Abstract
Microscopic analysis of the phytoplankton and other protist communities in High Arctic lakes has shown that they often contain taxa in the Chrysophyceae. Such studies have been increasingly supported by pigment analysis using high-performance liquid chromatography (HPLC) to identify the major algal groups. However, the use of 18S rRNA gene surveys in other systems indicates that many protists, especially small heterotrophs, are underreported or missed by microscopy and HPLC. Here, we investigated the late summer protist community structure of three contrasting lakes in High Arctic polar desert catchments (Char Lake at 74°42′ N, Lake A at 83°00′ N and Ward Hunt Lake at 83°05′ N) with a combination of microscopy, pigment analysis and small subunit 18S ribosomal RNA gene surveys. All three methods showed that chrysophytes were well represented, accounting for 50–70% of total protist community biomass and 25–50% of total 18S rRNA gene sequences. HPLC analysis supported these observations by showing the ubiquitous presence of chrysophyte pigments. The clone libraries revealed a greater contribution of heterotrophs to the protist communities than suggested by microscopy. The flagellate Telonema and ciliates were common in all three lakes, and one fungal sequence was recovered from Char Lake. The approaches yielded complementary information about the protist community structure in the three lakes and underscored the importance of chrysophytes, suggesting that they are well adapted to cope with the low nutrient supply and strong seasonality that characterize the High Arctic environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The protist taxonomic composition of lakes is highly variable and influenced by local conditions of irradiance and nutrient supply. The majority of high-latitude lakes are oligotrophic or even ultra-oligotrophic because slow weathering in cold polar soils results in low catchment inputs of nutrients (Vincent et al. 2008). Irradiance conditions of these lakes differ from temperate lakes, with continuous darkness throughout the winter months and low irradiance over much of the rest of the year due to ice cover. These particular features of Arctic lakes and their relative isolation provide an opportunity to investigate communities adapted to such low nutrient and energy conditions.
The phytoplankton community composition of Canadian High Arctic waters has been previously examined by microscopy (Kalff et al. 1975; Vallières et al. 2008), and pigment analysis using high-performance liquid chromatography (HPLC) has also been applied to infer the major phytoplankton groups present in several High Arctic lakes (Bonilla et al. 2005; Mueller et al. 2005). Both approaches have indicated the common occurrence of chrysophytes in high-latitude lakes in general (Panzenböck et al. 2000; Laybourn-Parry and Marshall 2003; Bonilla et al. 2005; Forsström et al. 2005). Short 18S rRNA gene sequences of chrysophytes from denaturing gradient gel electrophoresis (DGGE) bands have also been reported from oligotrophic Antarctic maritime lakes (Unrein et al. 2005). However, these techniques are thought to underestimate community diversity compared to the more recent environmental gene surveys (Moreira and López-Garcia 2002). Such molecular techniques can be used to identify smaller organisms than is possible using light microscopy, and with more precision than by pigment signatures alone (Vaulot et al. 2008). Hence, gene surveys possibly allow for a more complete assessment of the microbial community, including heterotrophs, living in these lakes. Sequence information may also enable phylogenetic comparisons of plankton from different studies and over global scales (Jungblut et al. 2010).
Although microscopy and pigment analyses have indicated the potential importance of chrysophytes in polar environments, there has been little recent taxonomic work on the organisms inhabiting Arctic lakes, and genetic investigations have been lacking. The aim of the present study was to identify and compare the contribution of different phytoplankton to the protist community structure of three limnologically contrasting High Arctic lakes using 18S rRNA gene surveys, classic microscopic observations and pigment analyses. Given the previous records based on microscopy from Arctic (Welch 1973; Panzenböck et al. 2000; Bonilla et al. 2005), Subarctic (Forsström et al. 2005) and Antarctic (Butler et al. 2000; Unrein et al. 2005) lakes, a second objective was to examine the detailed phylogeny of the chrysophytes in the different lakes. Heterotrophic protists have been little examined in such waters although heterotrophic nanoflagellates have been identified as an important food web component (Vincent et al. 2008). Hence, a third objective was to examine the biodiversity of heterotrophic protists in the lakes using both microscopy and gene surveys.
Materials and methods
Study sites
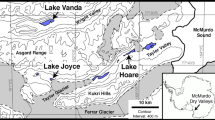
The three lake sites were located in Nunavut, in the Canadian Arctic Archipelago. Char Lake (CH) is on Cornwallis Island, near Resolute Bay, at latitude 74°42′ N and longitude 94°50′ W (Fig. 1). The limnology of the lake was extensively described during the Char Lake Project, as part of the International Biological Program (IBP), in the early 1970s (Rigler 1974). It has a drainage basin of 4.35 km2, a surface area of 0.53 km2, a maximum depth (z max) of 28 m and was considered ultra-oligotrophic (Schindler et al. 1974). The lake is normally ice-free for less than 2 months (Rigler 1974) during which time the water column is entirely mixed, and the water temperature rarely exceeds 4°C.
Lake A (LA) lies 1,000 km north of Cornwallis Island, on the northern coast of Ellesmere Island at latitude 83°00′ N, and longitude 75°30′ W (Fig. 1). It has a drainage basin of about 36 km2 (Jeffries et al. 1984), a surface area of 5 km2 and a maximum depth of 120 m (Van Hove et al. 2006). Lake A is a permanently stratified, meromictic lake and was classed as oligotrophic to ultra-oligotrophic by Van Hove et al. (2006). The upper 10 m is freshwater while the metalimnion is characterised by a conductivity of 4.5 mS cm−1 at 12 m and the monimolimnion reaches a maximum of 30.2 mS cm−1 in the deeper waters. Wind mixing is further inhibited by the perennial ice cover. During an exceptional period of warming in August 2008, this ice cover melted entirely (Vincent et al. 2009), but the salinity stratification was maintained (Veillette et al. 2011).
Ward Hunt Lake (WH) is located on Ward Hunt Island (Fig. 1), and at latitude 83°05′ N and longitude 74°10′ W is the northernmost lake of North America (Villeneuve et al. 2001). The lake’s maximum depth is 8 m, and with a total area of 0.37 km2, it was the smallest lake of this study. Ward Hunt Lake is ultra-oligotrophic and usually completely covered by thick perennial ice throughout the summer. However, in August 2008 for the first time on record, more than 25% of this ice had melted and detached from the eastern side, and the western side was entirely devoid of ice cover (Vincent et al. 2009).
Sampling and analyses
All three lakes were sampled in August 2008. Physico-chemical profiles of the water columns were taken using a conductivity–temperature–depth (CTD) profiler (XR-420 CTD-RBR profiler; RBR Ltd, Ottawa, Canada). Approximately 12 l of water for chemical and biological analyses were collected from the surface (1–2.5 m) using a closing Kemmerer bottle (Wildlife Supply Company, Yulee, FL, USA) emptied directly into lake-rinsed polypropylene containers, with no prefiltration; our experience is that prefiltration presents a contamination risk in these ultra-oligotrophic waters. The samples were kept cool and in the dark and transported back to a field laboratory, within 4 h. The water was subsampled for nutrients, microscopy, HPLC pigment determination and DNA analysis.
Nutrients
Aliquots of 120 ml of sampled water were stored in glass bottles with polypropylene caps. These were later analysed at the Canadian Center for Inland Waters (Burlington, Ontario) after being transported in the dark at ca. 4°C. Concentrations of nitrate and nitrite (NOx), total nitrogen (TN), ammonia (NH3) and soluble reactive phosphorus (SRP) were determined using standard colorimetric techniques (Gibson et al. 2002). Total phosphorus was determined from a separate 125-ml aliquot by the continuous flow analyser stannous chloride method. The detection limit was 5 μg N l−1 for NOx and 1 μg l−1 for SRP.
