Abstract
Cryoconite holes have biogeochemical, ecological and biotechnological importance. This communication presents results on culturable psychrophilic yeast and filamentous fungi from cryoconite holes at Midre Lovénbreen glacier. The identification of these microbes was achieved through conventional and DNA sequencing techniques. Effect of temperature, salt and media on growth of the cultures was studied. Measurements on the bioavailability of nutrients and trace metals were made through different methods including ICPMS (Inductively Coupled Plasma Mass Spectrometry). Colony forming unit (CFU) per gram of sediment sample was calculated to be about 7 × 103–1.4 × 104 and 4 × 103–1.2 × 104 of yeast and filamentous fungi, respectively. Based on morphology and sequence data, these were identified as Cryptococcus gilvescens, Mrakia sp., Rhodotorula sp., Phialophora alba and Articulospora tetracladia. Amongst these, Phialophora alba, Cryptococcus gilvescens and Mrakia sp. zhenx-1 are reported for the first time from Svalbard Arctic, while Rhodotorula sp. (95% gene similarity) is a new species, yet to be described. Rhodotorula sp. expressed high amylase, while Cryptococcus gilvescens showed high lipase activity. Mrakia sp. showed phosphate solubilization between 4 and 15°C, which is a first record. Chemical analysis revealed the presence of organic carbon, nitrogen and phosphorus in substantial amounts in the sediments. Filamentous fungi and yeast in the cryoconite holes drive the process of organic macromolecule degradation through cold-adapted enzyme secretion, thereby assisting in nutrient cycling in these subglacial environments. Further, these cold-adapted enzymes may provide an opportunity for the prospect of biotechnology in Arctic. This is the first report on mycological investigation into cryoconite holes from Midre Lovénbreen glacier.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cryoconite holes are variously shaped, organically rich, water filled depressions that are distributed over the glaciers and sea ice. They are formed when particulates present on the surface of the glacier are warmed by solar radiation and melt into the underlying ice (Christner et al. 2003). They contain a soft, dark coloured granular material, mostly consisting both organic and inorganic matters. The organic matter mainly includes algae, cyanobacteria, bacteria, fungi and rotifers, while the inorganic matter is a mixture of minerals and trace elements. During the polar summers, photosynthesis by algae and cyanobacteria within the holes provides environment for further colonization and succession of complex communities. Cryoconite holes due to their importance in biogeochemistry and ecology form a thrust area of research for biologists and glaciologists (Wharton et al. 1985; Takeuchi et al. 2001; Hodson et al. 2007; Stibal et al. 2009).
Evidence of colonization of Arctic glaciers by psychrophilic microbes has been recorded (Skidmore et al. 2000, 2005; Mindl et al. 2007). In a recent study, Butinar et al. (2007) isolated yeast from basal ice layers of high Arctic glacier of the Svalbard archipelago. However, studies on microbial ecology of cryoconite holes are limited (Säwström et al. 2002, 2007; Anesio et al. 2007, 2009; Hodson et al. 2008). Cyanobacteria and algae of cryoconite holes have been investigated by Mueller et al. (2001) and Kastovska et al. (2005). Interactions between bacterial diversity, microbial activity and supraglacial hydrology of cryoconite holes in Svalbard have been studied by Edwards et al. (2011). Studies on the occurrence of filamentous fungi in supraglacial cryoconite holes and their characterization for extracellular enzymes remain undescribed so far. In this communication, the results of a study on the isolation, identification and characterization of culturable yeasts and filamentous fungi from supraglacial Arctic cryoconite holes are presented.
Materials and methods
Sampling site
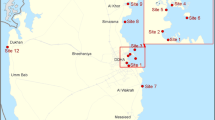
Midre Lovénbreen is a well-known polythermal valley glacier that is located in Ny-Ålesund (78°55′N, 11°56′E) on the west coast of Spitsbergen, Svalbard (Arctic) (Fig. 1a). It is a well-researched glacier and has a mass balance record since late 1960 s (Hagen et al. 2003). The mean temperature of the region in the coldest month (February) is −14°C, while it is +5°C in the warmest month (July). Like many other glaciers, melting of this glacier also occurs due to incident radiation, leaving the snow cover less than 10% of the glacier surface at the end of summer. This facilitates the formation of cryoconite holes all over the snow-free glacier surface. Cryoconite holes represent about 6% of the glacier surface with an average of about 12 holes/m2 (Säwström et al. 2002). Typically, the holes are about 8–30 cm deep and 5–50 cm in diameter and contain debris of about 0.1–1,000 g (Säwström et al. 2002; Hodson et al. 2007). The temperature of water in the cryoconite hole was between 0.2–1.9°C and pH 7.1–8.6 (measured using Thermo Orion 4Star, USA). Kastovska et al. (2005), however, has recorded the pH of cryoconite holes as 4.7. The organic matter in the holes is loose, rounded and brownish-black in colour (Fig. 1c, d).
