Abstract
At the arctic archipelago of Svalbard, bare glacier surfaces are populated by microalgae like Ancylonema nordenskiöldii (Zygnematales, Streptophyta). The resulting blooms cause, due to a vacuolar pigmentation, brownish colourations of the glacier surface. This freshwater ice alga has been described from several polar and alpine glaciers; however, these reports lacked data about the ecophysiology or ultrastructure. Considering the harsh environmental conditions of the exceptional habitat, such as permanently low temperatures, exposure to high irradiation or a short vegetation period, the aim of this study was to elucidate cellular adaptations of A. nordenskiöldii. Thus, samples were collected at two glaciers in Spitsbergen. The cytoarchitecture of the cylindrical cells, which are arranged in unbranched filaments, demonstrates active cells with Golgi bodies, mitochondria and rough endoplasmic reticulum close to the nucleus when investigated by transmission electron microscopy (TEM). The cell walls are pore less and only 90 nm thin. A. nordenskiöldii only sporadically produces oblong zygotes when two filaments conjugate. The most remarkable cytological feature is peripheral brownish vacuoles, appearing osmiophil and electron dense by TEM. Aqueous extracts of this pigmentation show a broad absorption in the visible light and in the UV. Consequently, a protection against excessive irradiation is provided. Photosynthesis measurements performed at different temperatures and light levels indicate that the metabolism is adapted to temperatures close to the freezing point as well as to high light conditions. Therefore, A. nordenskiöldii can be regarded as metabolically and cytological well adapted to live on glaciers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The surfaces of polar glaciers, where permanent or semipermanent covers of snow and ice prevail, were initially regarded as sterile deserts devoid of any life. During the last decades however, especially melting snow fields and wet glacier surfaces were revealed to be ecosystems occupied by a number of highly specialized psychrophilic organisms like bacteria, algae or fungi (Margesin and Schinner 1999; Mueller and Pollard 2004; Hodson et al. 2008). From early summer on, when liquid water becomes available between the ice crystals, blooms of algae can cause striking, macroscopically visible colourations depending on the prevailing cellular pigmentation. The probably best known phenomenon, encountered at Svalbard (79°N) and many other places worldwide, is so-called red snow, caused by Chlamydomonas cf. nivalis (Kol and Eurola 1974; Stibal et al. 2007). The taxonomy and ecology of such a polar or alpine eukaryotic cryoflora (defined by exclusively thriving in snow and ice) was summarized by Kol (1968) with a classical approach (determinations by light microscopy of mainly fixed material) and with recent methodology by Hoham and Duval (2001) and Komárek and Nedbalová (2008). Furthermore, Remias (2012) described cytological and physiological differences between species living either in snow or on glacier surfaces.
Noteworthy, virtually all photoautotrophs reported to thrive on bare glacier ice are members of a distinct class within the green algae, the non-flagellated Zygnematales (Streptophyta). These are two unicellular species, Mesotaenium berggrenii (Wittrock) Lagerheim 1892 and Cylindrocystis brebissonii fo. cryophila Kol 1942, as well as the filamentous Ancylonema nordenskiöldii Berggren 1871 (Kol 1968; Ling and Seppelt 1990; Leya 2004). The latter has been reported from several polar and alpine glaciers: Greenland (Uetake et al. 2010), Alaska (Takeuchi 2001), Chile (Takeuchi and Kohshima 2004), Himalaya (Yoshimura et al. 1997) or Maritime Antarctica (Komárek and Komárek 2001), thus indicating a cosmopolitan occurrence. Concerning Svalbard, reports of A. nordenskiöldii are sparse, although more than 60% of this arctic archipelago is glaciated (Kol and Eurola 1974; Leya 2004). Perhaps, the inconspicuousness of the habitat results in a lack of probing. This could explain that several floristic works about freshwater microalgae in Svalbard miss A. nordenskiöldii so far (e.g. Matuła et al. 2007; Kim et al. 2011).
One of the few works dealing with the cytology of a glacier dwelling alga investigated populations of M. berggrenii in the European Alps (Remias et al. 2009). Field populations were composed of vegetative cells throughout the season with chloroplasts containing a prominent starch sheath in a well-developed pyrenoid. Despite the harsh habitat, the alga obviously forms no spores (i.e. thickened cell walls) for overwintering, as it is common at species causing red snow (Remias 2012). Moreover, M. berggrenii possesses vacuoles abundantly filled with brownish, secondary phenols, which’s structure as purpurogallin derivatives recently has been described by Remias et al. (2011). Due to their absorption characteristics, the chloroplasts are protected against excessive irradiation. Vacuoles of A. nordenskiöldii have a similar dark pigmentation, but no report of structure determination is known so far. Further investigations of freshwater ice algae, like measurements of photosynthetic performance to evaluate the level of adaptation to the habitat, were, to our knowledge, never performed.