HPLC pigments
Samples for HPLC analyses were filtered (0.5–1 l) in our field laboratory under dim ambient light on GF/F 25-mm filters (Fisher Scientific), which were then folded and wrapped in aluminium foil and immediately placed in a Dry Shipper (nominal temperature −180°C). This was then shipped back for analysis at Université Laval, and the samples stored at −80°C until analysis. Pigments were extracted from frozen filters by sonication (3 times at 17 W, for 20 s) in 2.5 ml of 95% methanol, followed by centrifugation, and filtration with PTFE syringe filters (pore size, 0.2 μm) into HPLC vials. Shortly following extraction, 100 μl of the extracts were injected into a ProStar HPLC (Varian, Palo Alto, CA, USA) equipped with a Symmetry C8 column (3.5 μm pore size, 4.6 × 150 mm, Waters Corporation, Milford, MA, USA) with the solvent and detection protocols as in Bonilla et al. (2005).
Microscopy
Samples for microscopy were collected for analysis by the Fluorescence-Nomarski-Utermöhl (FNU; Lovejoy et al. 1993) technique. Briefly, 90 ml of lake water was fixed with 10 ml of a mix of buffered paraformaldehyde and glutaraldehyde (final concentrations of 0.1 and 1%, respectively; Tsuji and Yanagita 1981) and stored in the dark at 4°C. Samples were examined within 8 months at Université Laval, where 16–60 ml was left for 24 h in a sedimentation chamber (Hasle 1978). The sedimented samples were then stained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen Inc.; 5 μg/mL final concentration) and examined using a Zeiss Axiovert 100 inverted microscope at 400× for larger cells and 1,000× magnification for smaller cells, under visible light and ultraviolet excitation, to visualise DAPI-stained nuclei. Blue excitation was used to confirm the presence or absence of red fluorescing chloroplasts. Taxonomic identification of phytoplankton was based on Findlay and Kling (1979), Canter-Lund and Lund (1995) and Wehr and Sheath (2003) on specimens viewed at 1,000×. Heterotrophic protists were poorly identified using the above references, but they were separated by shape and size, and diversity estimates were based on the separate categories.
Taxon-specific biovolumes were estimated from the two visible dimensions measured directly with an ocular micrometer or from images captured using a Qimaging Fast 2000R system (Qimaging, Surrey BC, Canada) and processed using Image-Pro (v. 5.1.1, Acton MA). Geometry was inferred from the literature, for example, to differentiate between oblate spheres and ovoids. The biovolumes of complex cell shapes were estimated using the equations proposed by Hillebrand et al. (1999). Cell biovolumes were then transformed to carbon biomass (pg C l−1) based on the equations in Menden-Deuer and Lessard (2000).
DNA collection and clone libraries
To examine the nanoplankton community, approximately 3–4 l of water was filtered onto 47-mm-diameter 3.0-μm pore size polycarbonate filters (Millipore) with no initial prefiltration. The filters were then submerged in buffer (50 mM Tris, 40 mM EDTA, 0.75 M sucrose), in 2-ml cryovials before being stored at −80°C until further manipulation.
Community DNA was extracted using a salt (NaCl) extraction protocol modified from Aljanabi and Martinez (1997). Briefly, filters and buffer were transferred from cryovials to 15-ml tubes, with lysozyme (1 mg/ml) for 45 min at 37°C (Diez et al. 2001). Proteinase K (0.2 mg/ml) and SDS 1% were then added and cells were incubated for 1 h at 55°C (Diez et al. 2001). Concentrated NaCl (6 M) was then added to the tubes (final concentration of 2.3 M), which were vortexed for 1 min and centrifuged for 10 min at 7,000g. The supernatant was transferred into a new 15-ml tube, and 5 ml of cold 70% ethanol was added into each sample, mixed and left overnight at −20°C. For each sample, 1.8 ml of the total volume was transferred into a 2-ml microcentrifuge tube and centrifuged at 14,000 rpm for 10 min at 4°C and the supernatant discarded. This was repeated for the entire volume of the sample. The DNA was then washed with 200 μl of 70% ethanol and pellets dried and finally resuspended in 100 μl of 1× TE buffer (10 mM Tris–HCl, 1 mM EDTA). Genomic DNA was stored at −20°C until subsequent polymerase chain reaction (PCR) and cloning.
Clone libraries were constructed targeting the small subunit 18S ribosomal RNA gene. Community genomic DNA was amplified with the eukaryote specific primers NSF4/18 (5′-CTGGTTGATYCTGCCAGT-3′) and NSR 1787/18 (5′-CYGCAGGTTCACCTACRG-3′) (Hendriks et al. 1991) using the iCycler™ Thermal Cycler (Bio-Rad Laboratories, Inc., CA, USA). One PCR consisted of 1× Feldan PCR Buffer (Feldan Bio Laboratories, Inc., Québec, Canada), 200 μM of dNTPs, 0.3 μM of each primer, 1 μl of bovine serum albumin, 1.25 units of Feldan Taq polymerase and 1–4 ng of genomic DNA. The PCR product was purified using a QIAquick PCR Purification Kit (QIAGEN Sciences, Maryland, USA), and a polyadenosine tail was added on each end (24 μl of PCR product, 5 μl of 10× Feldan PCR buffer, 1 μl of 10 mM dATP and 0.2 μl Feldan Taq polymerase, for 10 min at 72°C). The ligation of the product into the StrataClone vector, pSC-A-amp/kan, and transformation of Escherichia coli competent cells with the recombinant vectors were done according to the StrataClone PCR Cloning Kit instruction manual (Stratagen, La Jolla, CA, USA). Cells were plated on Luria–Bertani (LB) and selected for recombinant transformants, which were then picked and grown as per Diez et al. (2001). The cloned PCR inserts were verified and screened as in Potvin and Lovejoy (2009), then sequenced at the Centre Hospitalier de l’Université Laval (CHUL, QC, Canada) using the forward (NSF4/18) and reverse (NSR1787/18) primers, which had been used to make the clone libraries, with an ABI 3730xl system (Applied Biosystems, Foster City, CA, USA).
The same protocols as above were used to obtain the 18S rRNA gene sequence from Kephyrion strain CCMP 3057, as a reference “cultured” sequence for the present study. This strain was isolated by J Boenigk in 2006 from a freshwater lake in Austria. We obtained the strain from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton (CCMP). It was cultured in DY-V for 5 weeks, then cells were centrifuged and DNA extracted, cloned and sequenced using the same 18S rRNA primers as above.
Forward and reverse sequence segments were compiled, edited and trimmed using ChromasPro (Technelysium Pty Australia, version 1.5). The approximately 1,700-nt sequences were then aligned with ClustalW multiple alignment tool and visually checked using the BioEdit Sequence Alignment Editor (Hall 1999). Operational taxonomic units (OTUs) were generated using MOTHUR (Schloss et al. 2009). Sequences were deposited in GenBank under accession numbers JF730750–JF730878. Reference alignments were constructed using additional sequences selected from the closest match to our sequences based on a BLAST search (Altschul et al. 1990) of GenBank. If the closest match was an uncultured clone, the closest isolated strain was also included. Phylogenetic trees were created using ClustalX (Thompson et al. 1997) and NJPlot (Perrière and Gouy 1996). OTUs with matches close to organisms reported from both marine and freshwater origins were further investigated with and aligned with additional sequences using the Multiple Sequence Comparison by Log-Expectation (MUSCLE; European Molecular Biology Laboratory).