Sampling methods
For the present study, four sampling sites were selected over the glacier at different altitudes, during the Indian Arctic Expedition 2009. The sampling locations were designated as G1, G2, G3 and G4 (Fig. 1b) and ranged from an altitude of 273 m (G1) to 164 m (G4). Samples were collected from cryoconite holes using a sterile syringe with a tube, placed in sterile ampules (Himedia) and stored at low temperature (−20°C) until analyses.
Sediment analysis
The cryoconite sediments were air dried and crushed gently using a wooden pestle and mortar. Organic carbon content was determined by wet digestion method (Walkley and Black 1934), available/mineralizable N by the method of Subbiah and Asija (1956) using VAP 30 distillation apparatus (Gerhardt), available P following Bray and Kurtz (1945) by extracting NaHCO3 and estimating spectrophotometrically. For elemental analyses, the cryoconite sediments were oven dried at 40°C, digested in HNO3 and HCl, and 0.5 g of sample was digested in a Teflon vessel at 180°C in Milestone make ‘Ethos’ advanced microwave digestion system. Elements were determined through ICPMS (inductively coupled plasma mass spectrometry, Thermo Scientific ICPMS-X series II) using Merck certiPUR ICP multi-element standard solution XXI for MS. All reagents used were suprapure Merck.
Isolation of yeast and filamentous fungi
One gram (wet weight) of cryoconite sediment was processed following the serial dilution method (Waksman 1916) and plated on six different media viz.YPD agar (yeast extract, 5 g/l; mycological peptone, 5 g/l; dextrose, 10 g/l; pH 6.5; agar, 20 g/l; chloramphenicol, 100 mg/l), MYP agar (malt extract, 7 g/l; yeast extract, 0.5 g/l; soytone, 2.5 g/l; agar ,15 g/l; pH 4; chloramphenicol, 100 mg/l), MEA (malt extract agar), PDA (potato dextrose agar), SDA (Sabouraud dextrose agar) and PCA (potato carrot agar) (Himedia, Mumbai), by pour plate as well as spread plate techniques. Viable counts of yeasts and filamentous fungi were measured according to Turchetti et al. (2008), using Rose Bengal agar (RB) + tetracycline. Plates were incubated in triplicates at 5, 10, 15, 20 and 25°C for 2–4 weeks. Plates were periodically examined, emerging yeast and filamentous fungal colonies counted, purified and stored at 4°C in MYP agar or PDA agar slants, respectively. Morphological, physiological and biochemical properties of the yeast isolates were determined as described by Yarrow (1998). All assimilation tests were performed twice, and results were scored after 1 and 3 weeks. Morphology of yeast cells and morphotaxonomic characters of filamentous fungi (Fig. 2a–e) were studied using OLYMPUS BX51 and IX71 microscopes (Japan). Extracellular enzymatic activity for lipase, protease, amylase, pectinase, catalase and cellulase was determined according to established procedures (Hankin and Anagnostakis 1975; Buzzini and Martini 2002). Suspensions of yeast cells (100 cells/ml) grown for 24–48 h or filamentous fungal spores (106 spores/ml) were used to inoculate the surface of agar plates using a multipoint inoculation device. Enzymatic activity was checked after 20 days of incubation at 4, 10, 15 and 20°C. The activity zones were qualitatively assessed as + (0.6–0.8 cm diam.) and ++ (1–1.2 cm diam.). Urease activity was tested on YNBG (6.7 g/l yeast nitrogen base, 20 g/l glucose), containing 1 g/l urea solution (pH 5.5). Change in colour from orange to pink was considered positive.
