn) in Arctic cod, B. saida, and selected zooplankton from Strathcona Sound, northern Batfin Island. J Fish Res Board Can 33:2836–2840
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Cortés E (1997) A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Can J Fish Aquat Sci 54:726–738
Coyle KO, Gillispie JA, Smith RL, Barber WE (1997) Food habits of four demersal Chukchi Sea fishes. Am Fish Soc Symp 19:310–318
Coyle KO, Bluhm B, Konar B, Blanchard A, Highsmith RC (2007) Amphipod prey of gray whales in the northern Bering Sea: Comparison of biomass and distribution between the 1980s and 2002–2003. Deep-Sea Res II 54:2906–2918
Craig PC, Griffiths WB, Haldorson L, McElderry H (1982) Ecology studies of Arctic cod (B. saida) in Beaufort Sea Coastal Waters, Alaska. Can J Fish Aquat Sci 39:395–406
Crawford RE, Jorgenson JK (1993) Schooling behaviour of arctic cod, B. saida, in relation to drifting pack ice. Environ Biol Fish 36:345–357
Cui X, Grebmeier JM, Cooper LW, Lovvorn JR, North CA, Seaver WL, Kolts JM (2009) Spatial distributions of groundfish in the northern Bering Sea in relation to environmental variation. Mar Ecol Prog Ser 393:147–160
Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S (1998) Making mistakes when predicting shifts in species range in response to global warming. Nature 391:783–786
Dolbeth M, Martinho F, Leitão R, Cabral H, Pardal MA (2008) Feeding patterns of the dominant benthic and demersal fish community in a temperate estuary. J Fish Biol 72:2500–2517
Gradinger R, Bluhm B (2004) In situ observations on the distribution and behaviour of amphipods and Arctic cod (B. saida) under the sea ice of the High Arctic Canada Basin. Polar Biol 27:595–603
Grebmeier JM (2012) Shifting patterns of life in the Pacific Arctic and Sub-Arctic Seas. Annu Rev Mar Sci 4:63–78. doi:10.1146/annurev-marine-120710-100926
Grebmeier JM, Dunton KH (2000) Benthic processes in the northern Bering Sea/Chukchi Seas: status and global changes. In: Huntington HP (ed) Impacts of changes in sea ice and other environmental parameters in the Arctic. Report of the Marine Mammal Commission workshop, 15–17 February 2000, Girdwood, Alaska, USA
Grebmeier JM, Harrison NM (1992) Seabird feeding on benthic amphipods facilitated by gray whale activity in the northern Bering Sea. Mar Ecol Prog Ser 80:125–133
Grebmeier JM, Overland JE, Moore SE, Farley EV, Carmack EC, Cooper LW, Frey KE, Helle JH, McLaughlin FA, McNutt SL (2006) A major ecosystem shift in the northern Bering Sea. Science 311:1461–1464
Heide-Jørgensen MP, Laidre KL, Litovka D, Villum Jensen M, Grebmeier JM, Sirenko BI (2012) Identifying gray whale (E. robustus) foraging grounds along the Chukotka Peninsula, Russia, using satellite telemetry. Polar Biol. doi:10.1007/s00300-011-1151-6
Highsmith RC, Coyle KO (1990) High productivity of northern Bering Sea benthic amphipods. Nature 344:862–864
Highsmith RC, Coyle KO (1992) Productivity of arctic amphipods relative to gray whale energy requirements. Mar Ecol Prog Ser 83:141–151
Hop H, Welch HE, Crawford RE (1997) Population structure and feeding ecology of Arctic cod schools in the Canadian high Arctic. Am Fish Soc Symp 19:68–80
Hunt GL, Coyle KO, Eisner LB, Farley EV, Heintz RA, Mueter F, Napp JM, Overland JE, Ressler PH, Salo S, Stabeno PJ (2011) Climate impacts on eastern Bering Sea foodwebs: a synthesis of new data and an assessment of the Oscillating Control Hypothesis. ICES J Mar Sci 68:1230–1243
Lawlor LR (1980) Overlap, similarity, and competition coefficients. Ecology 61(2):245–251
Linton RL, Davies RW, Wrona FJ (1981) Resource utilization indices; an assessment. J Anim Ecol 50:283–293