Diversity analyses and comparison of samples
UniFrac analyses were conducted with MOTHUR to compare the phylogenetic diversity among the protist communities (Lozupone and Knight 2005). The Bray–Curtis index was applied to abundance data from both microscopic cell counts and OTUs from the clone libraries, using the PAST software package (Hammer et al. 2001). The same data matrix was used to estimate the Shannon diversity index (Pielou 1966) and Simpson’s dominance index (Simpson 1949). For the microscopy data, indices were based on the proportion of individuals for each species to the total number of cells counted. For the 18S rRNA gene sequence data, indices were based on the proportion of sequences for each distinct OTU to the total number of sequences analysed.
Results
Physical and chemical properties
The physico-chemical results indicated different surface water properties among the three lakes (Table 1). Char Lake was warmest, whereas Ward Hunt Lake was still under its perennial ice cover and had the coldest waters. Conductivity was highest in Lake A, while Char Lake and Ward Hunt Lake both had similar values around 0.15 mS cm−1. Nutrient values were low in all three lakes, with total nitrogen (TN) concentrations <0.1 mg l−1 and total phosphorus (TP) <0.004 mg l−1. Char Lake had lower concentrations of TN compared to the other two lakes. Conversely, phosphorus concentrations were lowest in Lake A compared to Char Lake and Ward Hunt Lake. The Char Lake N: P ratio of 15:1 was close to the Redfield ratio, whereas Lake A (32:1) and Ward Hunt Lake (24:1) N: P ratios were much higher, implying a more severe phosphorus limitation in these latter two lakes.
General protist biomass and diversity
Chlorophyll (chl) a concentrations were extremely low (<1 μg l−1) as were biomass levels estimated from cell counts (Table 2). This, in combination with the low nutrient concentrations, would classify the lakes as ultra-oligotrophic. The accessory pigment data (Table 2) indicated extremely low concentrations of chlorophyll b, a pigment characteristic of green algae. Chlorophylls c2 and c3 concentrations were also low. Chl c1, characteristic of chrysophytes, diatoms and prymnesiophytes, had the highest concentration among the accessory chlorophylls. The dominant carotenoid was fucoxanthin, a pigment found in chrysophytes, diatoms and dinoflagellates. Char Lake also contained violaxanthin (11%) and diadinoxanthin (8%) as well as smaller concentrations of alloxanthin, zeaxanthin, lutein and β-carotene. Aside from the fucoxanthin, Ward Hunt Lake only contained violaxanthin, diadinoxanthin and zeaxanthin. The pigment diversity was lowest in Lake A, with zeaxanthin, a signature pigment of cyanobacteria (but also green algae), being the most prominent carotenoid. We looked for the dinoflagellate marker peridinin in the chromatograms but did not detect it in any of the lakes.
Microscopy and gene surveys revealed different aspects of the protist diversity (Fig. 2a, b). The microscopic analyses indicated that chrysophytes represented 50–70% of the total planktonic biomass, with dinoflagellates representing <1–3% in the three lakes (Fig. 2a). The Char Lake community included other identifiable photosynthetic groups including species of Bacillariophyceae, Dictyochophyceae, Chlorophyceae and Cryptophyceae. These classes were also detected in Ward Hunt Lake, albeit in much smaller proportions. In contrast, Lake A was less diverse with microscopic data indicating, aside from the different chrysophytes (Fig. 3a), there were Cryptophyceae (5%) and Chlorophyceae (2%). Dictyochophyceae (Pseudopedinella sp.) were observed in Char Lake (3%) and Ward Hunt Lake (2%) but not Lake A. Unidentified chloroplast-containing flagellates accounted for ca. 20% of the Char Lake and Ward Hunt Lake protist biomass, but only 5% of Lake A biomass. Heterotrophic protists including ciliates (<1%) accounted for the remaining small proportion of biomass in the three lakes, with unidentified colourless eukaryotic single cells relatively more important in Lake A and Ward Hunt Lake compared to Char Lake. The diversity indices (Table 3) were consistent with these observations, indicating that Char Lake and Ward Hunt Lake had the most diverse communities. Shannon’s index for Lake A was much lower, while the dominance index was two times higher than for Char Lake (Table 3).
Chrysophyte sequences were also predominant and diverse in the molecular surveys (Fig. 3b), representing 25–50% of the sequences. The diversity indices differed from those estimated with the microscopy (Table 3). The sequences of protists other than chrysophytes indicate that different organisms contributed to this diversity (Fig. 2b; Table 4). Dictyochophyceae were detected in clone libraries from Lake A and Ward Hunt Lake (Fig. 2b; Table 4). Cercozoa sequences were recovered from Lake A, rare in Char Lake and not found in Ward Hunt Lake. In contrast, Bacillariophyceae sequences were recovered from Char Lake and Ward Hunt Lake, but not from Lake A. Dinoflagellates accounted for 13–25% of the clones at the three sites, and ciliates represented over 20% of sequences from Char Lake and Lake A but were much less common in Ward Hunt Lake (Fig. 2b).
Most dinoflagellate operational taxonomic units (OTUs, defined at 98% similarity) had closest matches to either phototrophic (Gymnodinium aureolum; GeneBank accession AY999082) or heterotrophic (Pfiesteria-like dinoflagellate; AM050344) organisms, both usually reported from marine environments (Table 4). One Char Lake clone grouped with Woloszynskia pascheri, the only freshwater dinoflagellate that was related to any of our polar lake clones (ESM 1). Sequences closely matching the bipolar ice-associated Polarella glacialis were recovered from Lake A and Ward Hunt Lake (ESM 1). Most ciliates from Char Lake grouped within the Oligotrichia (ESM 2), with closest matches to uncultured strombidia, whereas Lake A sequences were clearly dominated by Halteria grandinella (Stichotrichia), which was also present in Ward Hunt Lake (Table 4).
The recently proposed phagotrophic eukaryote phylum Telonemia (Shalchian-Tabrizi et al. 2006) was represented by close sequence matches (95–96% similarity) to one of its two named species, Telonema antarcticum Thompsen. These sequences were detected in all three lakes, with a considerable proportion in Ward Hunt Lake. Fungi, however, were only detected in Char Lake.
Shannon diversity indices for the molecular analyses indicated that Char Lake was most diverse, while Lake A and Ward Hunt Lake had comparable Shannon indices (Table 3). The dominance index was also greater for the latter two lakes compared to Char Lake (Table 3). The Bray–Curtis cluster analysis based on microscopic data indicated that protist community compositions of the lakes were all >60% different from each other, with Char Lake and Ward Hunt Lake the most similar (40%) compared to Lake A; that is, this analysis implied that Char Lake and Ward Hunt Lake had more species in common than either had with Lake A. The molecular data suggested differently, with the cluster analysis grouping Lake A and Ward Hunt Lake although only at 20% similarity. UniFrac analysis indicated that each lake contained a distinct assemblage of species (P < 0.05).
Chrysophyte diversity
Results from both microscopy and molecular techniques indicated that chrysophytes largely dominated the protist community in all three lakes (Fig. 2a, b). The microscopic observations revealed that each lake was characterised by different genera (Fig. 3a). Ward Hunt Lake was dominated by the morphospecies Erkenia subaequiciliata Skuja, a small 3- to 5-μm spherical cell with one long and one short flagellum and two distinct chloroplasts, and Dinobryon sociale Ehrenberg, a colonial lorica-forming species with typical heterokont flagella. D. sociale also seemed to have formed cysts, which made up to 3.5% of the chrysophyte population in Ward Hunt Lake. The most prevalent Lake A chrysophytes were identified as either Pseudokephyrion Pascher, a small cell in a bottle shaped lorica that has two visible flagella, or Kephyrion Pascher, which looks similar to Pseudokephyrion except with a single visible flagellum. We were not able to consistently see this difference. The classical taxonomy of these two genera is based on lorica shape (Bourrelly 1968), and there were at least four distinct lorica forms in the Arctic samples. Chrysophyte cysts were common in Lake A, representing ca. 20% of the chrysophytes cells. Char Lake was dominated by a Dinobryon sp., most closely resembling D. sociale. Both Kephyrion/Pseudokephyrion spp. and E. subaequiciliata were also recorded in Char Lake. Chrysophyte cysts that could not be matched to a particular species constituted 5% of the Char Lake chrysophytes while Dinobryon sociale cysts, recognized by their association with D. sociale loricas, represented another 5% of chrysophyte cells.