Identification of yeast and filamentous fungi
Identification of yeast was achieved by polyphasic approach, combining conventional and molecular techniques. Initial identification was carried out based on morphological characteristics and physiological tests according to Yarrow (1998). Fungal cultures were identified on the basis of morphotaxonomy with the help of standard literature (Ellis 1971, 1976; Barnett 1960; Barron 1977; Carmichael et al. 1980; Kirk et al. 2008). Strains with identical morphological and physiological characteristics were grouped together, and a representative strain of each group was subjected to sequence analysis of D1/D2 region of the rDNA gene. BLAST search was carried out with all fungal sequences available in the NCBI database (http://www.ncbi.nlm.nih.gov/BLAST/). Pure cultures were deposited at the National Fungal Culture Collection of India (NFCCI-WDCM 932) Pune, India.
DNA extraction and rRNA gene sequence analysis
Total DNA extraction was performed according to Libkind et al. (2003) with modifications. Two loopful of cultures grown on MYP agar were suspended in eppendorf tubes containing 500 μl of lysis buffer [20 mM Tris, 250 mM NaCl, 50 mM EDTA, 0.3% SDS, pH 8] and 200 μl of 425–600 mm glass beads. The content was vortexed for 3 min, incubated for 1 h at 65°C, re-vortexed for 3 min and centrifuged for 30 min at 5200×g. The supernatants were collected and diluted. For PCR amplification, 5 ml of diluted sample was used. The leftover supernatants were preserved at −20°C. PCR amplification of the DNA was done using ITS5 (5′-CGC AGT AAA AGT CGT AAC AAG G-3′) and LR6 (5′-CGC CAG TTC TGC TTA CC-3′) primers as described by Libkind et al. (2003). Sequencing of the D1/D2 region of the 26S ribosomal subunit was performed directly from purified PCR products using ABI Sequencer.
Growth characteristics of yeast and filamentous fungi
An experiment was carried out to assess the influence of temperature on growth of the isolated strains. In order to determine minimum and maximum growth temperatures, all the 83 isolates were tested for the ability to grow at different temperatures (5, 10, 15, 20, 25 and 30°C) on MYP agar, YPD agar, PDA for yeast and PDA, MEA, SDA and PCA for filamentous fungi. The plates were inoculated with a suspension of yeast cells grown for 24–48 h and well sporulated cultures of filamentous fungi. Growth was monitored visually for 20 days on a daily basis. Salt tolerance in the isolates was measured by growing the cultures at various concentrations of salt ranging from 1 to 5 M.
Statistical analysis
In order to compare fungal diversity within the four localities following software’s were used: Shannon-Wiener Diversity Index/Shannon Entropy Calculator (http://www.changbioscience.com/genetics/shannon.html), Online Calculation (http://www2.biology.ualberta.ca/jbrzusto/rarefact.php) and Past software (Hammer et al. 2001).
Results
The average water content in the cryoconite sediments was found to be between 67.16–79.01%, and organic carbon content was between 1.07 and 1.86% per dry sediment. Certain key trace nutrients such as nitrogen, phosphate, calcium and magnesium were detected in the samples in varying amounts. The details of physical and chemical characteristics of cryoconite sediments are presented in Table 1.
A total of 43 yeast and 40 filamentous fungal strains, with viable counts ranging between 7 × 103–1.4 × 104 and 4 × 103–1.2 × 104, respectively, were isolated and purified from the four cryoconite sediment samples (G1, G2, G3 and G4) (Table 2). Phialophora alba occurred in all four studied sampling sites. Simpson’s and Shannon’s diversity index (H′) showed the diversity of G-4 to be the highest and that of G-1 to be the lowest as compared to the rest of sampling points (Table 3). Based on morphological, physiological and biochemical characteristics, the isolates were categorized into five groups (three yeast and two filamentous fungi). BLAST analysis based on sequence data of D1/D2 domain of 26S rDNA helped to identify the nearest phylogenetic neighbours (99% similarity in their D1/D2 sequence) (Table 4). Based on phylogenetic neighbours, the representative strains were identified as Cryptococcus gilvescens (99% AF181547.1), Mrakia sp. zhenx-1 (99% EU680778.1), Rhodotorula sp. (95% FN400942.1), Phialophora alba (99% AB100618.1) and Articulospora tetracladia (100% EU998921.1) (Figs. 3, 4). Identification of isolates based on ITS/D1/D2 region of 28S rDNA sequences similarity (%) obtained in this study is shown in Table 4. Strains differed from the closest related type strain by two or fewer nucleotides in the D1/D2 region and were considered to be the same species. Among the identified representative strains, three are presumably new species, viz. Rhodotorula sp. (CCP-II) showed 20 nucleotide substitutions compared to closest neighbour Rhodotorula sp. (FN400942.1), Mrakia sp. (CCP-III-WY) showed 3 nucleotide substitutions when compared to the closest sequence Mrakia sp. (EU680778.1) which has not yet been validly described. Isolate CCP-I showed the closest identity to Phialophora alba (AB 100618.1) with 3 nucleotide substitution.