In this study, deeper investigations of the physiology and cytology of A. nordenskiöldii were aimed because earlier reports showed that this species represents a remarkable extremophile, exclusively living on glacial surfaces, but the principles of coping with the habitat remained unknown.
Material and methods
Sampling sites and cell harvest
Midtre Lovenbreen, situated in the Kongsfjorden region, 7 km southeast of Ny Ålesund, was sampled at 01 August 2008 (N78°53.63, E12°03.08; 101 m a.s.l.) and 05 August 2008 (N78°53.34 E12°02.75; 178 m a.s.l.). Initial samples demonstrating the presence of algae were taken in summer 2007. The second glacier, Longyearbreen, is situated 110 km south from Midtre Lovenbreen in the Adventfjorden region, 4 km southwest of Longyearbyen, and sampling was done at 15 July 2010 (N78°11.01 E15°30.91; 375 m a.s.l.) and 17 July 2010 (N78°11.02 E15°30.914; 372 m a.s.l.). Also, this glacier was successfully tested for the existence of a cryoflora before (summer 2008). The presence of algae on the ice was checked visually (darkened ice) and with a portable field microscope (Pyser-SGI Ltd., UK). Spots containing high amounts of A. nordenskiöldii were harvested with a stainless-steel shovel, scratching off the approx. three to five centimetre of the surface glacier ice. The ice was transferred into buckets and then allowed to melt gently. The separation of algae from debris (mostly cryoconite particles or remnants of insects) was done by filtration with stainless-steel sieves (Retsch, Germany) with mesh apertures of 800, 400 and 200 μm. The cell concentration per melted ice volume was calculated by directly picking approximately 10 ml of surface ice volume into plastic vials. After the melt and measurement of the total liquid volume, the suspension was shaken and 0.5 ml was transferred into a plankton counting chamber (Hydrobios, Germany) with 1 mm2 grids.
Environmental parameters
The pH and electrical conductivity of the glacial meltwater were measured with WTW instruments, Germany. Ambient radiation was measured with a PMA2100 radiometer (Solar Light, USA) according to Remias et al. (2009), with the sensors placed 10 cm above the glacier surface. The glacier temperature was recorded with TidbiT v2 logger (Onset Computer Cooperation), dug in approx. 5 cm depth below the ice surface where also algae were present.
Light- and transmission electron microscopy
Samples were either photographed at a Zeiss Axiolab microscope with a Nikon Coolpix 8400 in Svalbard or transported to the laboratory in Austria. There, they were investigated at a Zeiss Axiovert 200 M, micrographs were captured with a Zeiss Axiocam MRc5 (Carl Zeiss AG, Germany). Fluorescence microscopic images were captured with a Zeiss filter set 15 (excitation BP 546/12 nm, emission LP 590 nm). The samples were observed at 1°C in a temperature-controlled chamber to prevent temperature stress artefacts during observation.
Freshly harvested field samples of the 2008 field campaign from the Midtre Lovenbreen were chemically fixed with modifications after Holzinger et al. (2009). Briefly, samples were concentrated by centrifugation of cell suspension at 4°C. The algal filaments in meltwater were mixed 1:1 with 10 mM cacodylate buffer (pH = 6.8) containing 2.5% glutaraldehyde (giving a final concentration of 5 mM cacodylate buffer containing 1.25% glutaraldehyde). Samples were exposed for 1 h at 4°C to this solution, rinsed in cooled cacodylate buffer and postfixed in 1% OsO4 in the same buffer for 12 h at 4°C. Samples were then dehydrated in increasing ethanol concentrations, transferred via propylene oxide to low viscosity embedding resin (Agar Scientific, England) and temperature polymerized. Ultrathin sections were stained with 1% uranyl acetate for 10 min and Reynold’s lead citrate for 2 min prior to examination at a Zeiss Libra 120 TEM at 80 kV. Images were captured digitally with a ProScan 2 k SSCCD camera and processed with Adobe Photoshop software.