The gene survey identified different chrysophyte taxa, relative to the microscopic analysis (Fig. 3b). Most sequences fell into two clusters, designated Cluster I and Cluster II (Fig. 4), which were not assignable to known taxonomic groups. Both clades were strongly supported by bootstrap values of 100% in the neighbour-joining (NJ) tree and 70–99% in the maximum-likelihood (ML) tree. Sequences from all three lakes were found in Cluster I, which had no matches closer than 92% similarity to any cultured organism. The highest BLAST match was to Cyclonexis annularis, but Cluster I did not group near the C. annularis sequence and even fell outside of the main chrysophyte phylogeny (Fig. 4). The second unidentified group, Cluster II, was predominantly found in Ward Hunt Lake and Char Lake. These sequences were 99% similar among themselves and were matched with 96% similarity to Ochromonas tuberculata (AF123293). In the NJ and ML trees, the cluster fell within a larger clade that included O. tuberculata and several other Char Lake clones (Fig. 4).
Chrysophyte 18S rDNA neighbour-joining phylogenetic tree. The outgroup (not shown) used to root this tree was the dinoflagellate Paulsenella vonstoschii. Bootstrap values (>50%) from the neighbour-joining tree are in bold, and those from the maximum-likelihood tree are in italics. Sequences from Char Lake (CH), Lake A (LA) and Ward Hunt Lake (WH) are in bold, with the number of clones for each sequence indicated in parentheses, CH8A2mG9 represents OTU Ch-OTU34, and CH8ASmE12 represents OTU Ch-OTU 25 referred to in Fig. 3
Four sequences from different lakes fell into different, previously identified environmental clades (Fig. 4). Three of these environmental clades contained sequences from oligotrophic Lake George, in Adirondack State Park (Richards et al. 2005). Other sequences from Ward Hunt Lake and Char Lake clustered with Dinobryon sequences from several different species (Fig. 4). The NCBI BLAST search indicated 96% similarity with the Dinobryon cylindricum sequence (EF165140), yet in the NJ and ML trees, all three sequences appeared more closely related to D. bavaricum and D. divergens (FN662758 and FN662756) than to D. cylindricum. Two of the Lake A sequences and one Char Lake sequence clustered with Kephyrion CCMP 3057 as part of a sub-cluster with several sequences of undescribed Ochromonas—Spumella-like flagellates (Fig. 4).
Discussion
Protist dominance and diversity
Irrespective of approach, chrysophytes dominated the three lakes examined. Chrysophytes are found across diverse aquatic habitats (Sandgren et al. 2009) but species composition varies, as do community associations of chrysophytes with other protists. Chrysophyte stomatocysts (Smol 1988), and silica-scaled chrysophytes (Wilken et al. 1995), accumulate in the sediments and are used to reconstruct past climate trends, illustrating the importance of these organisms in Arctic regions. Chrysophytes have been previously recorded in many High Arctic and subarctic lakes such as in the Franz-Joseph Archipelago (Panzenböck et al. 2000), oligotrophic Greenland lakes (Christoffersen et al. 2008) and pingos (Kristiansen et al. 1995), and Canadian subarctic lakes where chrysophytes and cryptophytes dominate the two annual phytoplankton peaks (Sheath 1986). Holmgren (1984) suggested that the phytoplankton communities in oligotrophic arctic and subarctic lakes could be classified as four distinct assemblages: (1) Chrysophyceae, (2) Chrysophyceae-Diatoms, (3) Chrysophyceae-Cryptophyceae, (4) Chrysophyceae-Dinophyceae. The oligotrophic High Arctic lakes of the present study all fall into the Type 1 assemblage category.
All methods utilized showed that chrysophytes were ubiquitous and abundant, suggesting their importance for biological productivity and trophic links in Arctic lakes. The HPLC results showing high levels of fucoxanthin supported the predominance of chrysophytes. This carotenoid is the signature pigment of chrysophytes, but Bacillariophyceae and Dictyochophyceae can also contribute to a fucoxanthin peak, and microscopy and clone libraries detected both classes. However, none of the signature pigments of diatoms (diatoxanthin, diadinoxanthin), Dictyochophyceae (19′-butanoyloxyfucoxanthin), or dinoflagellates (peridinin, peridinol, dinoxanthin) were detected, indicating that as recorded using microscopy, these groups were much less abundant, and that most of the fucoxanthin was from the chrysophytes.
The molecular and microscopic techniques both indicated that there were different chrysophyte genera in each of the three lakes. The two shallower lakes, Char Lake and Ward Hunt Lake, were dominated by Dinobryon spp. according to microscopic observations. Dinobryon and Uroglena are common in summer waters of Finnish Lake Saanajärvi (Forsström et al. 2005), which is limnologically similar to Char Lake. The gene surveys diverged from the microscopy to some extent, although they also detected Dinobryon spp. as being prominent in both Ward Hunt Lake and Char Lake. Microscopy indicated that Lake A was dominated by Pseudokephyrion-Kephyrion spp. with no Dinobryon recorded, despite its geographical proximity to Ward Hunt Lake. Similarly, Laybourn-Parry and Marshall (2003) reported the genus Dinobryon in some Spitzbergen lakes while in others Kephyrion was dominant.
Erkenia subaequiciliata was an important morphological species in all three lakes. The genus Erkenia was described by Skuja (1948), but has apparently not been deposited in any culture collection as yet. Furthermore, no record exists for Erkenia 18S rDNA, although it is often reported in plankton communities of freshwater lakes worldwide (Gerhart and Likens 1975; Pollingher 1981; Jacquet et al. 2005; Kozak 2005). In general, existing 18S rRNA gene records are far from exhaustive and lack many key morphospecies (Richards et al. 2005). Based on distribution patterns emerging from both the morphological and sequence data, we can speculate on the identity of our Cluster II (Fig. 4), which grouped with Ochromonas tuberculata. The distribution of Cluster II between the three lakes was similar to that of E. subaequiciliata (Figs. 2b, 3b), which has two visible flagella. O. tuberculata itself has a short second flagellum, but in phylogenetic studies it consistently groups with the Chrysosphaerales, which have one single visible flagellum (Andersen et al. 1999). This suggests that despite a similar distribution, it is unlikely that Cluster II corresponds to Erkenia. However, the morphologically based taxonomy of many single-cell chrysophytes hides much genetic diversity, and many current genera are polyphyletic (Andersen et al. 1999; Boenigk et al. 2005; Pfandl et al. 2009). O. tuberculata, for example, is phylogenetically dissimilar to any other species of the polyphyletic genus Ochromonas (Andersen et al. 1999).
The sequences in Cluster I did not group with any sequences currently in GenBank, whether cultured or uncultured. This Cluster I was more diverse than Cluster II and the Lake A and Char Lake sequences grouped apart. We speculated that Cluster I could represent a loricate organism related to Kephyrion, as this genus was frequently observed in our microscopic counts, especially in Lake A. We sequenced the 18S rRNA gene from the only well-identified culture listed in CCMP as Kephyrion (isolated by J. Boenigk from a high-altitude Austrian lake). However, this culture (CCMP3057) grouped far from Cluster I, within a clade of Spumella-like chrysophytes. Cluster I branched at the base of the chrysophytes in our phylogenies, implying that it may be ancestral or a sister clade to the Chrysophyceae. It possibly represents a novel taxon, which has previously been overlooked and is potentially restricted to Arctic freshwaters. The unresolved morphological identity of this cluster suggests it may be among the small nondescript flagellates and represented by the “unidentified flagellates” category recovered by microscopy in this study.
