Abstract
In order to reveal the structure of the sparsely known deeper sublittoral hard bottom communities of glacial Kongsfjorden, the macroepibenthos from six depth zones (30–200 m) was analysed. A total of 180 still images derived from 6-h video recorded at the Kongsfjordneset remotely operated vehicle station were assessed quantitatively. Overall 27 mainly suspension-feeding species/taxa were observed. Of these, two-thirds have an arcto-boreal distribution, while the remainder are cosmopolitan. The overall mean epibenthos abundance was 33 ind. m−2 with maximum values at 150 m depth (97.9 ind. m−2). The majority of the taxa inhabited the entire depth range. Encrusting red algae, an unidentified sponge and the sea anemone Urticina eques, characterized the assemblage of the shallow zone. The sea anemones Hormathia spp. were important below 30 m, the Serpulid polychaete Protula tubularia was characteristic for the community below 50 m and the demosponge Haliclona sp. was a key taxon between 100 and 200 m depth. Cluster analysis and non-metrical multidimensional scaling based on abundance data showed differences between the assemblages along the bathymetric gradient, but only in the shallower depths in relation to the substratum surface incline. As surface and tidal current impacts attenuate with increasing depth, there is a gradual trend from robust key species towards more fragile ones (i.e. P. tubularia), in line with the ‘Physical control hypothesis’.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kongsfjorden, on the west coast of Spitsbergen, is a valuable site for the study of complex environmental gradients and their responses to climate warming, and detailed information pertaining to its physical characteristics and ecological attributes already exist (for reviews see Svendsen et al. 2002; Hop et al. 2002). The hard bottom epifauna of the Kongsfjorden littoral zone between 5 and 30 m depth has been previously studied by Jørgensen and Gulliksen (2001), Sahade et al. (2004), Beuchel et al. (2006) and Beuchel and Gulliksen (2008), and the soft bottom macrobenthos has been investigated from the intertidal zone to 380 m depth (Włodarska-Kowalczuk et al. 1998, 2005; Kendall et al. 2003; Sahade et al. 2004; Włodarska-Kowalczuk and Pearson 2004; Gutt 2005; Kaczmarek et al. 2005; Bick and Arlt 2005; Laudien et al. 2007; Kędra et al. 2010). Macrofauna associated with macroalgae was analysed by Lippert et al. (2001). Investigations on deeper hard bottom communities in the Arctic are scarce, and existing knowledge is based on qualitative dredge hauls (e.g. for Kongsfjord-Renna 960 m see Piepenburg et al. 1996). Hop et al. (2002) stated that motile species and fragments of sessile organisms predominate in such samples, which frequently include the sea stars Crossaster papposus, Henricia sp. and Pteraster sp., the sponges Myxilla sp., Haliclona sp. and Polymastia sp. and other species that generally dominate rocky substrata within the euphotic zone, such as the barnacle Balanus balanus, the actinarian Hormathia nodosa, and the sedentary polychaete Thelepus cincinatus. Kaczmarek et al. (2005) dredged in Kongsfjorden down to 50 m and identified the chiton Tonicella rubra, the gastropod Erginus rubellus, the decapod Spirontocaris turgida, the amphipod Pleusymtes glabroides, and an unidentified polynoid polychaete as characteristic taxa for the rocky faunal assemblage. From the deep sea, the fauna colonizing hard substrata such as dropstones, sunken wood and anthropogenic material was described by Oschmann (1990), Von Juterzenka (2004), Schulz et al. (2010), and MacDonald et al. (2010).
These qualitative studies are informative, but for an understanding of faunal community responses to global and Arctic environmental change, quantitative inventories of species assemblages and biodiversity are essential (Bluhm et al. 2005; Renaud et al. 2007, 2008; Buchholz et al. 2010; Węsławski et al. 2010a, b; Kędra et al. 2010). The longevity of most of the species means that polar epibenthic community structures arise as a consequence of abiotic and biotic control over long periods of time (e.g. Mileikovsky 1971; Węsławski et al. 2010a). Investigation of community structures can therefore provide insights into persistent and/or recent environmental conditions (Klages et al. 2003) and will provide a baseline for research examining the impacts of climate change on polar biodiversity. Such impacts include effects relating to shifting Atlantic and Arctic water masses (e.g. Walczowski and Piechura 2006), warming (ACIA 2005), and the resulting species migrations (Berge et al. 2005; Węsławski et al. 2010a, b, Buchholz et al. 2010). Thus, there is a need for sound species inventories and information on the present status of community structure of the study location.
In addition to the potential changeability of the physical, chemical and biological factors, the topography of the substratum surface has an effect on the composition of benthic hard bottom communities. This is complex and not yet fully understood (Knott et al. 2004). Several studies have shown that algae are characteristic on upward-facing substrata, while suspension-feeders dominate vertical faces and overhangs (e.g. Logan et al. 1984; Sebens 1985; Witman and Sebens 1991; Baynes 1999; but see Knott et al. 2004). Even sessile carnivores, such as the cold-water coral Desmophyllum dianthus, are found exclusively on near-vertical walls and under overhanging rock walls or larger boulders in depths accessible by SCUBA diving (Häussermann and Försterra 2007). The direct effect of topography on community structure is probably insignificant, but the slope angle affects light incidence (Hartnoll 1983) and silt settling (Kukliński et al. 2005; Häussermann and Försterra 2007) upon the substratum. Although numerous observations have evaluated the community composition in relation to the substratum slope, few studies have evaluated the effect quantitatively (e.g. Jørgensen and Gulliksen 2001; Knott et al. 2004). For a site at 20 m depth in Kongsfjorden (Svalbard), Jørgensen and Gulliksen (2001) recorded that macrobenthic assemblages are more diverse on vertical hard bottom substrata than on horizontal ones; however at 30 m depth, there was a reduced disparity between the colonizing assemblages of horizontal, vertical and overhanging surfaces.
McLachlan’s (1990) “Physical control hypothesis”, which was originally proposed for sandy beach fauna, postulates that communities are structured by the responses of individual species to the physical environment, and that abundance and diversity should increase between harsh and more benign environments. A calming of abiotic environmental parameters with depth was noted by Svendsen et al. (2002). The differences in influence of such parameters on differently oriented substratum surfaces should thus decrease with increasing depth, resulting in greater homogeneity of locally occurring epibenthic assemblages than in shallower habitats. This holds true if there is no discriminative biological control.
The present study aims to quantitatively describe epibenthic hard bottom communities of rock faces with different substratum surface slope angles from Kongsfjorden at 30, 50, 75, 100, 150 and 200 m depth. The study focuses on species abundance, distribution, zoogeographical composition, diversity and feeding modes. Dissimilarities in assemblages are determined by cluster analysis using abundance data. To our best knowledge, this is the first quantitative assessment of epibenthic hard bottom communities of Svalbard from the perspective of a remotely operated vehicle. This study thus provides a humble, but valuable register of video and digital still photographs from this sparsely known habitat, as well as significantly extending the records of Jørgensen and Gulliksen (2001), Sahade et al. (2004), Beuchel et al. (2006), Beuchel and Gulliksen (2008) (5–30 m water depth).
Materials and methods
Study area
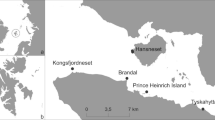
The study site Kongsfjordneset (78°58.37′N, 11°29.35′E, Fig. 1) is situated near Kvadehuken at the north-eastern tip of the Brøgger Peninsula, which forms the southern coast of Kongsfjorden (west coast of Spitsbergen, Arctic). Kongsfjorden has an extension of 30 km in length and between 4 and 10 km (at the mouth between Kvadehuken and Kapp Guissez) in width. It reaches depths of approximately 350 m (on average 200–300 m). The fjord is directly connected with the North Atlantic Ocean via the Kongsfjord-Renna trough (Bluhm et al. 2001; Jørgensen and Gulliksen 2001; Svendsen et al. 2002).
Semidiurnal tides of the fjord system range from 1.5 to 2 m and weak currents prevail. Mean sea temperature at 9 m water depth is 0.7°C, while maximal values may reach 7.4°C in summer and minimum values −2.3°C in winter (Laudien 2011a, b). The mean temperature at the 20 m isobath is 3.6°C (Bluhm et al. 2001). Two glaciers, Kronebreen and Kongsvegen, head the fjord and together with other large glaciers (i.e. Conwaybreen and Blomstrandbreen) discharge about 1 km3 of fresh water annually into the central and inner parts of Kongsfjorden with a seasonal peak in July (Lefauconnier et al. 1994; Węsławski et al. 1995; Beszcynska et al. 1997). During summer, the salinity occasionally decreases to 28 at 9 m depth, whereas a value of 33 is maintained throughout the winter (Laudien 2011a, b). The water column is usually stratified during the summer season but becomes mixed during autumn (Normann and Pettersen 1984). Ice cover is common throughout the winter, with marked inter-annual variations (Mehlum 1991). A comprehensive description of the physical environment can be found in Svendsen et al. (2002) and Hanelt et al. (2004); ecological information on Kongsfjorden is broadly compiled in Hop et al. (2002).