Phylogenetic tree constructed according to maximum parsimony method using D1/D2 domain of 26S rRNA gene sequence showing relationship of Cryptococcus, Mrakia and Rhodotorula yeast strains from Arctic. Candida sp. was used as out group. Bootstrap value for 1,000 replication is given in the branch nodes. The figures in parenthesis refer to the accession numbers of the D1/D2 domin of the 26 S rRNA gene of the various yeasts obtained from GeneBank
Phylogenetic tree constructed according to maximum parsimony method using D1/D2 domain of 26S rRNA gene sequence showing relationship in filamentous fungi Phialophora and Articulospora strains from Arctic. Acrophialophora nainana was used as out group. Bootstrap value for 1,000 replication is given in the branch nodes. The figures in parenthesis refer to the accession numbers of the D1/D2 domain of the 26 S rRNA gene of the various strains obtained from GeneBank
The extracellular enzymatic activity of the strains is listed in Table 5. Almost all tested strains showed at least one extracellular enzyme activity. Lipase activity (hydrolysis of tributyrin/Tween-80) was the most widely expressed extracellular enzyme among the cultures tested. Almost all strains showed the ability to hydrolyse at least one of the two lipophilic substrates, regardless of the temperature at which it was tested. Mrakia sp. (CCP-III-WY) was the only isolate that exhibited phosphate solubilizing ability at 4 and 10°C. Rhodotorula sp. (CCP-II) expressed good amylase activity at 10 and 15°C. All yeast strains expressed catalase activity at 10°C. Extra cellular pectinase activity was seen in Mrakia sp. (CCP-III-WY), while extracellular cellulase activity was seen only in Phialophora alba (CCP-I), at 15°C.
Effect of temperature variation on growth of cultures showed that all filamentous fungal strains were able to grow at temperatures below 20°C but not at or above 25°C. Growth rate of cultures at 20°C was slow as compared to the lower temperatures. The yeast strains grew luxuriantly at 10 and 15°C but did not grow above 20°C. The optimum temperature for growth of Cryptococcus gilvescens (dry biomass wt. 1.2 g/l) and Rhodotorula sp. (dry bio mass wt. 2.3 g/l) was 15°C while that of Mrakia sp. (dry bio mass wt.1.5 g/l) was 10°C, after 30 days of incubation in YMP broth. The effect of growth medium and temperatures are shown in Fig. 5. The optimum temperature for growth of filamentous fungi Phialophora alba was 20°C (colony diam. 3.0 mm) while that of Articulospora tetracladia was 15°C (colony diam. 3.5 mm) on PCA medium. Of the four different media tested all showed good growth, but the luxuriant growth and sporulation were observed on potato carrot agar medium. Salt tolerance studies showed that the cultures showed varying response. Mrakia sp. was the highest salt-tolerant culture (3.5 M), while Phialophora alba (1 M) was the least tolerant (Table 5).
Growth of filamentous fungi at different temperature on different media after 30 days. (SDA, Sabouraud dextrose agar; CMA, corn carrot agar; PCA, potato carrot agar; PDA, potato dextrose agar; CCP-I, Phialophora alba; CCP-II, Rhodotorula sp.; CCP-III, Cryptococcus gilvescens and Mrakia sp.; CCP-V, Articulospora tetracladia)
Discussion
Although microbial populations of the supra and subglacial habitats of Midre Lovénbreen glacier have been studied in the past (Kastovska et al. 2005; Hodson et al. 2008; Säwström et al. 2002, 2007), mycological investigation into the cryoconite holes have not been carried out so far. The viable cell count made in the present study showed that the cryoconite holes at higher latitudes (G1) support more yeast species than fungi. The number decreased as one move down the glacier. At the lowermost point (G4), filamentous fungi were more abundant than the yeast. This could be likely because the temperature at higher altitudes is lower than the downstream of the glacier and yeast rather than filamentous fungi prefer lower temperatures. This is in agreement with Gostinĉar et al. (2006) and Butinar et al. (2007) who described the evolution of yeast and yeast-like fungal populations in the basal Arctic ice.