The number of environmental sequences in publically available databases continues to grow, and although there is scant morphological information to be inferred from the small ribosomal subunit gene, biogeographical studies are possible by comparing sequences from different regions. In addition, some ecological information can be gleaned by comparing environmental conditions where specific sequences and clades are found. The grouping of some of our sequences with other environmental sequences (Environ 1–4) hints at the distribution and ecology of these organisms. Three of these environmental clades were first recovered from the temperate oligotrophic Lake George, in Adirondack State Park (Richards et al. 2005), suggesting that they are common in low nutrient freshwater environments. The fourth clade, Environ-3, included marine sequences from the South-Eastern Pacific, but these were only 96% similar to our sequence, which could therefore represent a novel Arctic group. However, the number of 18S rRNA gene studies of freshwater lakes is far fewer than those from marine systems, and many of these taxa could well be globally dispersed.
Chrysophyte dominance in Arctic lakes may be due to a number of adaptations to low nutrient availability or to the pronounced seasonal changes in light availability. For example, chrysophytes form cysts, protecting cells during unfavourable conditions (Nicholls 2009), which in Arctic lakes could include nutrient exhaustion and winter darkness. We found that cysts represented 7–20% of total chrysophytes in the three lakes, with the greatest proportion in Lake A. Yubuki et al. (2008) suggested that formation of spores in planktonic chrysophytes is directly influenced by cell density and that cells encyst once a critical density is reached. Nicholls (2009) also noted that the small size of many chrysophytes compared to diatoms and dinoflagellates affords a relatively high surface to volume ratio, which could favour the uptake of nutrients at low concentrations. The most common chrysophyte found in Ward Hunt Lake, the morphospecies Erkenia subaequiciliata, was 2–5 μm in diameter, and as few picocyanobacteria were present in that lake, it may have been the dominant small phytoplankton cell type. Lake A has high pelagic concentrations of picocyanobacteria (Van Hove et al. 2008), and picocyanobacteria were frequently observed in Char Lake (Sophie Charvet personal observations). HPLC pigment profiles also indicated the presence of cyanobacteria in these two lakes. These 1- 2-μm-diameter prokaryotic cells would outcompete the chrysophytes for nutrients on the basis of size since chrysophytes in the two lakes were generally larger than 10 μm in diameter; however, these abundant picocyanobacteria could provide an N- and P-containing food source for mixotrophic chrysophytes.
Another factor that could favour chrysophytes in oligotrophic waters is their capacity to swim. Several genera (Dinobryon, Synura, Uroglena and Mallomonas) can actively maintain their position at strategic depths. Motility confers a relative advantage over sessile algae in seeking favourable irradiance conditions (Pick and Lean 1984) or avoiding zooplankton predators (Nicholls 2009). Furthermore, cells able to migrate to deeper depths where nutrients are more abundant have an advantage within a stratified water column. Another potential adaptation to low nutrients and periodic light limitation is phagotrophic mixotrophy, whereby nutrients and energy can be obtained from bacterial prey, circumventing direct energy dependency on solar radiation and the reliance on dissolved inorganic nutrients (Raven 2009). A number of studies have documented that phototrophic chrysophytes prey on bacterial or algal cells (Bird and Kalff 1986; Rothhaupt 1996; Katechakis and Stibor 2006). Hence, when nutrients are scarce at the end of the growing season, as in Lake A (Veillette et al. 2011), chrysophyte mixotrophy would be favoured.
While many OTUs recovered from the present study were closest to sequences from freshwater and ice, others grouped closest to sequences previously reported only from marine systems. The transition from marine to freshwater is thought to be rare (Logares et al. 2007), and the putative marine groups recovered from the freshwater lakes may well have been transient. Among the alveolates, all were from nominal marine groups, with the exception of one freshwater dinoflagellate Woloszynskia pascheri. Another dinoflagellate Polarella glacialis was the sole known cold ecotype represented and was recovered from Ward Hunt Lake and Lake A. P. glacialis was originally isolated from the sea ice in the Ross Sea, Antarctica and subsequently from Northern Baffin Bay, in the Arctic Ocean (Montresor et al. 1999, 2003). Using PCR and cloning techniques, it has also been detected in saline Antarctic lakes (Rengefors et al. 2008) and, notably, in Arctic snow on Ward Hunt Island (Harding et al. 2011). This ice-associated dinoflagellate readily encysts and local atmospheric transport from the sea or sea ice to the lakes is likely. Other dinoflagellate sequences, with matches to Gymnodinium aureolum (AY99082), and the Pfiesteria group, as well as ciliate sequences belonging to the marine Strombidium, are nearly always picked up in Arctic marine 18S rRNA gene surveys (Lovejoy et al. 2006; Lovejoy and Potvin 2011), and their presence in our clone libraries could indicate local transport as well.
Overall, Char Lake was more taxonomically diverse than the other two lakes. This was the southernmost lake we examined, with a longer growing season that lasts from April to the end of August (Schindler et al. 1974). The N:P ratio of inorganic nutrients in Char Lake corresponded to the Redfield ratio, while the ratios of the other lakes indicated strong phosphorus limitation. These observations imply that protist diversity might increase with increasing nutrient levels in our three oligotrophic arctic systems; however, much wider sampling is required to test this relationship. Furthermore, this difference in diversity could be due to the geographic distance between Cornwallis Island and the northern coast of Ellesmere Island. Char Lake is not influenced by the same winds as Lake A and Ward Hunt Lake, nor is it subjected to the same allochthonous inputs. For example, Char Lake lies closer to anthropogenic activity, near the hamlet of Resolute Bay.
Comparison of methods
There have been few studies where both detailed microscopy and small subunit rRNA gene surveys have been carried out on the same sample. Jungblut et al. (2010) targeted polar cyanobacteria living in microbial mats using a multiphasic approach. Even within this narrow taxon sampling and the addition of many new sequences from morphologically well-identified polar cultures, there were mismatches between environmental sequences and the morphotypes within the mats. That study did, however, imply a global distribution of cyanobacterial genotypes throughout the cold biosphere. The low similarity of the environmental sequences to cultured representatives of most of the protists in our Arctic lakes reflects the poor representation of different groups that have been sequenced and may also indicate that as for cyanobacteria, some protists may be characteristic of polar or cold regions and these represent genuine new records.
Pigment analysis, microscopy and the 18S rRNA gene survey identified chrysophytes as common, but there were differences in taxonomic detail. Microscopy also highlighted the abundance of cysts, which would not be separated from vegetative cells by their 18S rRNA gene sequences. Other less common taxa such as dictyochophytes were noted by both methods but again the resolution of the species present was poor, with Pseudopedinella noted from Char Lake under light microscopy, while Pedinella was the closest match to dictyochophyte sequences from Lake A and Ward Hunt Lake. Whether this species difference is real or due to inadequate reference sequences available cannot be resolved without additional culturing and sequencing studies. Microscopy and HPLC pigment analyses are intrinsically more quantitative than 18S rRNA gene surveys because copy number of 18S rRNA genes among different groups varies widely (Zhu et al. 2005). As has been found in other gene surveys, we recovered higher proportions of dinoflagellates and ciliates compared to microscopic observations. Dinoflagellates and ciliates have many more copies of this gene than most small algae, so even if rare, they are more likely to be detected using PCR-based cloning and sequencing (Potvin and Lovejoy 2009). Because of this, the 18S rRNA gene detected species likely to be rare, such as Polarella, and the ciliates Halteria and Strobilidium that were not recorded in our microscopy survey.