Analysis of the secondary pigmentation
For analytical purposes, the cleaned algal suspension was filtered through glass fibre filters (Whatman GF/C) by mild vacuum filtration with a Sterifil system (Millipore). Then, the filters were immediately frozen and lyophilized for 48 h to total dryness. The freeze-dried cells were broken in a Mikro-Dismembrator S mill (Sartorius, Germany) with an agate-stone grinding ball in 5-ml Teflon jars, the latter cooled for 10 min in liquid nitrogen prior to use. Afterwards, the powdery material was suspended and extracted in 2 ml distilled (Millipore) water. Preliminary tests showed that the brownish pigment is very hydrophilic and hardly resolves in organic solvent. To remove apolar pigments (chlorophylls, carotenoids), a phase separation with hexane was performed. Afterwards, the aqueous phase was centrifuged, and the absorption of the clear supernatant was measured with a Lambda 20 spectrophotometer (Perkin Elmer).
Photosynthesis
Light-dependent photosynthesis- and dark-respiration measurements were performed with a oxygen-optode (Fibox 3 with PSt3 sensor; PreSens, Germany) in a 3-ml acryl chamber (DW1; Hansatech Instruments, UK), thermostated to 1, 10 and 20°C. The mean oxygen performance (n = 3) and the calculated standard deviation per time was normalized to the amount of chlorophyll per sample. The amount of chlorophyll was determined spectrophotometrically, using N–N-dimethylformamid (Sigma Aldrich) for extraction (further details see Remias et al. 2010).
Results
Habitat description
At both glaciers, large areas of the surface were covered by dark and grainy material, mainly comprised of cryoconite particles (Fig. 1). The ideal spots for collecting A. nordenskiöldii were composed of firn-like ice in the middle parts of both glaciers (approximately between the accumulation and ablation zones), especially next to small glacier streams, which’s vicinity was sprayed with water. There, the algal populations caused a brownish colouration mainly in the top 2–3 cm of crispy ice, and they could be harvested relatively monospecific (almost free of typical snow algae). Furthermore, dark brown spots (with diameters of some millimetres) were found at several locations, where clumps of A. nordenskiöldii had been frozen in solid ice, most likely pools of cells originating from the season before. These spots were washed free by meltwater, potentially dispersing the algae along the ice. At the lower parts of both glaciers, Chlamydomonas cf. nivalis caused patches of red snow, which gradually mixed with A. nordenskiöldii on the ice surface after complete snow melt. Contrary, the upper parts of both glaciers were still covered with white, algal-free snow even during the late arctic summer (July, August), which was checked by a field microscope. Furthermore, the samples contained some scattered cells of a third psychrophilic species, the unicellular Mesotaenium berggrenii var. alaskana. In 2010, the cell concentrations per ml meltwater at the middle part of Longyearbreen were approx. 23.500 cells for A. nordenskiöldii, 1.140 for C. nivalis and 300 for M. berggrenii.
The ambient irradiation values of Longyearbreen were recorded at 13 July 2010 during a sunny day between 12 and 13 h CET (Central European Time). The values varied between 995 and 1,104 μmol PAR m−2 s−1. UVA was in the range of 20.8–23.1 W m−2 and UVB between 49.0 and 63.3 mW m−2. At Midtre Lovenbreen, only the VIS values were recorded: on 05 August 2008, around noon, the irradiation varied between 850 and 1,120 μmol PAR m−2 s−1 at sunny conditions and dropped to approximately 160 μmol when overcast. The pH values of ice meltwater varied between 4.7 and 6.0, and the electrical conductivity ranged from 3.7 to 20.1 μS cm−1. The abiotic factors of the melted ice are summarized in Table 1, along with data from other references. Furthermore, the diurnal temperature variations of Midtre Lovenbreen were recorded with a logger. During night (18 h CET to 6 h CET), the temperature varied around 0.4 ± 0.2°C and raised during day (6 h CET to 18 h CET) to 1.9 ± 0.4°C.
Cellular appearance by light microscopy
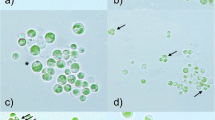
Light microscopy (LM) revealed the characteristic brownish-green appearance of field samples of A. nordenskiöldii with mean cell sizes of 19.0 ± 7.8 μm for length and 8.1 ± 1.7 μm for width (see also Table 2, together with results from other locations and authors). The number of cells per filament varied between 2 and approximately 64, sometimes containing obviously dead cells with a totally bleached interior. With mean diameters of 6.3 ± 1.2 μm after cell division, the similar pigmented unicellular M. berggrenii was significantly smaller. In the top view (Fig. 2a), the brownish vacuoles of A. nordenskiöldii covered other cellular structures, but in the side view (Fig. 2b, c), the cytoarchitecture with two parietal chloroplasts per cell (Fig. 2b) and the central translucent area where the nucleus was located became obvious (Fig. 2c). When cells were viewed with the fluorescence mode, the chloroplasts became visible, despite frequently not observable in bright field mode (top view) (Fig. 2d, e). In Midtre Lovenbreen samples of 2007, the conjugation process (illustrated in Fig. 2f, g) and mature, irregularly oblong zygotes were found (Fig. 2h), however in low numbers. Details how the sexual process was actually induced were not observed.