Off Kongsfjordneset (Fig. 1), the hard bottom slopes gradually with the 15-m isobath about 200–300 m from shore before it inclines steeply (slope angle app. 30–45°) with occasional step-like terraces, down to the maximum depth of the fjord. The area is characterized by pure bedrock, but ice-rafted material and silt accumulates according to the topography (Beuchel and Gulliksen 2008).
Recording macrozoobenthos and abiotic parameters
Macrobenthic communities were recorded in June 2009 with the remotely operated vehicle (ROV) ACHILLE M4 (Institut Polaire Français Paul Emile Victor, France) from aboard the research boat Teisten (Kings Bay AS, Norway). The primary video camera was a SONY HVR-A1E HDV 1080i fitted with a 300 m Extreme Vision Lens, set to its widest angle and focus was adjusted to give the largest depth of field from 1 to 4 m in front of the ROV. For quantitative monitoring, two halogen lights were arranged to provide broad coverage in the direction of travel and three laser pointers (3 cm horizontal distance and 2 cm vertical distance) provided the scale. The surface boat proceeded along the same vector and at about the same forward speed as the ROV, thus preventing the boat from dragging the ROV off course. Video targets smaller than about 5 mm could not be reliably identified with this setup (see also Barthel et al. 1991; Jørgensen and Gulliksen 2001; Beuchel and Gulliksen 2008). Video images were recorded on high-definition tapes (SONY PHDVM-63DM, Sony Corporation, Tokyo, Japan). Temperature, salinity and depth were logged with a salinity, conductivity, temperature and depth sensor system (STD/CTD model SD204; SAIV A/S, Bergen, Norway), and time codes linked the video data frame by frame to the simultaneous CTD measurements.
The quantitative video surveys were run along six isobathic transects (30, 50, 75, 100, 150, 200 m; Fig. 1) during daytime hours. The recording time was 1 h. Minimum surface area observed per transect was 19.5 m2. Table 1 lists the date of dive, dive depth, start and end position as well as the transect length.
Quantitative image analysis
Video sequences were digitized (Adobe Premiere, MasterCollection CS5) ashore and viewed with the software Avidemux 2.5 (Mean, France). Frame numbers of sequences showing bare rock colonized by epifauna on (1) vertical (80° to 100°), (2) horizontal (−10° to 10°) or (3) diagonal (35° to 55°) substratum surfaces (defined as >50% of the surface aligned, respectively) were noted in a text file. Faces with apparent silt deposition were not included as the focus was on typical hard bottom communities. Random freeze-images (Bitmap format) were generated with the help of a Python script and the open source software FFmpeg (Licensed by GNU General Public License 2.0 or later and GNU Lesser General Public License 2.1 or later; http://www.ffmpeg.org/, download 20.08.2010). Images were named according to time, depth and topography, and thereafter ten images of each of the three substratum slopes (vertical, diagonal and horizontal) and depths (ten × three slopes × six depths = 180 in total) were analysed.
Image analysis was carried out on a Samsung Aura Epon E172 T 6400 Notebook with windows vista home premium and a high-resolution monitor using CoralCount®. Individual epibenthic organisms were identified visually to the lowest level possible, counted and archived (supplementary data including the pictures and quantification of identified taxa are available at doi:10.1594/PANGAEA.753197). Due to the high variability in terms of coloration and length of spines, the two sea urchin species Strongylocentrotus droebachiensis and the scarce Strongylocentrotus pallidus were identified to genus level only. The anthozoans Hormathia digitata and H. nodosa were pooled as the two species were not always clearly distinguishable. Colonial species were counted as one organism, since the colony forms after the successful settlement of only one individual. As colonial hard bottom species may colonize large areas of rocky substratum, the percentage cover of ground was additionally estimated for the numerically important sponge Haliclona sp. and for red corallines. To this end, the area of the picture covered by the respective taxon was stained in a colour not present in the respective picture and copied in a new layer using Adobe Photoshop CS V8.0.1. Its histogram option provides the number of pixels in the respective layer and thus the base to calculate the percentage cover of the taxon of interest in relation to the overall image selection.
For each frame, organism counts were standardized to one square meter before the Shannon–Wiener diversity index (H′, Log e), species richness and evenness were determined. Thereafter, data were square-root transformed to lessen the contribution of rare species, without excluding them from the analysis. Multi-dimensional scaling was used to elucidate patterns in faunal composition, abundance and distribution over depth utilizing the PRIMER v6 package (Clarke and Warwick 2001; Clarke and Gorley 2006). Frame classifications were achieved and thereafter assemblages visible on the distinct frames related to each other and identified from the resulting dendrogram based on group average linkage. Statistical differences were identified by means of a similarity analysis (one-way ANOSIM, 95% confidence interval, Clarke and Gorley 2006). SIMPER (Clarke and Gorley 2006) was then used to identify characteristic species, which contribute most to the statistical dissimilarity among samples. Differences in species richness, species diversity and evenness between the six bathymetric transects were tested using one-way ANOVA. Furthermore, species were classified into four feeding modes (active and passive filter feeders, omnivores and carnivores) according to the literature (Syvitski et al. 1989; Aitken 1990; Gromisz and Legezynska 1992; Schmid and Piepenburg 1993; Gulliksen and Svensen 2004). Species were attributed to the two location modes “sessile” and “mobile” and to four zoogeographical groups (arctic, arcto-boreal, boreal and cosmopolitan) in accordance with appropriate publications (Gajewska 1948; Rózycki 1991; Węsławski 1991; Anisimova et al. 1992; Gromisz and Legezynska 1992; Włodarska et al. 1996).
Results
Hydrographic characteristics of the water column
The typical water column profile (Fig. 2), measured during the descent of the ROV at the study site, showed a decline of the water temperature from 6.5°C at the surface to 1.1°C at 20 m, an increase to 2.2°C at 50 m and a sudden drop to 0.2°C around 60 m. Thereafter, the temperature increased irregularly to 2.8°C at app. 100 m and decreased thereafter to 2°C. Salinity increased abruptly from a surface value of 23–34.8 and was fairly constant thereafter.
Benthic community
Species–accumulation curves (sensu Gray 2001; Fig. 3) show that ten replicates were sufficient to include a high percentage of the hard bottom epifaunal organisms as the curves level at three to six replicates.
In total, 13,065 individuals were identified. Echinoderms comprise 24%, sponges and crustaceans each 16%, and Anthozoans, molluscs, bryozoans, ascidians each 8%. Others (including Phaeophyta, Rhodophyta and Polychaeta) contributed 12% of the fourteen species, seven additional genera, three additional families, one porifera and one asteroidean species not further identified. In numerical abundance, polychaetes contributed 79% of the specimens, Rhodophyta 9%, Anthozoa 7%, Porifera 3% and others less than 2%. The crustaceans B. balanus, Pagurus sp. and Lebbeus polaris, as well as an unidentified species of the family Holothuroidea were not captured by the freeze-images but were scarcely recorded in the video sequences. Table 2 summarizes the abundances of all taxa. The bulk of Phaeophycea and Corallinacea (99.6 and 82.0%, respectively) were found at 30 m in association with the Polyplacophora Tonicella sp., which was only found in shallow water, and the ascidian Halocynthia pyriformis, which was only found down to 50 m water depth. In contrast, the serpulid polychaet Protula tubularia was much more abundant below 100 m water depth.
Figure 4 illustrates the abundances of Rhodophyta, Polychaeta, Anthozoa, Porifera and others (Phaeophyta, Bryozoa, Ascidiacea, Echinodermata, Bivalvia, Asteroidea, Decapoda, Cirripedia, Holothuroidea and Polyplacophora) along the depth gradient 30–200 m.