A major insight provided by the 18S rRNA gene libraries, in contrast to microscopy, was in the diversity of heterotrophic protists. Most heterotrophic protists are colourless, and many have little in the way of distinguishing morphological features (Caron 1983) and are vulnerable to loss from fixation and preservation processes (Hara et al. 1986). Furthermore, taxonomic studies have previously concentrated on phototrophs, and the number of classic phycologists exceeds the number of protozoologists. This has resulted in a limited number of reference texts on heterotrophic protists, which has impeded their identification (Vørs et al. 1995; Packroff and Woelfl 2000). Clone libraries also detected more heterotrophic protists than microscopy from Lake Stechlin (Luo et al. 2011).
The comparison of sequences from different geographic regions and habitats is less ambiguous than comparing microscopically identified morphospecies that depend on expert identification (Jungblut et al. 2010). For example, Telonema has been previously reported from marine, ice and freshwater environments (Bråte et al. 2010). This genus was present in Char Lake and relatively common in both Lake A and Ward Hunt Lake. Three of the four Telonema OTUs recovered from our sites had best matches to Telonema antarcticum (98%), which was first recorded in microscope studies from Antarctica but was also isolated and described from coastal waters of Norway (Klaveness et al. 2005). When aligned in MUSCLE with the sequences used in Bråte et al. (2010), our Telonema OTUs all grouped within the sole freshwater clade (Bråte et al. 2010). Telonemia cells were probably among the unidentified heterotrophic nanoflagellates in the microscopy data. The sole fungal sequence, detected from Char Lake, was 94% similar to Rhizophlyctis rosea, a fungus that is mostly reported from soil environments (Willoughby 2001). There exists scant information about fungi in Arctic aquatic environments (Voronin 1997; Hodson et al. 2008), and as in the case of heterotrophic protists, few researchers study these and they have been largely ignored in microscopic surveys of plankton.
Overall, the combined approach provided deeper insight into the lake communities than any single analysis. HPLC provides some taxonomic information at the phylum level for photosynthetic taxa and information that can be used to infer adaptation to different irradiance regimes. Microscopy was biased against heterotrophs, whereas the 18S rRNA gene survey was limited by the lack of reference sequences to known organisms. This may be a temporary shortcoming since as sequences are added to reference databases, more species names will be matched to environmental sequences. The 18S rRNA gene survey approach may be biased against autotrophic communities (Vaulot et al. 2008), accurate assessments in the future will therefore require both microscopy and genetic analysis.
Implications for food webs and climate change
Polar lakes are diverse and harbour a continuum of food webs, from systems that support both zooplankton and fish communities to those where ciliates and rotifers comprise the highest trophic level (Vincent et al. 2008). Cyanobacteria often dominate polar freshwater productivity in these extreme ecosystems, either as benthic microbial mats in streams, lakes and ponds (Jungblut et al. 2010) or as picocyanobacteria in the phytoplankton communities (Lizotte 2008; Van Hove et al. 2008). The high zeaxanthin concentrations from Lake A were consistent with previous reports of large picocyanobacterial populations in this lake (Veillette et al. 2011). These picocyanobacteria likely represent the primary food source of phagotrophic protists, belonging to both heterotrophic and mixotrophic groups. The dominance of chrysophytes, accompanied by the significant presence of heterotrophs, in these aquatic ecosystems suggests their importance for the local food webs. Chrysophytes, such as Dinobryon spp. and Ochromonas tuberculata, feed on bacterial cells (Bird and Kalff 1986; Rothhaupt 1996). In these lakes, we also recovered phagotrophic species such as Telonema antarcticum, and ciliates that feed on flagellates and small algae. The different phagotrophs may differ in their trophic position within the food web of High Arctic lakes. From the data presented here, we can deduce a simple food web that would be based on the primary production of picocyanobacteria, consumed by the mixotrophic chrysophytes, which in turn likely may become prey to the larger Telonema and ciliates.
Arctic aquatic food webs are likely to be altered by climate change. The increases in temperature and precipitation result in thawing of permafrost, followed by increasing terrestrial vegetation in the surrounding catchments. These changes will likely result in major shifts in allochthonous carbon and nutrient inputs (Vincent et al. 2008) that may stimulate the production of bacterial prey for microbial grazers. Longer ice-free periods and thermal stratification of the lakes may result in earlier nutrient depletion in the upper waters, creating favourable conditions for mixotrophic chrysophytes. Alternatively, these conditions may cause a shift towards co-dominance with dinoflagellates, as suggested by Holmgren (1984). How such shifts in lake trophic status influence higher food webs, nutrient cycling and carbon fluxes will be important questions for future studies.
References
Aljanabi SM, Martinez I (1997) Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res 25:4692
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andersen RA, Van de Peer Y, Potter D, Sexton JP, Kawachi M, LaJeunesse T (1999) Phylogenetic analysis of the SSU rRNA from members of the Chrysophyceae. Protist 150:71–84. doi:10.1016/S1434-4610(99)70010-6
Bird DF, Kalff J (1986) Bacterial grazing by planktonic lake algae. Science 231:493–495
Boenigk J, Pfandl K, Stadler P, Chatzinotas A (2005) High diversity of the ‘Spumella-like’ flagellates: an investigation based on the SSU rRNA gene sequences of isolates from habitats located in six different geographic regions. Environ Microbiol 7:685–697. doi:10.1111/j.1462-2920.2005.00743.x
Bonilla S, Villeneuve V, Vincent WF (2005) Benthic and planktonic algal communities in a High Arctic lake: pigment structure and contrasting responses to nutrient enrichment. J Phycol 41:1120–1130. doi:10.1111/j.1529-8817.2005.00154.x
Bourrelly P (1968) Les algues d’eau douce II: Les algues jaunes et brunes. Société Nouvelle des Éditions Boubée, Paris
Bråte J, Klaveness D, Rygh T, Jakobsen KS, Shalchian-Tabrizi K (2010) Telonemia-specific environmental 18S rDNA PCR reveals unknown diversity and multiple marine-freshwater colonizations. BMC Microbiol 10:168. doi:10.1186/1471-2180-10-168
Butler HG, Edworthy MG, Ellis-Evans JC (2000) Temporal plankton dynamics in an oligotrophic maritime Antarctic lake. Freshw Biol 43:215–230. doi:10.1046/j.1365-2427.2000.00542.x
Canter-Lund H, Lund WGJ (1995) Freshwater algae: their microscopic world explored. Biopress Limited, Bristol