Light micrographs of field material of A. nordenskiöldii. a top view showing the dark vacuoles, b side view demonstrating the two parietal chloroplasts (arrows), c side view demonstrating translucent areas where the nucleus is located (arrow), d, e corresponding light and fluorescence image in top view, while in the light micrograph, only the dark pigment of the vacuoles is visible (d), in the fluorescence image the, two chloroplasts become obvious (e), f conjugating filaments (arrow), g conjugation leads to a fusion of the protoplasts (arrows), h mature zygote (arrow), the lower one and the vegetative cell above are dead. Scale Bars = 10 μm
Ultrastructural appearance by transmission electron microscopy
Field samples investigated by transmission electron microscopy (TEM) showed prominent electron-dense vacuoles, covering a large proportion of the cell body (Fig. 3a–e). Depending on the direction of the section, the vacuoles of A. nordenskiöldii were arranged either equally peripheral (Fig. 3a) or lying at one side of the cell (Fig. 3b). The vacuoles had a high electron density, but showed small electron-translucent portions, likely representing cytoplasmic strands penetrating the vacuoles (Fig. 3d). The cytoplasm contained several, medium-electron-dense lipid bodies with diameters below 1 μm (Fig. 3d). The chloroplast contained a central pyrenoid surrounded by starch grains (Fig. 3d, e). Thylakoid membranes were parallel oriented in the chloroplast, and numerous plastoglobules could be observed (Fig. 3f). The cells contained Golgi bodies, mostly in close vicinity to the chloroplasts (Fig. 3f, g), mitochondria and dense accumulations of rough ER close to the nucleus (Fig. 3g). The cell walls were thin (approx. 90 nm) and did not contain pores (Fig. 3h).
Transmission electron micrographs of A. nordensköldii from freshly harvested samples of the field campaign 2008 on Midtre Lovenbreen. Abbreviations: Chl chloroplast, CW cell wall, ER endoplasmic reticulum, G Golgi body, L lipid droplet, PG plastoglobule, M mitochondrion, Py pyrenoid, N nucleus, S starch, V vacuole. a longitudinal section, chloroplast and cytoplasm are covered by the electron-dense vacuole, b intermediate section, with nucleus and chloroplast, c cross-section with nucleus in the centre, d pyrenoid with starch grains, lipid bodies, vacuoles with electron-translucent cytoplasmic strands (arrows), e cytoplasm in the centre surrounded by electron-dense vacuole and numerous lipid bodies, f detail of the chloroplast with thylakoid membranes and plastoglobules, Golgi bodies close to the chloroplast, g detail with nucleus, Golgi body, mitochondria and accumulation of rough endoplasmic reticulum, h detail of two adjacent cells with cell wall, lipid droplets and electron-dense vacuole. Scale Bars a–c = 2 μm; d, e = 1 μm; f–h = 0.5 μm
Secondary pigmentation and photosynthesis
The spectral absorption of the aqueous extract of A. nordenskiöldii containing the secondary pigmentation is shown in Fig. 4. It had, on the one hand, a maximum at 339.7 nm, causing a high UV A & B absorption. On the other hand, a lower but broad shoulder of absorbance in the VIS region from 400 to 700 nm was present, evidently causing the brownish aspect.
Photosynthesis and respiration measurements of the alga were performed at different, short-term applied irradiation and temperature levels (Fig. 5). At 1°C, oxygen production was most effective at lower light conditions (<400 μmol photons m−2 s−1), whereas at 10 and 20°C, in combination with high light conditions (>400 μmol PAR), the photosynthesis showed a better performance. However, respiration (oxygen consumption in the dark) significantly increased when compared to conditions close to ambient (1°C), and this resulted in very high light compensation points (e.g. approx. 100 μmol PAR m−2 s−1 at 10°C). Generally, no decline of photosynthesis (e.g. due to photorespiration) took place, even at more than 1,900 μmol PAR m−2 s−1.