Abundances (ind. m−2) of the dominating taxonomic groups Polychaeta, Rhodophyta, Anthozoa, Porifera and others (<5% contribution to total abundance, i.e. Phaeophyta, Bryozoa, Ascidiacea, Echinoidea, Bivalvia, Asteroidea, Decapoda, Cirripedia, Holothuroidea and Polyplacophora) recorded during ROV-dives at a vertical rock face at Kongsfjordneset (Spitsbergen, Arctic) along the depth gradient 30–200 m
In 30 m depth, Rhodophyta numerically dominated the community (88% of the total abundance) followed by Phaeophyta (7%). The latter are very rare down to 75 m and are not found deeper. In 50 m, Rhodophyta are still the numerically dominant taxon (77%) but were not observed below 75 m. This is also reflected in the percentage of ground covered, which decreases significantly with depth (ANOVA P < 0.05). Anthozoans increase in abundance down to 75 m (62%) and show less abundance on the rock faces below 100 m. The same trend was observed for sponges, which increase in abundance down to 100 m depth (19%), but are less numerous in 150 and 200 m depth. Polychaetes inhabited the entire bathymetric gradient with increasing importance as depth increases (at 150 m 98% of the total abundance, 95.7 ind. m−2).
Only the euphausids Thysanoessa inermis and T. raschii (ground-truthing via plankton samples at the same day and location followed by microscopic identification, pers. comm. C. and F. Buchholz, AWI) commonly observed at 75 m and below are true arctic species; however, they were not included in the analyses as they are epibenthic/pelagic species. In total, 15 taxa were categorized as arcto-boreal, five as cosmopolitan and two as boreal. The entire depth range was characterized by a similar zoogeographical species composition with around 60–75% arcto-boreal and 20–30% cosmopolitans.
The overall mean epibenthic abundance was 33 ind. m−2 with maximum values at 150 m depth (97.9 ind. m−2, eleven species), followed by the 200 m transect (52.1 ind. m−2, eleven species). Lowest abundance values were recorded at 50 m depth (four ind. m−2, ten species) (Fig. 5; Table 2).
Mean (filled circle) and maximum (open square) abundance and number of taxa, Shannon diversity (H′, Log e) and evenness (J′) of hard bottom epibenthos at six different depths (30–200 m) of rock faces at Kongsfjordneset (Spitsbergen, Arctic). Associations of the respective depth sharing a letter (corresponding top line) do not differ significantly (ANOVA, Fisher LSD post hoc test, P > 0.05)
Active filter feeders were the major proportion of the macrofauna at all depths (30–60%) (Fig. 6). Carnivores are also important (15–50%). Passive filter feeders are present in all depths, however, most important at 30 m and below 75 m. The frequency of omnivores is positively correlated with depth (R = 0.90). In all depths, sessile and mobile fauna were present and did not show significant differences along the depth gradient.
Species richness differed significantly between the shallowest and all other depths; it was significantly lowest at 150 m (ANOVA P < 0.05, Fisher LSD P < 0.05; Fig. 5). Mean diversity was 0.73 (0.07 SE) and ranged between 0.39 (±0.07 SE) at 150 m and 0.99 (±0.05 SE) at 30 m. Lowest evenness was detected at 150 m (0.65 ± 0.05 SE), and the highest at 50 m (0.92 ± 0.01 SE) (Fig. 5).
The cluster analysis of abundance data indicated a gradient between the assemblages from 30 to 200 m (Figs. 7, 8). While the assemblage at 30 m depth is clearly different from the ones below 75 m (100 m: R = 0.78; 150 m: R = 0.90; 200 m: R = 0.83), it overlaps but is still different from the assemblage at 75 m (R = 0.72) but only barely separable (R = 0.30) from the neighbouring assemblage at 50 m.
nmMDS plots resulting from cluster analysis of Bray–Curtis similarities using abundance data of hard bottom epibenthos inhabiting vertical rock faces at Kongsfjordneset (Spitsbergen, Arctic) along the depth gradient 30–200 m (a), and comparing the epibenthic community between the 30 and 200 m transect (b, border line = 15% similarity), and 50 and 200 m transect, respectively (c, border line = 20% similarity). Different grey shadings indicate the depth (30, 50, 75, 100, 150 and 200 m), symbols represent the substrate angle (triangular diagonal, circle horizontal, square vertical orientation)
Dendrogram resulting from cluster analysis of Bray–Curtis similarities using abundance data of hard bottom epibenthos inhabiting differently inclined rock faces at Kongsfjordneset (Spitsbergen, Arctic) along the depth gradient 30–200 m. Depth zones sharing a letter do not differ significantly (one-way ANOSIM, P < 0.05)
The SIMPER analyses (Table 3) revealed that Corallinacea (75%; [at 50 m still 66%]), Phaeophycea (10%) and the sea anemone Urticina eques (8%) predominantly account for the similarity of the community in the shallow water. Below 30 m, the sea anemones Hormathia spp. are important (50 m: 31%, 75 m: 65%, 100 m: 13%, 200 m 7%). P. tubularia is characteristic for the community below 50 m (75 m: 25%, 100 m: 62%, 150 m 92%, 200 m 76%). The demosponge Haliclona sp. is important in 100 and 200 m (16 and 10%, respectively).
While the distinction between assemblages colonizing differently inclined surfaces in 200 m water depth is not clear, at 30 m, assemblages living on horizontal substratum surfaces may be distinguished from those covering diagonal and vertical substratum surfaces (at 46% similarity only three assemblages living on vertical and one on a horizontal surface group with all replicates from horizontal surfaces). Red corallines colonizing vertical surfaces (mean coverage 24.86% ± 2.97% SE at 30 m, and 9.06% ± 2.62% SE at 50 m, respectively) reveal a significantly higher percentage of cover than on horizontal (mean 9.91% ± 1.32% SE at 30 m, 2.90% ± 0.81% SE at 50 m) or diagonal surfaces (18.04% ± 1.76% SE at 30 m, 4.02% ± 1.34% SE at 50 m) (ANOVA P < 0.05, Fisher LSD P < 0.05). Only two replicates with horizontal surfaces were colonized in 75 m depth, accordingly the percentage cover was very low (0.12% ± 0.08% SE). The demosponge Haliclona sp. covered a significantly larger area of vertical (0.102% ± 0.060% SE) and diagonal (0.100% ± 0.047% SE) substratum surfaces than of horizontal ones (0.003% ± 0.002% SE) (ANOVA P < 0.05, Fisher LSD P < 0.05).
Discussion
Between 30 and 200 m depth, the epibenthos off Kongsfjordneset comprises at least 27 taxa, this is comparable with the 22 taxa found by Sahade et al. (2004) at the same site in 5–30 m depth. At a neighbouring, but shallower site (15 m depth) 23 taxa were identified by Beuchel and Gulliksen (2008) and 31 taxa in 20 and 30 m depths by Jørgensen and Gulliksen (2001) using underwater photography. All taxa had been previously recorded for Svalbard (Gulliksen et al. 1999; Sahade et al. 2004; Palerud et al. 2004; Beuchel et al. 2006; Beuchel and Gulliksen 2008). In respect to species number, sponges, crustaceans and sea stars (four species each) dominate the hard bottom epifauna of Kongsfjordneset. Polychaetes (95.7 Ind. m−2 at 150 m) and Corallinacea (14.9 Ind. m−2 at 30 m) are the most abundant. In a 24-years study of the epibenthic community at the nearby site, Beuchel and Gulliksen (2008) found several components of the presently recorded community (i.e. the anthozoans H. nodosa and U. eques, the molluscs Tonicella sp. and Chlamys islandica, the crustaceans Balanus sp. and Hyas sp., the sea urchin Strongylocentrotus sp., the sea star Henricia sp. and the ascidians H. pyriformis and Styela rustica). Several sponges, the serpulid polychaete P. tubularia, bryozoans and Holothuroidea, seem to be characteristic for intermediate (Jørgensen and Gulliksen 2001; Sahade et al. 2004) and deeper sublittoral epibenthic communities (present results).
The total number of individuals was highest at 150 and 200 m, mainly due to P. tubularia, although a taxa minimum was found in these two depths. In contrast, at 50 and 75 m, the total number of individuals was lowest but the number of taxa was highest.
While some rare taxa were found inhabiting depths down to 50 m only (the polyplacophora Tonicella sp., the barnacle B. balanus, the sea stars Henricia sp. and Solaster sp. and the ascidian H. pyriformis), the bulk of the taxa inhabited the entire depth range (with the exception of an unidentified sea cucumber found only at 150 and 200 m depth). The actinians Hormathia spp. dominate the intermediate zone, while P. tubularia is the most abundant species at 150 and 200 m.