Caron DA (1983) Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol 46:491–498
Christoffersen KS, Amsinck SL, Landkildehus F, Lauridsen TL, Jeppesen E (2008) Lake flora and fauna in relation to ice-melt, water temperature and chemistry at Zackenberg. Adv Ecol Res 40:371–389. doi:10.1016/S0065-2504(07)00016-5
Diez B, Pedròs-Aliò C, Massana R (2001) Study of genetic diversity of eukaryotic picoplankton in different oceanic regions by small-subunit rRNA gene cloning and sequencing. Appl Env Microbiol 67:2932–2941
Findlay DL, Kling HJ (1979) A species list and pictorial reference to the phytoplankton of Central and Northern Canada: Part I. Department of Fisheries and the Environment, Winnipeg
Forsström L, Sorvari S, Korhola A, Rautio M (2005) Seasonality of phytoplankton in subarctic Lake Saanajärvi in NW Finnish Lapland. Polar Biol 28:846–861. doi:10.1007/s00300-005-0008-2
Gerhart DZ, Likens GE (1975) Enrichment experiments for determining nutrient limitation: four methods compared. Limnol Oceanogr 20:649–653
Gibson JAE, Vincent WF, Van Hove P, Belzile C, Wang X, Muir D (2002) Geochemistry of ice-covered, meromictic Lake A in the Canadian High Arctic. Aquat Geochem 8:97–119
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hara S, Tanoue E, Zenimoto M, Komaki Y, Takahashi E (1986) Morphology and distribution of heterotrophic protists along 75°E in the Southern Ocean. Mem Natl Inst Polar Res Spec Issue 40:69–80
Harding T, Jungblut AD, Lovejoy C, Vincent WF (2011) Microbes in high arctic snow and implications for the cold biosphere. Appl Environ Microbiol 77:3234–3243. doi:10.1128/AEM.02611-10
Hasle GR (1978) Settling: the inverted microscope method. In: Sournia A (ed) Phytoplankton manual, UNESCO Monographs on Oceanographic Methodology, Paris, vol 40, pp 88–96
Hendriks L, De Baere R, Van de Peer Y, Neefs J, Goris A, De Wachter R (1991) The evolutionary position of the rhodophyte Porphyra umbilicalis and the basidiomycete Leucosporidium scottii among other eukaryotes as deduced from complete sequences of small ribosomal subunit RNA. J Mol Evol 32:167–177
Hillebrand H, Dürselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Hodson A, Anesio AM, Tranter M, Fountain A, Osborn M, Priscu J, Laybourn-Parry J, Sattler B (2008) Glacial ecosystems. Ecol Monogr 78:41–67
Holmgren SK (1984) Experimental lake fertilization in the Kuokkel area, Northern Sweden: Phytoplankton biomass and algal composition in natural and fertilized subarctic lakes. Int Revue ges Hydrobiol 69:781–817
Jacquet S, Briand J, Leboulanger C, Avoisjacquet C, Oberhaus L, Tassin B, Vinconleite B, Paolini G, Druart J, Anneville O (2005) The proliferation of the toxic cyanobacterium following restoration of the largest natural French lake (Lac du Bourget). Harmful Algae 4:651–672. doi:10.1016/j.hal.2003.12.006
Jeffries MO, Krouse HR, Shakur MA, Harris SA (1984) Isotope geochemistry of stratified Lake ‘A’, Ellesmere Island, N.W.T., Canada. Can J Earth Sci 21:1007–1017
Jungblut AD, Lovejoy C, Vincent WF (2010) Global distribution of cyanobacterial ecotypes in the cold biosphere. ISME J 4:191–202. doi:10.1038/ismej.2009.113
Kalff J, Kling HJ, Holmgren SH, Welch HE (1975) Phytoplankton, phytoplankton growth and biomass cycles in an unpolluted and in a polluted polar lake. Verh int Ver Limnol 19:487–495
Katechakis A, Stibor H (2006) The mixotroph Ochromonas tuberculata may invade and suppress specialist phago- and phototroph plankton communities depending on nutrient conditions. Oecologia 148:692–701. doi:10.1007/s00442-006-0413-4
Klaveness D, Shalchian-Tabrizi K, Thomsen HA, Eikrem W, Jakobsen KS (2005) Telonema antarcticum sp. nov., a common marine phagotrophic flagellate. Int J Syst Evol Microbiol 55:2595–2604. doi:10.1099/ijs.0.63652-0
Kozak A (2005) Seasonal changes occurring over four years in a reservoir’s phytoplankton composition. Pol J Environ Stud 14:451–465
Kristiansen J, Wilken LR, Jürgensen T (1995) A bloom of Mallomonas acaroides, a silica-scaled chrysophyte, in the crater pond of a pingo, Northwest Greenland. Polar Biol 15:319–324
Laybourn-Parry J, Marshall WA (2003) Photosynthesis, mixotrophy and microbial plankton dynamics in two high Arctic lakes during summer. Polar Biol 26:517–524. doi:10.1007/s00300-003-0514-z
Lizotte MP (2008) Phytoplankton and primary production. In: Vincent WF, Laybourn-Parry J (eds) Polar Lakes and Rivers: Limnology of Arctic and Antarctic Aquatic Ecosystems. University Press, London, pp 157–178
Logares R, Shalchian-Tabrizi K, Boltovskoy A, Rengefors K (2007) Extensive dinoflagellate phylogenies indicate infrequent marine-freshwater transitions. Mol Phylogenet Evol 45:887–903. doi:10.1016/j.ympev.2007.08.005
Lovejoy C, Potvin M (2011) Microbial eukaryotic distribution in a dynamic Beaufort Sea and the Arctic Ocean. J Plankton Res 33:431–444
Lovejoy C, Vincent WF, Frenette JJ, Dodson JJ (1993) Microbial gradients in a turbid estuary: Application of a new method for protozoan community analysis. Limnol Oceanogr 38:1295–1303
Lovejoy C, Massana R, Pedrós-Alió C (2006) Diversity and distribution of marine microbial eukaryotes in the Arctic Ocean and adjacent seas. Appl Environ Microbiol 72:3085. doi:10.1128/AEM.72.5.3085
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi:10.1128/AEM.71.12.8228
Luo W, Bock C, Li HR, Padisàk J, Krienitz L (2011) Molecular and microscopic diversity of planktonic eukaryotes in the oligotrophic Lake Stechlin (Germany). Hydrobiologia 661:133–143
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45:569–579
Montresor M, Procaccini G, Stoecker DK (1999) Polarella glacialis, Gen. Nov., Sp. Nov. (Dinophyceae): Suessiaceae are still alive! J Phycol 35:186–197. doi:10.1046/j.1529-8817.1999.3510186.x
Montresor M, Lovejoy C, Orsini L, Procaccini G, Roy S (2003) Bipolar distribution of the cyst-forming dinoflagellate Polarella glacialis. Polar Biol 26:186–194. doi:10.1007/s00300-002-0473-9
Moreira D, López-Garcia P (2002) The molecular ecology of microbial eukaryotes unveils a hidden world. Trends Microbiol 10:31–38
Mueller DR, Vincent WF, Bonilla S, Laurion I (2005) Extremotrophs, extremophiles and broadband pigmentation strategies in a high arctic ice shelf ecosystem. FEMS Microbiol Ecol 53:73–87. doi:10.1016/j.femsec.2004.11.001
Nicholls KH (2009) Chrysophyte blooms in the plankton and neuston of marine and freshwater systems. In: Sandgren CD, Smol JP, Kristiansen J (eds) Chrysophyte algae: ecology, phylogeny and development. Cambridge University Press, New York, pp 181–213
Packroff G, Woelfl S (2000) A review on the occurrence and taxonomy of heterotrophic protists in extreme acidic environments of pH values ≤3. Hydrobiologia 433:153–156
Panzenböck M, Möbes-Hansen B, Albert R, Herndl GJ (2000) Dynamics of phyto- and bacterioplankton in a high Arctic lake on Franz Joseph Land archipelago. Aquat Microb Ecol 21:265–369
Perrière G, Gouy M (1996) WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78:364–369
Pfandl K, Chatzinotas A, Dyal P, Boenigk J (2009) SSU rRNA gene variation resolves population heterogeneity and ecophysiological differentiation within a morphospecies (Stramenopiles, Chrysophyceae). Limnol Oceanogr 54:171–181