Light- and temperature-dependent photosynthesis and respiration of A. nordenskiöldii at different short-time applied light- and temperature levels. The samples were collected at Midtre Lovenbreen, the performance is normalized to the amount of chlorophyll. At 1°C (solid line), the oxygen production performs best at irradiances less than 400 μmol PAR m−2 s−1. At 10 (dashed) and 20°C (dotted), only high light conditions show higher oxygen formation, because dark respiration rose significantly at the later two situations, caused by heat stress
Discussion
Until now, A. nordenskiöldii has been found exclusively at glacier surfaces, but it seems to be widely distributed because it occurs in many mountainous and polar regions of the world (see introduction). Interestingly, this species has never been reported from the European Alps despite these glaciers are comparably well investigated. An old record of Chodat (1896) from the Mont Blanc massif (Switzerland) seems to be questionable, because it lacks any drawing or distinct morphological description. Moreover, the term Ancylonema was used misleadingly for the similar but unicellular M. berggrenii. While the latter seems to be common in the European Alps (Remias et al. 2009; Remias et al. 2011), the reason of absence of A. nordenskiöldii is unknown.
As shown by Takeuchi and Kohshima (2004) and in this work, the habitat meltwater pH can be acidic (4.7–6.0), but also reach alkaline conditions (8.6 at King George Island; Komárek and Komárek 2001). Most likely, the pH is influenced by the type of surrounding bedrock, but photosynthesis of the population may also cause local changes due to CO2 consumption (Hoham and Duval 2001). Moreover, Hoham et al. (2007) have shown that snow algae can have certain pH optima for their growth. The amount of dissolved ions is generally low when taking into account the measured electrical conductivities (2.5–20.1 μS cm−1). The values are in a similar range like reported by other authors (see Table 1; Hoham and Duval 2001) and typical for locations that are not influenced by sea spray or animal colonies. The measured VIS intensity was in the range described for Svalbard, as reported by Hanelt et al. (2001) for a sunny day in August 1995. The total UV radiation was approximately one-third lower than in this work (14 W m−2), however measured with different instrumentation, including UV only in a narrower range of 300–370 nm.
The reported average cell sizes of A. nordenskiöldii vary significantly (see Table 2). For example, populations from a Chilean glacier have lengths more than twofold compared with Svalbard in this work (Takeuchi and Kohshima 2004). This raises the question whether there is truly one cosmopolite species with varying cell sizes or a group of morphologically similar taxa share the same kind of habitat. Interestingly, Ling and Seppelt (1990) reported two distinct size classes in Antarctic populations of M. berggrenii and argued that this could be caused be either haplo- and diploid populations (the latter being the larger ones). Maybe a similar phenomenon exists in A. nordenskiöldii, causing such varying cell sizes. Unfortunately, no strains of Ancylonema are available in culture collections to allow detailed cytological investigations. Our own culturing experiments failed, the filaments ceased any growth when transferred to diluted medium at conditions close to the freezing point. Also, an institution specialized in culturing snow algae had no success with this species (T. Leya, CCCryo Germany, pers. comm.). Obviously, simulating the conditions prevailing on glacier surfaces seems to be a challenging task. Also, the question when and under which conditions the formation of zygotes is induced remains open, but it is clearly not a frequent process occurring in every population. Elicitors like daylight duration, nutrients or liquid water availability may be discussed, and closer observations lasting the whole arctic summer season would be necessary.
The question of polar and high alpine biodiversity should be resolved by molecular methods with field samples taken from many different regions. So far, Remias et al. (2011) showed by comparing 18S rRNA sequences that M. berggrenii from the Austrian Alps and A. nordenskiöldii from Svalbard are closely relative, but distinct species.
The photosynthesis of A. nordenskiöldii is well adapted to conditions at arctic glacier surfaces, where temperatures are constantly around 0–1°C during summer, as shown in the results. The large increase of dark respiration at higher temperatures, resulting into the need of relatively high amounts of light for a positive energy balance (light compensation point more than 100 μmol PAR m−2 s−1), indicates that this species is stressed at warmer conditions. This has to be considered when interpreting Fig. 5, where photosynthetic oxygen production was higher at 10 and 20°C during the experiments. However, the cells’ metabolism would burn too much energy as indicated by elevated amounts of respiration when long-term exposed to non-glacial temperatures. This could explain why this species has never been found in soils in the vicinity of retreated glaciers (Kaštovská et al. 2007; own negative observations of several sites by LM). Generally, short-time measurements as performed in this study only indirectly point to the psychrophilic nature of A. nordenskiöldii. For a direct proof, growth experiments with cultures would be necessary. The good photosynthesis performance at higher irradiation levels (>400 μmol PAR m−2 s−1) can be explained either by the presence of high-light-adapted photosystems or by protection of the chloroplast against effects of photoinhibition due to the secondary pigmentation.