Lonne OJ, Gulliksen B (1989) Size, age and diet of polar cod, B. saida (Lepechin 1773), in ice covered waters. Polar Biol 9:187–191
Loughlin TR, Sukhanova IN, Sinclair EH, Ferrero RC (1999) Summary of biology and ecosystem dynamics in the Bering Sea. In: Loughlin TR, Ohtani K (eds) Dynamics of the Bering Sea. University of Alaska Sea Grant, Fairbanks, Alaska, pp 387–407
Lovvorn JR, Richman SE, Grebmeier JM, Cooper LW (2003) Diet and body condition of spectacled eiders wintering in pack ice of the Bering Sea. Polar Biol 26:259–267
Lovvorn JR, Grebmeier JM, Cooper LW, Bump JK, Richman S (2009) Modeling marine protected areas for threatened eiders in a climatically changing Bering Sea. Ecol Appl 19:1596–1613
Lowry LF, Frost KJ (1981) Distribution, growth, and foods of Arctic cod (B. saida) in the Bering, Chukchi, and Beaufort Seas. Can Field-Nat 95:186–191
Mecklenburg CW, Mecklenburg TA, Thorsteinson LK (2002) Fishes of Alaska. American Fisheries Society, Bethesda, Maryland
Moore SE, Grebmeier JM, Davies JR (2003) Gray whale distribution relative to forage habitat in the northern Bering Sea: current conditions and retrospective summary. Can J Zool 81:734–742
O’Connell CP (1980) Percentage of starving northern anchovy, Engraulis mordax, larvae in the sea as estimated by histological methods. Fish Bull 78:475–489
Obst BS, Hunt GL Jr (1990) Marine birds feed at gray whale mud plumes in the Bering Sea. Auk 107:678–688
Overland JE, Stabeno PJ (2004) Is the climate of the Bering Sea warming and affecting the ecosystem? EOS Trans Am Geophys Union 85:309–316
Sigler MF, Renner M, Danielson SL, Eisner LB, Lauth RR, Kuletz KJ, Logerwell EA, Hunt GL (2011) Fluxes, fins, and feathers. Relationships among the Bering, Chukchi, and Beaufort Seas in a time of climate change. Oceanography 24:113–127
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci 60:1129–1157
Stabeno PJ, Overland JE (2001) Bering Sea shifts toward an earlier spring transition. EOS Trans Am Geophys Union 82:317–321
Tynan C, DeMaster DP (1997) Observations and predictions of Arctic climate change: potential effects on marine mammals. Arctic 50:308–322
Warburton K, Blaber SJM (1992) Patterns of recruitment and resource use in a shallow-water fish assemblage in Moreton Bay, Queensland. Mar Ecol Prog Ser 90:113–126
Wiens JA (1993) Fat times, lean times and competition among predators. Trends Ecol Evol 8:348–349
Woodward G, Hildrew AG (2002) Body-size determinants of niche overlap and intraguild predation within a complex food web. J Anim Ecol 71:1063–1074
Wyllie-Echeverria T, Wooster WS (1998) Year-to-year variations in Bering Sea ice cover and some consequences for fish distributions. Fish Oceanogr 7:159–170
Zaret TM, Rand AS (1971) Competition in tropical stream fishes: support for the competitive exclusion principle. Ecology 52:336–342
Acknowledgments
We thank Dr. R. Brown for lending her taxonomic expertise in the identification of fish prey. We thank the crew, officers, and particularly the marine science technicians from the USCGC Healy for sampling support at sea. We thank the chief editor and three anonymous reviewers for constructive comments that helped us to improve a prior version of the manuscript. Support for this research was provided by the U.S. National Science Foundation through OPP-ARC-0454454. The State of Tennessee also provided fellowship support to X. Cui through the University of Tennessee, Knoxville.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, X., Grebmeier, J.M. & Cooper, L.W. Feeding ecology of dominant groundfish in the northern Bering Sea. Polar Biol 35, 1407–1419 (2012). https://doi.org/10.1007/s00300-012-1180-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-012-1180-9