Pick FR, Lean DRS (1984) Diurnal movements of metalimnetic phytoplankton. J Phycol 20:430–436
Pielou E (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144. doi:10.1016/0022-5193(66)90013-0
Pollingher U (1981) The structure and dynamics of the phytoplankton assemblages in Lake Kinneret, Israel. J Plankton Res 3:93–105. doi:10.1093/plankt/3.1.93
Potvin M, Lovejoy C (2009) PCR-based diversity estimates of artificial and environmental 18S rRNA gene libraries. J Eukaryot Microbiol 56:174–181. doi:10.1111/j.1550-7408.2008.00386.x
Raven JA (2009) Comparative aspects of chrysophyte nutrition with emphasis on carbon, phosphorus and nitrogen. In: Sandgren CD, Smol JP, Kristiansen J (eds) Chrysophyte algae: ecology, phylogeny and development. Cambridge University Press, New York, pp 95–118
Rengefors K, Laybourn-Parry J, Logares R, Marshall WA, Hansen G (2008) Marine-derived dinoflagellates in Antarctic saline lakes: community composition and annual dynamics. J Phycol 44:592–604. doi:10.1111/j.1529-8817.2008.00517.x
Richards TA, Vepritskiy AA, Gouliamova DE, Nierzwicki-Bauer SA (2005) The molecular diversity of freshwater picoeukaryotes from an oligotrophic lake reveals diverse, distinctive and globally dispersed lineages. Environ Microbiol 7:1413–1425. doi:10.1111/j.1462-2920.2005.00828.x
Rigler FH (1974) The Char Lake project: a study of energy flow in a high Arctic lake. In: Hillbrecht-Ilkowska KA (ed) Productivity problems of freshwaters. Polish Scientific Publishers, Warsaw, pp 287–300
Rothhaupt KO (1996) Laboratory experiments with a mixotrophic chrysophyte and obligately phagotrophic and phototrophic competitors. Ecology 77:716–724
Sandgren CD, Smol JP, Kristiansen J (2009) Chrysophyte algae: ecology, phylogeny and development. Cambridge University Press, New York
Schindler DW, Welch HE, Kalff J, Brunskill GJ, Kritsch N (1974) Physical and chemical limnology of Char Lake, Cornwallis Island (75′N lat.). J Fish Res Board Can 31:585–607
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA et al (2009) Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Env Microbiol 75:7537. doi:10.1128/AEM.01541-09
Shalchian-Tabrizi K, Eikrem W, Klaveness D, Vaulot D, Minge MA, Le Gall F, Romari K et al (2006) Telonemia, a new protist phylum with affinity to chromist lineages. Proc R Soc B 273:1833–1842. doi:10.1098/rspb.2006.3515
Sheath RG (1986) Seasonality of phytoplankton in northern tundra ponds. Hydrobiologia 138:75–83
Simpson EH (1949) Measurement of diversity. Nature 163:688
Skuja H (1948) Taxonomie des phytoplankton einiger seen in Uppland, Schweden. Symbolae Botan Upsalienses 9:1–399
Smol J (1988) Chrysophycean microfossils in paleolimnological studies. Palaeogeogr Palaeocl 62:287–297. doi:10.1016/0031-0182(88)90058-2
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tsuji T, Yanagita T (1981) Improved fluorescent microscopy for measuring the standing stock of phytoplankton including fragile components. Mar Biol 64:207–211
Unrein F, Izaguirre I, Massana R, Balagué V, Gasol JM (2005) Nanoplankton assemblages in maritime Antarctic lakes: characterisation and molecular fingerprinting comparison. Aquat Microb Ecol 40:269–282. doi:10.3354/ame040269
Vallières C, Retamal L, Ramlal P, Osburn C, Vincent WF (2008) Bacterial production and microbial food web structure in a large arctic river and the coastal Arctic Ocean. J Marine Syst 74:756–773. doi:10.1016/j.jmarsys.2007.12.002
Van Hove P, Belzile C, Gibson JAE, Vincent WF (2006) Coupled landscape-lake evolution in High Arctic Canada. Can J Earth Sci 43:533–546. doi:10.1139/E06-003
Van Hove P, Vincent WF, Galand PE, Wilmotte A (2008) Abundance and diversity of picocyanobacteria in High Arctic lakes and fjords. Algol Stud 126:209–227. doi:10.1127/1864-1318/2008/0126-0209
Vaulot D, Eikrem W, Viprey M, Moreau H (2008) The diversity of small eukaryotic phytoplankton (≤3 μm) in marine ecosystems. FEMS Microbiol Rev 32:795–820. doi:10.1111/j.1574-6976.2008.00121.x
Veillette J, Martineau MJ, Antoniades D, Sarrazin D, Vincent WF (2011) Effects of loss of perennial lake ice on mixing and phytoplankton dynamics: Insights from High Arctic Canada. Ann Glaciol 51:56–70
Villeneuve V, Vincent WF, Komàrek J (2001) Community structure and microhabitat characteristics of cyanobacterial mats in an extreme high Arctic environment: Ward Hunt Lake. Nova Hedwigia 123:199–224
Vincent WF, Hobbie JE, Laybourn-Parry J (2008) Introduction to the limnology of high-latitude lake and river ecosystems. In: Vincent WF, Laybourn-Parry J (eds) Polar lakes and rivers: limnology of Arctic and Antarctic aquatic ecosystems. Oxford University Press, London, pp 1–24
Vincent WF, Whyte LG, Lovejoy C, Greer CW, Laurion I, Suttle CA, Corbeil J, Mueller DR (2009) Arctic microbial ecosystems and impacts of extreme warming during the International Polar Year. Polar Sci 3:171–180. doi:10.1016/j.polar.2009.05.004
Voronin LV (1997) Aquatic and aero-aquatic hyphomycetes in small lakes from Vorkuta vicinities. Mikol Fitopatol 31:9–17
Vørs N, Buck KR, Chavez FP, Eikrem W, Hansen LE, Ostergaard JB, Thomsen HA (1995) Nanoplankton of the equatorial Pacific with emphasis on the heterotrophic protists. Deep-Sea Res II 42:585–602
Wehr JD, Sheath RG (2003) Freshwater algae of North America: ecology and classification. Academic Press, Elsevier Science, Amsterdam
Welch HE Jr (1973) Emergence of Chironomidae (Diptera) from Char Lake, Resolute, Northwest Territories. Can J Zool 51:1113–1123
Wilken LR, Kristiansen J, Jürgensen T (1995) Silica-scaled chrysophytes from the peninsula of Nuusuaq/Nûugssuaq. Nova Hedwigia 61:355–366
Willoughby LG (2001) The activity of Rhizophlyctis rosea in soil: some deductions from laboratory observations. Mycologist 15:113–117
Yubuki N, Nakayama T, Inouye I (2008) A unique life cycle and perennation in a colorless chrysophyte Spumella sp. J Phycol 44:164–172. doi:10.1111/j.1529-8817.2007.00441.x
Zhu F, Massana R, Not F, Marie D, Vaulot D (2005) Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microb Ecol 52:79–92. doi:10.1016/j.femsec.2004.10.006
Acknowledgments
We are grateful to C. Vallières for microscopic counts, to M.-J. Martineau for the HPLC analyses and to J. Veillette, D. Sarrazin and S. Bourget for help in the field. This research was supported by the Natural Sciences and Engineering Research Council of Canada, the Network of Centres of Excellence ArcticNet, the Canada Research Chair program and the Fonds Québécois de la Recherche sur la Nature et les Technologies, with logistic support from Polar Continental Shelf Project (this is PCSP contribution no 027-11). We also thank Parks Canada for the use of their facilities in Quttinirpaaq National Park.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Charvet, S., Vincent, W.F. & Lovejoy, C. Chrysophytes and other protists in High Arctic lakes: molecular gene surveys, pigment signatures and microscopy. Polar Biol 35, 733–748 (2012). https://doi.org/10.1007/s00300-011-1118-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1118-7