The latter serpulid has been recorded globally; i.e. from the Arctic Ocean, the Mediterranean, the Red Sea, South Africa, India, Australia, Japan, Korea, the Pacific coast off Mexico and Ceylon (Kupriyanova and Jirkov 1997 and references therein); however, the authors suggest a worldwide revision to resolve a possible taxonomic confusion.
Encrusting red algae dominate down to 50 m depth. Corallinacea are, however, present down to 100 m depth. They are able to utilize light in the short wavelength range (500–650 nm)—which is inaccessible to chlorophyll—through their phycobilisomes. These are protein complexes anchored in a particular arrangement to thylakoid membranes. Each phycobiliprotein has its distinct fluorescence and absorption maximum. Thus, the phycobilisomes allow absorption and unidirectional transfer of light energy to chlorophyll a of photosystem II (Su et al. 2010). Although, in theory, there is still enough light energy in 200 m water depth, the light absorption by plankton organisms and the turbidity of the fjord water during Arctic summer may result in a reduction of the depth zone, colonizable by encrusting red algae. Kongsfjorden is influenced by glacier runoff and melting calved icebergs throughout the year (Svendsen et al. 2002). Although the inner parts of the fjord are much more affected (Hop et al. 2002; Svendsen et al. 2002), the present study area in the central part of the fjord may still be impacted by an inorganic suspension of 20 mg dm−2 day−1 and a sedimentation rate ranging between 7 and 31 g m−2 day−1 (Zajączkowski 2000, 2008). Sedimentation rates amount to 200 g m−2 year−1 in the outer fjord (Svendsen et al. 2002). Turbidity increased from 100 m depth downward, this may be a result of phytoplankton mortality due to light limitation. Lüning and Dring (1979) indicate that the lower depth limit of crustose coralline algae is approximately the depth where 0.05–0.01% of the surface irradiance is still available.
Another explanation for the disappearance of encrusting red algae in 100 m water depth may be weaker competition for settlement substratum in relation to the numerically dominating P. tubularia (in 150 m depth 98% of the overall abundance of all taxa). Colonies of the encrusting red algae Lithothamnion sp. from Kongsfjordneset grew only 133 μm year−1 (Laudien and Brey, unpubl. data). Unfortunately, autecological information on cold-water Corallinales is still not available (Adey et al. 2005). The observation of an almost monoculture of P. tubularia may be the result of hierarchical competition and the formation of a mature stage in the progress of successional colonization (Valdivia et al. 2005; Pacheco et al. 2010) without leaving free settlement substratum for corallines.
H. digitata and H. nodosa were recorded along the entire depth gradient, with higher abundance in intermediate depths. In 75 m, the two Hormathia species account for 60% of the total abundance of the macrofauna. The numerical dominance is in agreement with Beuchel and Gulliksen (2008), who, however, found much higher abundances of the combination of H. nodosa and U. eques at their 15 m depth site (144.2 ± 57.1 Ind. m−2), while Sahade et al. (2004) found H. nodosa at other sites in the fjord, but not at Kongsfjordneset between 5 and 30 m depth. This illustrates the patchiness of the system.
Zoogeography
The hard bottom epifauna of Kongsfjordneset is dominated by arcto-boreal species (70% of all organisms that could be identified to species or genus level, the latter were only included if the known species have the same zoogeographical distribution), followed by cosmopolitans. The present results are in agreement with those of Beuchel and Gulliksen (2008) and with a classification of the infauna from a soft bottom habitat of Kongsfjorden, where Laudien et al. (2007) recorded 58% arcto-boreal, 34% cosmopolitan and only 8% arctic species. They are also consistent with biogeographical relationships of macroalgae in the same fjord (Wiencke et al. 2004). The rarity of arctic species classified as pure arctic (the epibenthic euphausids T. inermis and T. raschii were presently recorded in 150 and 200 m depth) is typical for benthos of the Arctic and is explained by the short phase of its evolution, which did not allow much specification (Curtis 1975). When ice masses were displaced at the end of the Pleistocene, thermophile arcto-boreal species from the North Atlantic and North Pacific migrated in northern direction along the continental shelf and colonized the newly available habitat (Zenkevich 1963). In comparison with the macrozoobenthos of Tikhaia Bay off Franz Josef Land (Hooker Island, 80°N), which is influenced by cold, arctic water with a temperature peak of only −0.7°C in summer, the hard bottom macroepibenthos of Kongsfjorden is composed of even fewer arctic elements (Franz Josef Land: 45% arcto-boreal, 11% cosmopolitan, 28% arctic species; Włodarska et al. 1996). This is due to the influence of warmer, saline Atlantic Water, as the outer edges of the Gulf Stream—the Western Spitsbergen Current—reaches the study area at the western coast of Spitsbergen (Svendsen et al. 2002; Cottier et al. 2007). Accordingly, despite its geographic location in the Arctic, Kongsfjorden, is not a pure arctic but rather a subarctic fjord (see also Hop et al. 2002; Herrmann 2004; Herrmann and Laudien 2004; Buchholz et al. 2010; Wallace et al. 2010). The benthic species inventory of Kongsfjorden—or of a larger area—may, however, vary over decadal time scales due to climate variability (mainly temperature rise) (e.g. Kröncke et al. 1998; Beuchel et al. 2006; Narayanaswamy et al. 2010). The regional temperature increase can impact both physical (e.g. stratification) and biological (e.g. primary production) parameters thus influencing reproduction, recruitment and persistence in the system (Renaud et al. 2009). Since the 1990s a species increase of about 9% was recorded for Svalbard waters, which may partly be due to more intensive sampling effort, however, results are consistent with predicted impacts of climate warming (Narayanaswamy et al. 2010). Climate change is expected to have strong effects in the Arctic (ACIA 2005) and is most likely to result in a regime shift towards a more boreal European Arctic. The last hemispherical warming (in the 1920s and 1930s) was reflected in a significant northward shift of the distributions of several benthic species, especially on the western coasts of Greenland and Svalbard, and in the Barents Sea (Blacker 1957; Drinkwater 2006).
Trophic structure
Active and passive filter feeders dominate (61–74%) the epibenthic community of the entire depth profile as also found by Sahade et al. (2004) from 20 and 30 m water depth at the same location. This indicates that organic particles are available in all depths. However, filter feeders are most abundant at vertical and diagonal substratum surfaces due to the reduced impact of inorganic sedimentation. The latter may bring about a decrease in the number of filter feeders (Görlich et al. 1987; Włodarska et al. 1996; Włodarska-Kowalczuk and Pearson 2004; Włodarska-Kowalczuk et al. 2005) because (1) it hinders larval settlement and juvenile survival (e.g. ascidians: Young and Chia 1984; corals: Bak and Engel 1979), (2) of the dilution of the nutritious suspensions by inorganic particles, mostly non-selectively ingested by the specimens (e.g. Görlich et al. 1987; Syvitski et al. 1989) and (3) of the risk of gill clogging, which affects feeding and respiration (Moore 1977; Kükenthal et al. 2005; Ambrose et al. 2006).
Carnivores were rarer than expected for Arctic waters (Thorson 1957; Gulliksen 1979) and were replaced successively by omnivores with increasing depth. This was also detected for the soft bottom community of Kongsfjorden (Herrmann 2004) and is in contrast to findings from a high-arctic soft bottom community of an East Greenlandic fjord, where omnivores decreased with depth and carnivores showed no clear depth trend (Sejr et al. 2000).
As no algae were recorded below 100 m, the occurrence of the sea urchin Strongylocentrotus droebachiensis at this depth indirectly supports the findings of Briscoe and Sebens (1988), derived from gut analysis of sea urchins from the Gulf of Maine, that this species is not obligate herbivorous but may prey on sponges, hydrozoans, bryozoans and tunicates.