The high abundance of the brownish vacuoles with the combined UV/VIS absorption excludes most likely excessive irradiation. Thus, the chloroplast and other organelles were protected against UV, which can cause severe damages in algae (Holzinger and Lütz 2006). Remias et al. (2010) showed that two microalgae from Svalbard, when exposed to increased levels of UVB, raise their contents of secondary pigmentation. However, the absolute irradiation values on both sampled arctic glaciers were significantly lower to those reported from a population of the unicellular relative M. berggrenii on glaciers at 3,000 m a.s.l. in the Austrian Alps (Remias et al. 2009). The alpine VIS was nearly twice of the arctic maximum values, whereas UVA was 2.6-fold and UVB even 4.5-fold higher than in the coastal regions of Svalbard. In general, arctic freshwater ice algae would need less protection against excessive UV/VIS, but the extent of brownish pigmentation of alpine M. berggrenii and arctic A. nordenskiöldii looks virtually the same. Consequently, there may be further reasons for the abundant secondary pigmentation, such as the quenching of reactive oxygen species (ROS), which otherwise would cause destructive intracellular effects (Hadacek et al. 2011).
Similar secondary vacuole-bound pigmentations like in A. nordenskiöldii or M. berggrenii have been found in further members of the Zygnematales, i.e. C. brebissonii (Kol 1968) or Zygogonium ericetorum collected from alpine soils (Holzinger et al. 2010). In the latter species, field samples of the top layers appeared particularly purple, whereas this colouration was lost under culture conditions lacking UV irradiation (Holzinger et al. 2010). Recently, Remias et al. (2011) elucidated the chemical structure of the main component causing the brownish vacuoles of the alpine M. berggrenii. Unexpectedly, it was a glycosylated purpurogallin derivative, a polyphenol that has been known only from higher plants so far. This finding strengthens the close relationship of the conjugating algae to the land plants, which are now discussed to represent the next living relatives of higher plants, even closer than stoneworts (Wodniok et al. 2011). Since the spectral absorptions of aqueous extracts of M. berggrenii (Remias 2012) and of A. nordenskiöldii (this work) are similar, the second species most likely contains large amounts of putative purpurogallin-derived phenolics, too.
The most prominent ultrastructural feature of A. nordenskiöldii was the electron dense, osmiophilic vacuoles, likely protecting other compartments such as the nucleus or chloroplasts against UV irradiation due to their arrangement. In M. berggrenii however, the vacuoles appeared unstained, possibly due to a different fixation procedure (Remias et al. 2009). In other Zygnematales like Zygnema sp. collected from soils in Svalbard, smaller vacuoles, only a few micrometres in diameter, showed a similar electron-dense contrast as in A. nordenskiöldii (Holzinger et al. 2009). In TEM studies of field samples of Zygogonium ericetorum, the vacuoles appeared electron-translucent, and electron-dense particles were mostly found in desiccated samples, typically reaching diameters of 1–2 μm (Holzinger et al. 2010), but never covering larger parts of the cytoplasm. In contrast, neither the Trebouxiophycean green alga Prasiola crispa from the arctic (Holzinger et al. 2006) nor the Streptophycean Klebsormidium crenulatum (Holzinger et al. 2011) and K. dissectum (Karsten and Holzinger 2011) from the high Alps showed similar electron-dense vacuolar contents as A. nordenskiöldii.
Other ultrastructural details in A. nordenskiöldii point towards a highly metabolizing organism in its cold habitat: a prominent starch sheet around the pyrenoid, numerous Golgi bodies, accumulations of rough ER close to the nucleus and many mitochondria are characteristic for active cells, similarly to what was observed in M. berggrenii (Remias et al. 2009) for a comparable habitat in the Austrian Alps. This is in contrast to snow algae like Chlamydomonas cf. nivalis, which stay in a robust spore stage for most of the season to outlast unfavourable conditions. While in the spore stages, the cell walls were thick and sculptured (Remias 2012), in A. nordenskiöldii, extremely thin and smooth cell walls of only 90 nm diameter were observed. The latter did not contain pores or plasmodesmata, which would provide movement by slime secretion or metabolic interchange within the filaments. This situation is typical for Zygnematales, as already demonstrated by Mix (1972). This feature, as well as new molecular data, clearly separates Zygnematales from the Desmidiales (e.g. McCourt et al. 2000; Gontcharov et al. 2003).