Substratum topography
Although P. tubularia is dominant on vertical and diagonal but does not occur on horizontal substratum faces the epifauna associations colonizing differently declined substratum surfaces between 30 and 200 m depth were rather similar. This indicates that the effect of substratum orientation is not as consistent as previously thought: Results from the 20 m depth line off Kvadehuken indicate that assemblages are more diverse on vertical hard bottom faces compared to horizontal ones (Jørgensen and Gulliksen 2001). However, the authors report that at 30 m depth, the horizontally orientated association showed reduced dissimilarity to vertically orientated associations and those that colonize overhangs. This is explained by the substratum heterogeneity of the horizontal sites (base rock, pebbles, stones and accumulations of sediment). The presence of infauna in samples from the latter sites accounts for differences between the distinct faces. In the present study, only pure rock faces apparently exposed to currents preventing particle deposition were considered, which makes siltation as a structuring factor non-relevant. Thus, all three analysed substratum faces are available to typical sessile, rocky bottom taxa (e.g. Haliclona sp., H. digitata, H. nodosa, U. eques, P. tubularia, Crisia denticulate). Slope preferences of benthic organisms are generally due to light conditions in shallow sublittoral habitats because of the preference of strong competing algae on upward-facing surfaces (Hartnoll 1983; Logan et al. 1984; Sebens 1985; Witman and Sebens 1991; Baynes 1999). This effect diminishes with increasing depth when light diffuses (Hartnoll 1983). For instance on the Isle of Man the Dead man’s finger Alcyonium digitatum only colonizes overhangs down to 10 m, from 10 to 20 m, it extends onto verticals and steep slopes, while below 20 m, it also colonizes horizontals (Hartnoll 1975). Noble et al. (1976) observed that the Terebratulina septentrionali community inhabits the underside of boulders in shallow water but becomes more emergent with increasing depth. Logan et al. (1984) distinguished between communities colonizing well-illuminated upward-facing surfaces from those of shaded steeply inclining cliff faces at 10 m depth but did not find dissimilarities in 18 m depth. Crisp and Ghobashy (1971) reported that the ascidian Diplosoma listerianum was found on the underside of settlement plates in shallow water, but with increasing depth this preference for the underside was reduced and sometimes reversed, which correlates with the natural adult distribution pattern. However, even assemblages on vertical surfaces were not always found to be different from those on horizontal surfaces (e.g. at Bare Island, Sydney-Illawarra, Australia, four to eight meter), indicating that the effect of substratum declination may not be as consistent as previously thought (Knott et al. 2004).
Ecological indices
The 27 documented taxa found in this study underestimate biodiversity along the bathymetric gradient, especially that of highly mobile, tiny and infauna species, which were difficult to recognize from photographs. This was also observed by Barthel et al. (1991), Roberts et al. (1994), Jørgensen and Gulliksen (2001), Beuchel and Gulliksen (2008). Shannon diversity (H′, abundance) ranged between 0.39 and 0.99 with a maximum at 30 m and a minimum at 150 m depth. The latter is due to the numerical dominance of P. tubularia, which comprise app. 90% of all individuals at that depth (evenness value of only 0.65). The present diversity values are less than those calculated by Beuchel et al. (2006) from their nearby station ‘Kvadehuken’, ranging between 1.53 and 2.21, and are also lower than estimates calculated from results of photographs (16 and 32 m depth; H′ between 1.17 and 2.24) taken by Jørgensen and Gulliksen (2001). The latter authors calculated even higher diversity values including their abundance data collected by suction sampling, which integrated infauna dwelling in cavities of the rocky bottom filled by sedimentation (Table 4).
However, Jørgensen and Gulliksen (2001) confirmed that large epibenthic species (e.g. Tonicella sp., Balanus sp., U. eques and H. nodosa) were found in similar abundances by both picture analyses and quantification following identification after suction sampling. It is therefore reasonable to assume that large and non-dwelling species recorded during the ROV-dives would be registered in the same range of abundance values by destructive sampling. For pure soft bottom habitats of Kongsfjorden, Shannon diversity varies between 0.57 and 2.19 (Włodarska-Kowalczuk et al. 1998: 1.49; Kaczmarek et al. 2005: 0.57–2.84; Bick and Arlt 2005: 1.64; Laudien et al. 2007: 1.85–2.19), suggesting that the substratum heterogeneity (rock and soft sediment in cavities) at the Kvadehuken station maintains the elevated diversity.
On a larger spatial scale, the biodiversity of macrobenthic hard bottom communities is further increased. Alone 62 bryozoan taxa colonizing larger rock substratum in 10 m depth at nine stations of the 89.6 km coastline of Kongsfjorden were identified by Kukliński et al. (2006), 137 bryozoan taxa were identified between the surface and 296 m inhabiting different habitats of Kongsfjoden (Kukliński et al. 2005). Furthermore, 23 hydroid species were found from Kongsfjorden’s head to mouth between the surface and 30 m depth by Voronkov et al. (2010) and 20 hydroid taxa on deeper subtidal hard substrata in tidewater glacier influenced Hornsund (Ronowicz et al. 2011) amounting to 127 hydroid species for Svalbard waters (Palerud et al. 2004; Ronowicz 2007).
Figure 7 indicates a gradual change in community structure related to water depth. When comparing the organism assemblage at 30 m with that of 200 m, the nmMDS-Plot indicates two groups compiling exclusively samples from one depth, which are already separated at the 15% similarity level (2D Stress: 0.08). The same holds true for a comparison between the assemblages at 50 m with those of 200 m, where two groups discriminate at the 20% level. The R-statistics of the ANOSIM 1 supports this result (Table 3); clear differences were detected between the assemblages at 30 m and those below 75 m. There was an increase in similarity between neighbouring depths with increasing depth as also observed by Gulliksen et al. (1980) for a 10-year old benthic community at Jan Mayen (70°49′N–71°10′N). The minor horizontal distance between the present transects (<500 m between the 30 and 200 m transect) makes spatial differences, e.g. differences in sedimentation levels, negligible (Zajączkowski and Włodarska-Kowalczuk 2007). Elsewhere this leads to distinct latitudinal changes in soft bottom macrofaunal assemblages within glacial fjords (e.g. Kendall and Aschan 1993; Włodarska et al. 1996; Kukliński 2002; Włodarska-Kowalczuk and Pearson 2004; Kukliński et al. 2005). The linkage between community structure and bathymetry is in contrast to observations from soft bottom macrofauna of Kongsfjorden, where regression analyses indicated that patterns of change were unrelated to depth (38–380 m) (Włodarska-Kowalczuk et al. 2005). They are, however, in agreement with observations of Laudien et al. (2007), indicating that the shallower (5 and 30 m) soft bottom macrofaunal diversity of one site was also directly or indirectly related to water depth. Furthermore, bryozoan assemblages of Kongsfjorden (surface to 296 m) are structured primarily by processes related to depth and sedimentation (Kukliński 2002; Kukliński et al. 2005). Analyses of data spanning the latitudinal extent of European continental shelf waters (36° to 81° N) archived in the MarBEF (EU Network of Excellence for Marine Biodiversity and Ecosystem Function) database indicate relatively strong and complex relationships between diversity and water depth (Renaud et al. 2009). 20–40% of the variability in community diversity measures is explained by water depth. However, different phyla show distinct patterns: While the taxonomic distinctness of annelids declines from 0 m to approximately 200 m and thereafter showed a positive relationship with water depth, a weaker but similar relationship was apparent for crustaceans. In contrast, molluscs showed poor relations with water depth. The observed variability of diversity with water depth may be due to changing food supply and/or disturbance frequency (Levin et al. 2001).
The “Physical Control Hypothesis” (McLachlan 1990), originally proposed for sandy beaches, states that communities are shaped (among other factors) by the individual responses of each species to the physical environment. As surface and tidal currents are muted with increasing depth, the key species gradually change with fragile species (i.e. P. tubularia) substituting the more robust ones. By analysing still photographs from video transects carried out at northern Georges Bank (East coast of North America), Collie et al. (2000) also observed that P. tubularia was much more abundant in depths below 80 m, compared to sites between 42 and 49 m. In line with the Physical Control Hypothesis, this polychaete was recorded in significantly lower numbers at physically impacted locations. Furthermore, the variability in the light regime and salinity, as well as the obvious impact through wind-driven resuspension of sediment due to waves is reduced with increasing profundity (Svendsen et al. 2002). Additionally, factors such as light exposition of differently orientated substratum surfaces are more relevant in the illuminated shallow zone in comparison with the depth characterized by diffuse light. With increasing depth, this results—at least on a larger spatial scale—in an increasingly homogeneous environment (Kukliński et al. 2005).
According to Whittaker (1960, 1972), species populations have scattered centres and usually overlap broadly along such habitat gradients. The extent of differentiation of communities along the latter is called beta diversity and may be expressed in standard error units. While highest alpha diversity (the diversity at each respective depth) was found for the assemblage at 30 m, its beta differentiation was lowest (SE: 0.049 vs. 100 m: SE = 0.086 and 200 m: SE = 0.083, Fig. 5). The gradual change in the more uniform deeper sublittoral is apparently predominantly biologically controlled as also suggested for the subarctic macrobenthic community of Jan Mayen (Gulliksen et al. 1980).
The present inventory bridges the gap of knowledge on community structure of deeper sublittoral high latitude hard bottoms. In order to achieve a more comprehensive picture of which factors drive the community structure, further studies should focus on the temporal variability of the physical environment along a wide bathymetrical range covering a larger spatial scale. Furthermore, in situ experiments are necessary, e.g. to reveal if hierarchical competition explains the dominance of Hormathia spp. in intermediate depths repressed by P. tubularia below 100 m depth. This should lead to a broad understanding of system functioning and thus provide a base for the detection of climatic changes in the arctic benthic realm.
References
ACIA (2005) Arctic climate impact assessment. Cambridge University Press, New York
Adey WH, Chamberlain YM, Irvine LM (2005) Morphology: an SEM-based analysis of the morphology, anatomy, and reproduction of Lithothamnion tophiforme (Esper) Unger (Corallinales, Rhodophyta), with a comparative study of associated North Atlantic Arctic-Subarctic Melobesioideae. J Phycol 41:1010–1024
Aitken AE (1990) Fossilization potential of Arctic fjord and continental shelf benthic macrofaunas. Geol Soc Spec Publ 53:155–180
Ambrose WG Jr, Carroll ML, Greenacre M, Thorrold SR, McMahon KW (2006) Variation in Serripes groenlandicus (Bivalvia) growth in a Norwegian high-Arctic fjord: evidence for local- and large-scale climatic forcing. Glob Change Biol 12:1595–1607
Anisimova NA, Presler P, Węsławski JM (1992) Echinodermata. In: Klekowski RZ, Węsławski JW (eds) Atlas of marine fauna of southern Spitsbergen. Inst Oceanol, PAS, Sopot, pp 153–155
Bak RPM, Engel MS (1979) Distribution, abundance and survival of juvenile hermatypic corals (Scleractinia) and the importance of life history strategies in the parent coral community. Mar Biol 54:341–352
Barthel D, Gutt J, Tendal OS (1991) New information on the biology of Antarctic deep-water sponges derived from underwater photography. Mar Ecol Prog Ser 69:303–307
Baynes TW (1999) Factors structuring a subtidal encrusting community in the southern gulf of California. Bull Mar Sci 64:419–450
Berge J, Johnsen G, Nilsen F, Gulliksen B, Slagstad D (2005) Ocean temperature oscillations enable reappearance of blue mussel Mytilus edulis in Svalbard after 1000 years of absence. Mar Ecol Prog Ser 303:167–175
Beszcynska A, Węsławski JM, Walczowski W, Zajaczkowski M (1997) Estimation of glacial meltwater discharge into Svalbard coastal waters. Oceanologia 39:289–298
Beuchel F, Gulliksen B (2008) Temporal patterns of benthic community development in an Arctic fjord (Kongsfjorden, Svalbard): results of a 24-year manipulation study (1980–2003). Polar Biol 31:913–924
Beuchel F, Gulliksen B, Carroll ML (2006) Long-term patterns of rocky bottom macrobenthic community structure in an Arctic fjord (Kongsfjorden, Svalbard) in relation to climate variability (1980–2003). J Marine Syst 63:35–48
Bick A, Arlt G (2005) Intertidal and subtidal soft-bottom macro- and meiofauna of the Kongsfjord (Spitsbergen). Polar Biol 28:550–557
Blacker RW (1957) Benthic animals as indicators of hydrographic conditions and climatic change in Svalbard waters. Fish Invest Ser 2:1–59
Bluhm B, Iken K, Laudien J, Lippert H (2001) German activity in cold water scientific diving. In: Jewett SC (ed) Cold water diving for science. Proceedings of the 21st annual scientific diving symposium, American Academy of Underwater Sciences. University of Alaska Sea Grant, AK-SG-01-06, Fairbanks pp 1–4
Bluhm B, MacDonald IR, Debenham C, Iken K (2005) Macro- and megabenthic communities in the high Arctic Canada Basin: initial findings. Polar Biol 28:218–231
Briscoe CS, Sebens KP (1988) Omnivory in Strongylocentrotus droebachiensis (Müller) (Echinodermata: Echinoidea): predation on subtidal mussels. J Exp Mar Biol Ecol 115:1–24
Buchholz F, Buchholz C, Węsławski JM (2010) Ten years after: krill as indicator of changes in the macro-zooplankton communities of two Arctic fjords. Polar Biol 33:101–113
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. Natural Environmental Research Council, Plymouth
Collie JS, Escanero GA, Valentine PC (2000) Photographic evaluation of the impacts of bottom fishing on benthic epifauna. ICES J Mar Sci 57:987–1001
Cottier FR, Nilsen F, Inall ME, Gerland S, Tverberg V, Svendsen H (2007) Wintertime warming of an Arctic shelf in response to large-scale atmospheric circulation. Geophys Res Lett 34:L10607
Crisp DL, Ghobashy AFAA (1971) Responses of the larvae of Diplosoma listerianum to light and gravity. In: Crisp DL (ed) Proceedings of the fourth European marine biology symposium, Bangor, 1969, pp 443–465. Cambridge University Press
Curtis MA (1975) The marine Benthos of Arctic and Sub-Arctic continental shelves. Polar Rec 17:595–626
Drinkwater KF (2006) The regime shift of the 1920s and 1930s in the North Atlantic. Prog Oceanogr 68:134–151
Gajewska NS (1948) Opredelitel’ Fauny I Flory Severnych Morej SSSR [Key to Fauna and Flora of North Seas of Soviet Union]. Gos. Lzd. ‘Sovetskaja Nauka’, Moskva
Görlich K, Węsławski JM, Zajaczkowski M (1987) Suspension settling effect on macrobenthos biomass distribution in the Hornsund fjord, Spitsbergen. Polar Res 5:175–192
Gray JS (2001) Marine diversity: the paradigms in patterns of species richness examined. Sci Mar 65(2):41–56
Gromisz S, Legezynska E (1992) Polychaeta. In: Klekowski RZ, Węsławski JW (eds) Atlas of marine fauna of Southern Spitsbergen. Inst Oceanol, PAS, Sopot, pp 8–111
Gulliksen B (1979) Shallow water benthic fauna from Bear Island. Astarte 12:5–12
Gulliksen B, Svensen E (2004) Svalbard and life in polar oceans. Kom forlag, Kristiansund
Gulliksen B, Haug T, Sandnes OK (1980) Benthic macrofauna of new and old lava grounds at Jan Mayen. Sarsia 65:137–148
Gulliksen B, Palerud R, Brattegard T, Sneli J-A (eds) (1999) Distribution of marine benthic macroorganisms at Svalbard (including Bear Island) and Jan Mayen. Research report for DN 1999-4. Directorate for Nature Management, Trondheim, pp 1–148
Gutt, J (2005) Sea-bed photographs (benthos) from the shelf west of Svalbard along ROV profile HE153_1289-4. doi:10.1594/PANGAEA.320062
Hanelt D, Bischof K, Wiencke C (2004) The radiation, temperature and salinity regime in Kongsfjorden. In: Wiencke C (ed) The coastal ecosystem of Kongsfjorden, Svalbard. Synopsis of biological research performed at the Koldewey Station in the years 1991–2003. Ber Polarforsch Meeresforsch, vol 492, pp 14–25
Hartnoll RG (1975) The annual cycle of Alcyonium digitatum. Estuar Coast Mar Sci 3:71–78
Hartnoll RG (1983) Substratum. In: Earll R, Erwin DG (eds) Sublittoral ecology: the ecology of the shallow sublittoral benthos. Clarendon Press, Oxford, pp 97–124
Häussermann V, Försterra G (2007) Large assemblages of cold-water corals in Chile: a summary of recent findings and potential impacts. In: Gorge RY, Crains SD (eds) Conservation and adaptive management of seamount and deep-sea coral ecosystems. University of Miami, Rosentiel School of Marine and Atmospheric Sciente, Florida, pp 195–207
Herrmann M (2004) Macrozoobenthic communities of Arctic soft bottoms: Structure and importance as food source for demersal fishes (in German). University of Kiel (IFM-GEOMAR)
Herrmann M, Laudien J (2004) Data from soft bottom community structure and diversity in Arctic Kongsfjord. http://www.marbef.org