This study represents the first report of ultrastructure and photosynthesis of the arctic glacial alga A. nordenskiöldii, which shows that this species is adapted well to its harsh habitat. Therefore, during summer, the bare glacier surfaces of Svalbard cannot be ignored in terms of biological activity. The fact that specialized photoautotrophs form a simple but stable ecosystem at such locations has been neglected for long time, most probably because icy surfaces were expected to be virtually devoid of eukaryotic life. Only molecular studies could reveal if the different populations of A. nordenskiöldii at locations very remote from each other truly represent one species. Future investigations should also deal with the structure determination and biochemical characterization of the brownish pigment of this species.
Abbreviations
- ER:
-
Endoplasmic reticulum
- VIS:
-
Visible light
- TEM:
-
Transmission electron microscopy
- UV:
-
Ultraviolet light
References
Chodat R (1896) La Flore des Neiges du Col des Ecandies. Bull Herb Boiss 4:879–889
Gontcharov AA, Marin B, Melkonian M (2003) Molecular phylogeny of conjugating green algae (Zygnematophyceae, Streptophyta) inferred from SSU rDNA sequence comparisons. J Mol Evol 56:89–104
Hadacek F, Bachmann G, Engelmeier D, Chobot V (2011) Hormesis and a chemical raison d’être for secondary plant metabolites. Dose-Response 9:79–116
Hanelt D, Tüg H, Bischof K, Gross C, Lippert H, Sawall T, Wiencke C (2001) Light regime in an Arctic fjord: a study related to stratospheric ozone depletion as a basis for determination of UV effects on algal growth. Mar Biol 138:649–658
Hodson A, Anesio AM, Tranter M, Fountain A, Osborn M, Priscu J, Laybourn-Parry J, Sattler B (2008) Glacial ecosystems. Ecol Monogr 78:41–67
Hoham RW, Duval B (2001) Microbial ecology of snow and freshwater ice with emphasis on snow algae. In: Jones HG, Pomeroy JW, Walker DA, Hoham RW (eds) Snow ecology. Cambridge University Press, New York, pp 168–228
Hoham RW, Filbin RW, Frey FM, Pusack TJ, Ryba JB, McDermott PD, Fields RA (2007) The optimum pH of the green snow algae, Chloromonas tughillensis and Chloromonas chenangoensis, from Upstate New York. Arct Antarct Alp Res 39:65–73
Holzinger A, Lütz C (2006) Algae and UV irradiation: effects on ultrastructure and related metabolic functions. Micron 37:190–207
Holzinger A, Karsten U, Lütz C, Wiencke C (2006) Ultrastructure and photosynthesis in the supralittoral green macroalga Prasiola crispa (Lightfoot) Kützing from Spitsbergen (Norway) under UV exposure. Phycologia 45:168–177
Holzinger A, Roleda MY, Lütz C (2009) The vegetative arctic green alga Zygnema is insensitive to experimental UV exposure. Micron 40:831–838
Holzinger A, Tschaikner A, Remias D (2010) Cytoarchitecture of the desiccation-tolerant green alga Zygogonium ericetorum. Protoplasma 243:15–24
Holzinger A, Lütz C, Karsten U (2011) Desiccation stress causes structural and ultrastructural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust. J Phycol 47:591–602
Karsten U, Holzinger A (2011) Light, temperature and desiccation effects on photosynthetic activity and drought-induced ultrastructural changes in the green alga Klebsormidium dissectum (Streptophyta) from a high alpine soil crust. Microb Ecol. doi:10.1007/s00248-011-9924-6
Kaštovská K, Stibal M, Šabacká M, Černá B, Elster J (2007) Microbial community structure and ecology of subglacial sediments in two polythermal Svalbard glaciers characterized by epifluorescence microscopy and PLFA. Polar Biol 30:277–287
Kim GH, Klochkova TA, Han WH, Kang SH, Choi HG, Chung KIW, Song JK (2011) Freshwater and terrestrial algae from Ny-Alesund and Blomstrandhalvoya Island (Svalbard). Arctic 64:25–31
Kol E (1968) Kryobiologie. Biologie und Limnologie des Schnees und Eises. I. Kryovegetation. Die Binnengewässer, Band XXIV. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart
Kol E, Eurola S (1974) Red snow algae from Spitsbergen. Astarte 7:61–66 (J Arct Biol)
Komárek O, Komárek J (2001) Contribution to the taxonomy and ecology of cryosestic algae in the summer season 1995–96 at King George Island, S. Shetland Islands. Beih Nova Hedwigia 123:121–140
Komárek J, Nedbalová L (2008) Green cryosestic algae. In: Seckbach J (ed) Cellular origin, life in extreme habitats and astrobiology (volume 11): algae and cyanobacteria in extreme environments, part 4: phototrophs in cold environments. Springer, Dordrecht, pp 323–344
Leya T (2004) Feldstudien und genetische Untersuchungen zur Kryophilie der Schneealgen Nordwestspitzbergens. Dissertation. Shaker, Aachen
Ling HU, Seppelt RD (1990) Snow algae of the Windmill Islands, continental Antarctica. Mesotaenium berggrenii (Zygnematales, Chlorophyta) the alga of grey snow. Antarct Sci 2:143–148
Margesin R, Schinner F (1999) Cold-adapted organisms. Ecology, physiology, enzymology and molecular biology. Springer, Berlin
Matuła J, Pietryka M, Richter D, Wojtuń B (2007) Cyanoprokaryota and algae of Arctic terrestrial ecosystems in the Hornsund area, Spitsbergen. Polish Polar Res 28:283–315
McCourt RM, Karol KG, Bell J, Helm-Bychowski KM, Grajewska A, Wojciechowski MF, Hoshaw RW (2000) Phylogeny of the conjugating green algae (Zygnemophyceae) based on rbcL sequences. J Phycol 36:747–758
Mix M (1972) Die Feinstruktur der Zellwände bei Mesotaeniaceae und Gonatophyceae mit einer vergleichenden Betrachtung der verschiedenen Wandtypen der Conjugatophyceae und über deren systematischen Wert. Arch Mikrobiol 81:197–220
Mueller DR, Pollard WH (2004) Gradient analysis of cryoconite ecosystems from two polar glaciers. Polar Biol 27:66–74
Remias D (2012) Cell structure and physiology of alpine snow and ice algae. In: Lütz C (ed) Plants in alpine regions. Cell physiology of adaption and survival strategies. Springer, Wien, pp 175–186
Remias D, Holzinger A, Lütz C (2009) Physiology, ultrastructure and habitat of the ice alga Mesotaenium berggrenii (Zygnemaphyceae, Chlorophyta) from glaciers in the European Alps. Phycologia 48:302–312
Remias D, Albert A, Lütz C (2010) Effects of realistically simulated, elevated UV irradiation of photosynthesis and pigment composition of the alpine snow alga Chlamydomonas nivalis and the arctic soil alga Tetracystis sp. (Chlorophyceae). Photosynthetica 48:302–312
Remias D, Schwaiger S, Aigner S, Leya T, Stupper H, Lütz C (2011) Characterization of an UV- and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Zygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiol Ecol. doi:10.1111/j.1574-6941.2011.01245.x
Stibal M, Elster J, Šabacká M, Kaštovská K (2007) Seasonal and diel changes in photosynthetic activity of the snow alga Chlamydomonas nivalis (Chlorophyceae) from Svalbard determined by pulse amplitude modulation fluorometry. FEMS Microbiol Ecol 59:265–273
Takeuchi N (2001) The altitudinal distribution of snow algae on an Alaska glacier (Gulkana Glacier in the Alaska Range). Hydrol Process 15:3447–3459
Takeuchi N, Kohshima S (2004) A snow algal community on Tyndall Glacier in the Southern Patagonia Icefield, Chile. Arct Antarct Alp Res 36:92–99
Uetake J, Naganuma T, Hebsgaard MB, Kanda H, Kohshima S (2010) Communities of algae and cyanobacteria on glaciers in west Greenland. Polar Sci 4:71–80
Wodniok S, Brinkmann H, Glöckner G, Heidel AJ, Philippe H, Melkonian M, Becker B (2011) Origin of land plants: do conjugating green algae hold the key? BMC Evol Biol 11:104–113
Yoshimura Y, Kohshima S, Ohtani S (1997) A community of snow algae on a Himalayan glacier: change of algal biomass and community structure with altitude. Arct Antarct Alp Res 29:126–137
Acknowledgments
The authors thank Dr. Birgit Sattler, Institute of Ecology, University of Innsbruck, for collecting ice samples at Midtre Lovenbreen in 2007. This study has been supported by the Austrian Science Fund (FWF, P20810 to C.L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Remias, D., Holzinger, A., Aigner, S. et al. Ecophysiology and ultrastructure of Ancylonema nordenskiöldii (Zygnematales, Streptophyta), causing brown ice on glaciers in Svalbard (high arctic). Polar Biol 35, 899–908 (2012). https://doi.org/10.1007/s00300-011-1135-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1135-6