Hop H, Pearson T, Hegseth EN, Kovacs KM, Wiencke C, Kwaśniewski S, Eiane K, Mehlum F, Gulliksen B, Włodarska-Kowalczuk M, Lydersen C, Węsławski JM, Cochrane S, Gabrielsen GW, Leakey RJG, Lønne OJ, Zajaczkowski M, Falk-Petersen S, Kendall M, Wängberg S-A, Bischof K, Voronkov AY, Kovaltchouk NA, Wiktor J, Poltermann M, di Prisco G, Papucci C, Gerland S (2002) The marine ecosystem of Kongsfjorden, Svalbard. Polar Res 21(1):167–208
Jørgensen LL, Gulliksen B (2001) Rocky bottom fauna in arctic Kongsfjord (Svalbard) studied by means of suction sampling and photography. Polar Biol 24:113–121
Kaczmarek H, Włodarska-Kowalczuk M, Legezynska J, Zajaczkowski M (2005) Shallow sublittoral macrozoobenthos in Kongsfjord, West Spitsbergen, Svalbard. Pol Polar Res 26:137–155
Kędra M, Włodarska-Kowalczuk M, Węsławski JM (2010) Decadal change in macrobenthic soft-bottom community structure in a high Arctic fjord (Kongsfjorden, Svalbard). Pol Biol 33:1–11
Kendall MA, Aschan M (1993) Latitudinal gradients in the structure of macrobenthic communities: a comparison of Arctic, temperate and tropical sites. J Exp Mar Biol Ecol 172:157–169
Kendall MA, Widdicombe S, Węsławski JM (2003) A multi-scale of the biodiversity of benthic infauna of the high-latitude Kongsfjord, Svalbard. Polar Biol 26:283–388
Klages M, Boetius A, Christensen JP, Deubel H, Piepenburg D, Schewe I, Soltwedel T (2003) The benthos of Arctic seas and its role for the organic carbon cycle at the seafloor. In: Stein R, Macdonald RW (eds) The organic carbon cycle in the Arctic Ocean. Springer, Berlin, pp 139–167
Knott NA, Underwood AJ, Chapman MG, Glasby TM (2004) Epibiota on vertical and on horizontal surfaces on natural reefs and on artificial structures. J Mar Biol Ass UK 84:1117–1130
Kröncke I, Dippner JW, Heyen H (1998) Long-term changes in the macrofaunal communities off Norderney (East Frisia, Germany) in relation to climate variability. Mar Ecol Prog Ser 167:25–36
Kükenthal W, Storch V, Welsch U (2005) Kükenthal Zoologisches Praktikum Spektrum Akademischer Verlag, pp 149–164
Kukliński P (2002) Bryozoa of the high arctic fjord-a preliminary study. In: Wyse Jackson P, Buttler C, Spencer-Jones M (eds) Bryozoan studies 2001. Balkema, Abingdon, pp 175–182
Kukliński P, Gulliksen B, Lønne OJ, Węsławski JM (2005) Composition of bryozoan assemblages related to depth in Svalbard fjords and sounds. Polar Biol 28:619–630
Kukliński P, Gulliksen B, Lønne OJ, Węsławski JM (2006) Subsratum as a structuring influence on assemblages of Arctic bryozoans. Polar Biol 29:652–661
Kupriyanova E, Jirkov IA (1997) Serpulidae (Annelida, Polychaeta) of the Arctic Ocean. Sarsia 82:203–236
Laudien J (2011a) Physical oceanography at time series station Brandal in the Kongsfjorden (Spitsbergen, Arctic) in 2008/2009. Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, doi:pangaea.de/10.1594/PANGAEA.742768
Laudien J (2011b) Physical oceanography at time series station Brandal in the Kongsfjorden (Spitsbergen, Arctic), 2009-09 to 2011-06. Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, doi:pangaea.de/10.1594/PANGAEA.761660
Laudien J, Herrmann M, Arntz WE (2007) Soft bottom species richness and diversity as a function of depth and iceberg scour in Arctic glacial Kongsfjorden (Svalbard). Polar Biol 30:1035–1046
Lefauconnier B, Hagen JO, Rudant JP (1994) Flow speed and calving rate of Kongsbreen glacier, Svalbard, using SPOT images. Polar Res 10:56–65
Levin LA, Etter RJ, Rex MA, Gooday AJ et al (2001) Environmental influences of regional deep-sea species diversity. Annu Rev Ecol Syst 32:51–93
Lippert H, Iken K, Rachor E, Wiencke C (2001) Macrofauna associated with macroalgae in the Kongsfjord (Spitsbergen). Polar Biol 24:512–522
Logan A, Page FH, Thomas MLH (1984) Depth zonation of epibenthos on sublittoral hard substrates off Deer Island, Bay of Fundy, Canada. Estuar Coast Shelf S 18:571–592
Lüning K, Dring MJ (1979) Continuous underwater light measurement near Helgoland (North Sea) and its significance for characteristic light limits in the sublittoral region. Helgoland Wiss Meer 32:403–424
MacDonald IR, Bluhm BA, Iken K, Gagaev S, Strong S (2010) Benthic macrofauna and megafauna assemblages in the Arctic deep-sea Canada Basin. Deep Sea Res II 57:136–152
McLachlan A (1990) Dissipative beaches and macrofauna communities on exposed intertidal sands. J Coast Res 6:57–71
Mehlum F (1991) Eider studies in Svalbard. Norsk Polarinstitutt Skrifter, vol 195
Mileikovsky SA (1971) Types of larval development in marine bottom invertebrates, their distribution and ecological significance: a re-evaluation. Mar Biol 10:193–213
Moore PG (1977) Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanogr Mar Biol Annu Rev 15:225–363
Narayanaswamy BE, Renaud PE, Duineveld GC, Berge J, Lavaleye MS, Reiss H, Brattegard T (2010) Biodiversity trends along the western European margin. PLoS One. 13;5(12):e14295
Noble JPA, Logan A, Webb GR (1976) The recent Terebratulina Community in the rocky subtidal zone of the Bay of Fundy, Canada. Lethaia 9:1–17
Normann U, Pettersen F (1984) Hydrografiske observasjoner (havmiljødata) fra Svalbard 1979–1983 (in Norwegian). University of Tromso, Tromura, vol 40
Oschmann W (1990) Dropstones—rocky mini-islands in high-latitude pelagic soft substrate environments. Senkenbergiana Marit 21:55–75
Pacheco AS, Laudien J, Thiel M, Oliva M, Heilmayer O (2010) Succession and seasonal onset of colonization in subtidal hard-bottom communities off northern Chile. Mar Ecol 32:75–87
Palerud R, Gulliksen B, Brattegard T, Sneli J-A, Vader W (2004) The marine macro-organisms in Svalbard waters. In: Prestrud P, Strøm H, Goldman HV (ed) A catalogue of the terrestrial and marine animals of Svalbard. Norsk Polarinstitutt. Skrifter, vol 201, pp 5–56
Piepenburg D, Chernova NV, von Dorrien CF, Gutt J, Neyelov AV, Rachor E, Saldanha L, Schmid MK (1996) Megabenthic commmunities in the waters around Svalbard. Polar Biol 16:431–446
Renaud PE, Włodarska-Kowalczuk M, Trannum H, Holte B, Węsławski JM, Cochrane S, Dahle S, Gulliksen B (2007) Multidecadal stability of benthic community structure in a high-Arctic glacial fjord (van Mijenfjord, Spitsbergen). Polar Biol 30:295–305
Renaud PE, Carroll ML, Ambrose WG Jr (2008) Effects of global warming on Arctic sea-floor communities and its consequences for higher trophic levels. In: Duarte C (ed) Impacts of global warming on polar ecosystems. Fundacion BBVA, Bilbao
Renaud PE, Webb TJ, Bjørgesæter A, Karakassis I, Kędra M, Kendall MA, Labrune C, Lampadariou N, Somerfield PJ, Włodarska-Kowalczuk M, Vanden Berghe E, Claus S, Aleffi IF, Amouroux JM, Bryne KH, Cochrane SJ, Dahle S, Degraer S, Denisenko SG, Deprez T, Dounas C, Fleischer D, Gil J, Grémare A, Janas U, Mackie ASY, Palerud R, Rumohr H, Sardá R, Speybroeck J, Taboada S, Van Hoey G, Węsławski JM, Whomersley P, Zettler ML (2009) Continental-scale patterns in benthic invertebrate diversity: insights from the MacroBen database. Mar Ecol Prog Ser 382:239–252
Roberts DE, Fitzhenry SR, Kennelly SJ (1994) Quantifying subtidal macrobenthic assemblages on hard substrata using a jump camera method. J Exp Mar Biol Ecol 177:157–170
Ronowicz M (2007) Benthic hydroids (Cnidaria: Hydrozoa) from Svalbard waters biodiversity and distribution. J Mar Biol Ass UK 87:1089–1094
Ronowicz M, Włodarska-Kowalczuk M, Kukliński P (2011) Patterns of hydroid (Cnidaria, Hydrozoa) species richness and distribution in an Arctic glaciated fjord. Polar Biol. doi:10.1007/s00300-011-0999-9
Rózycki O (1991) Mollusca. In: Klekowski RZ, Węsławski JW (eds) Atlas of marine fauna of Southern Spitsbergen. Inst Oceanol, PAS, Sopot, pp 358–531
Sahade R, Stellfeldt A, Tatián M, Laudien J (2004) Macro-epibenthic communities and diversity of Arctic Kongsfjorden, Svalbard, in relation to depth and substrate. In: Wiencke C (ed) The coastal ecosystem of Kongsfjorden, Svalbard. Synopsis of biological research performed at the Koldewey Station in the years 1991–2003. Ber Polarforsch Meeresforsch, vol 492, pp 103–111
Schmid MK, Piepenburg D (1993) The benthos zonation of the Disko Fjord, West Greenland. Medd Gronland 37:21
Schulz M, Bergmann M, von Juterzenka K, Soltwedel T (2010) Colonisation of hard substrata along a channel system in the deep Greenland Sea. Polar Biol 33:1359–1369
Sebens KP (1985) Community ecology of vertical rock walls in the Gulf of Maine, USA: small-scale processes and alternative community states. In: Moore PG, Seed R (eds) The ecology of rocky coasts. Hodder & Stoughton, Sevenoaks, pp 346–371
Sejr MK, Jensen KT, Rysgaard S (2000) Macrobenthic community structure in a high-arctic East Greenland fjord. Polar Biol 23:792–801
Su H-N, Xie B–B, Zhang X-Y, Zhou B-C, Zhang Y-Z (2010) The supramolecular architecture, function, and regulation of thylakoid membranes in red algae: an overview. Photosynth Res 106:73–87
Svendsen H, Beszczynska-Møller A, Hagen JO, Lefauconnier B, Tverberg V, Gerland S, Ørbæk JB, Bischof K, Papucci C, Zajaczkowski M, Azzolini R, Bruland O, Wiencke C, Winther J-G, Dallmann W (2002) The physical environment of Kongsfjorden-Krossfjorden, an Arctic fjord system in Svalbard. Polar Res 21:133–166
Syvitski JPM, Farrow GE, Atkinson RJA, Moore PG, Andrews JT (1989) Baffin Island fjord macrobenthos: bottom communities and environmental significance. Arctic 42:232–247
Thorson G (1957) Bottom communities (sublittoral or shallow-shelf). In: Ladd HS (ed) Treatise on marine ecology and palaeoecology Mem Geol Soc Am 67:461–534
Valdivia N, Heidemann A, Thiel M, Molis M, Wahl M (2005) Effects of disturbance on the diversity of hard-bottom macrobenthic communities on the coast of Chile. Mar Ecol Prog Ser 299:45–54
Von Juterzenka K (2004) Notes on benthic mega-/epifauna and small-scale habitat diversity in the longterm observation area. In: Klages M, Thiede J, Foucher JP (eds) The expedition ARKTIS XIX/3 of the research vessel POLARSTERN in 2003. Reports of legs 3a, 3b and 3c. Reports on polar and marine research, vol 488, p 355
Voronkov A, Stepanjants SD, Hop H (2010) Hydrozoan diversity on hard bottom in Kongsfjorden, Svalbard. J Mar Biol Ass UK 90:1337–1352
Walczowski W, Piechura J (2006) New evidence of warming propagating toward the Arctic Ocean. Geophys Res Lett 33:L12601
Wallace MI, Cottier FR, Berge J, Tarling GA, Griffiths C, Brierley AS (2010) Comparison of zoo-plankton vertical migration in an ice-free and seasonally ice-covered Arctic fjord: an insight into the influence of sea ice cover on zooplankton behavior. Limnol Oceanogr 55:831–845
Węsławski JW (1991) Malacostraca. In: Klekowski RZ, Węsławski JW (eds) Atlas of marine fauna of southern Spitsbergen. Inst Oceanol, PAS, Sopot, pp 118–332
Węsławski JM, Koszteynm J, Zajaczkowski M, Wiktor J, Kwaśniewski S (1995) Fresh water in Svalbard fjord ecosystems. In: Skjoldal HR, Hopkins CCE, Erikstad KE, Leinaas HP (eds) Ecology of Fjords and coastal waters. Elsevier, Amsterdam, pp 229–241
Węsławski JM, Wiktor J, Kotwicki L (2010a) Increase in biodiversity in the arctic rocky littoral, Sorkappland, Svalbard, after 20 years of climate warming. Mar Biodiv 40:123–130
Węsławski JM, Kendall MA, Włodarska-Kowalczuk M, Iken K, Kędra M, Legezynska J, Sejr MK (2010b) Climate change effects on Arctic fjord and coastal macrobenthic diversity—observations and predictions. Mar Biodiv. doi:10.1007/s12526-010-0073-9
Whittaker RH (1960) Vegetation of the Siskiyou mountains, Oregon and California. Ecol Monogr 30:279–338
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Wiencke C, Vögele B, Kovaltchouk NA, Hop H (2004) Species composition and zonation of marine benthic macroalgae at Hansneset in Kongsfjorden, Svalbard. In: Wiencke C (ed) The coastal ecosystem of Kongsfjorden, Svalbard. Synopsis of biological research performed at the Koldewey Station in the years 1991–2003. Ber Polarforsch Meeresforsch, vol 492, pp 55–62
Witman JD, Sebens KP (1991) Distribution and ecology of sponges at a subtidal rock ledge in the Central Gulf of Maine. In: Rützler K (ed) New perspectives in sponge biology. Smithsonian Institution Press, Washington, pp 391–396
Włodarska M, Węsławski JM, Gromisz S (1996) A comparison of the macrofaunal community structure and diversity in two arctic glacial bays—a ‘cold’ one off Franz Josef Land and a ‘warm’ one off Spitsbergen. Oceanologia 38:251–283
Włodarska-Kowalczuk M, Pearson TH (2004) Soft-bottom macrobenthic faunal associations and factors affecting species distributions in an Arctic glacial fjord (Kongsfjord, Spitsbergen). Polar Biol 27:155–167
Włodarska-Kowalczuk M, Węsławski JM, Kotwicki L (1998) Spitsbergen glacial bays macrobenthos—a comparative study. Polar Biol 20:66–73
Włodarska-Kowalczuk M, Pearson TH, Kendall MA (2005) Benthic response to chronic natural physical disturbance by glacial sedimentation in an Arctic fjord. Marine Ecol Prog Ser 303:31–41
Young CM, Chia F-S (1984) An experimental test of shadow response function in ascidian tadpoles. J Exp Mar Biol Ecol 85:165–175
Zajączkowski M (2000) Doplyw i sedymentacja zawiesiny w wybranych fiordach zachodniego Spitsbergenu. PhD dissertation, Uniwersytet Gdanski, Gdynia (in Polish), p 91
Zajączkowski M (2008) Sediment supply and fluxes in glacial and outwash fjords, Kongsfjorden and Adventfjorden, Svalbard. Pol Polar Res 29:59–72
Zajączkowski M, Włodarska-Kowalczuk M (2007) Dynamic sedimentary environments of an Arctic glacier-fed river estuary (Adventfjorden, Svalbard). I. Flux, deposition, and sediment dynamics. Estuar Coast Shelf S 74:285–296
Zenkevich L (1963) Biology of the seas of the U.S.S.R. George Allen & Unwin, London, pp 956–995
Acknowledgments
Our deepest thanks are expressed for support during the fieldwork by the captain of the research boat Teisten Arne-Kristoffer Olstad (Kings Bay AS, Norway) and the two ROV pilots Dominique Fleury and Alain Pottier (IPEV, France). We are in dept to Maike Thomsen, who transferred the video material in a digital format. We are also very grateful to Jørgen Berge, Cornelia and Friedrich Buchholz, Bjørn Gulliksen and Christian Wiencke who helped with the identification of organisms and to Rainer Sieger, who archived the data in the database PANGAEA of the World Data Centre for Marine Environmental Sciences (WDC-MARE). We are in dept to Emma Plotnek and Ruth Alheit for English copy editing, and thank Amy-Jane Beer, Bjørn Gulliksen and two anonymous reviewers, who gave valuable comments improving the original manuscript considerably.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laudien, J., Orchard, JB. The significance of depth and substratum incline for the structure of a hard bottom sublittoral community in glacial Kongsfjorden (Svalbard, Arctic)—an underwater imagery approach. Polar Biol 35, 1057–1072 (2012). https://doi.org/10.1007/s00300-011-1153-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1153-4