Taxonomic studies based on morphology and sequence data showed the presence of Cryptococcus gilvescens, Mrakia sp., Rhodotorula sp., Phialophora alba and Articulospora tetracladia from the cryoconite sediments. Species of C. gilvescens, Mrakia sp. and Rhodotorula sp. have been reported previously from glacier sediments of Alpine region (Turchetti et al. 2008). Taxonomic identification based on sequence data was following the study by Fell et al. (2000), who suggested that strains that differed from the closest related type strain by two or fewer nucleotides in the D1/D2 region are the same species. Simpson’s and Shannon’s diversity index (H′) showed that the diversity at higher altitudes of the glacier was less and increased downstream as observed at G-4 (Table 3).
Chemical analysis of cryoconite sediments revealed the presence of organic carbon, nitrogen and phosphorus in substantial amounts that may support the growth and activity in the sediments. Similar nutrient presence was observed in the subglacial sediments by Sharp et al. (1999), Foght et al. (2004) and Turchetti et al. (2008). From the soil data and from the observations related to fungal diversity, it is evident that fungi prefer to grow and proliferate in organic soils as compared to ahumic soils. Similar observations have also been made by Stonehouse (1989).
Presence of heterotrophic organisms like filamentous fungi and yeasts in the cryoconite holes drives the process of degradation of organic macromolecules through the secretion of extracellular hydrolytic cold-adapted enzymes and thus assists in nutrient cycling in subglacial environments. Evidence of cold-adaptation in the cryoconite isolates is evident from the fact that these isolates do not survive above 20°C (Table 4). Exhibition of cold-adapted extracellular enzymatic activity further confirms this observation.
Though cold-active enzymes such as amylases, catalases, cellulases, invertase, lactase, lipases, pectinases and proteases produced by Arctic fungal strains find potential applications in food, medicine and detergent industries, as of now only catalase (Fiedurek et al. 2003) and invertase (Skowronek et al. 2003) have been recorded from Spitsbergen fungi. Extreme habitats as cryoconites holes are, therefore, unique for further exploration to understand the molecular adaptations of these microbes and their biotechnological potentials.
References
Anesio AM, Mindl B, Laybourn-Parry J, Hodson AJ, Sattler B (2007) Viral dynamics in cryoconite holes on a high Arctic glacier (Svalbard). J Geophys Res doi:10.1029/2006JG000350
Anesio AM, Hodson AJ, Fritz A, Psenner R, Sattler B (2009) High microbial activity on glaciers: importance to the global carbon cycle. Global Change Biol 15:955–960
Barnett HL (1960) Illustrated genera of imperfect fungi, 2nd edn. Burgess Publishing Company, USA
Barron GL (1977) The genera of hyphomycetes from soil. Robert E. Krieger Pub. Comp, INC. Huntington, New York
Bray RH, Kurtz LT (1945) Determination of total organic and available forms of phosphorus in soils. Soil Sci 59:39–45
Butinar L, Spencer-Martins I, Gunde-Cimerman N (2007) Yeasts in high Arctic glaciers: the discovery of a new habitat for eukaryotic microorganisms. Antonie Leeuwenhoek 91:277–289
Buzzini P, Martini A (2002) Extracellular enzymatic activity profiles in yeast and yeast-like strains isolated from tropical environments. J Appl Microbiol 93:1020–1025
Carmichael JW, BryceKendrick W, Conners IL, Sigler L (1980) Genera of hyphomycetes. The University of Alberta Press, Canada
Christner BC, Kvitko BH, Reeve JN (2003) Molecular identification of bacteria and eukarya inhabiting an Antarctic cryoconite hole. Extremophiles 7:177–183
Edwards A, Anesio AM, Rassner SM, Sattler B, Hubbard B, Perkins WT, Young M, Griffith GW (2011) Possible interactions between bacterial diversity, microbial activity and supraglacial hydrology of cryoconite holes in Svalbard. ISME J 5:150–160
Ellis MB (1971) Dematiaceous hyphomycetes. CMI, Kew, England
Ellis MB (1976) More dematiaceous hyphomycetes. CMI, Kew, England
Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A (2000) Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int J Syst Evol Micr 50:1351–1371
Fiedurek J, Gromada A, Saomka A, Korniaowicz-Kowalska T, Kurek E, Melke J (2003) Catalase activity in Arctic microfungi grown at different temperatures. Acta Biol Hung 54:105–112
Foght J, Aislabie J, Turner S, Brown CE, Ryburn J, Saul DJ, Lawson W (2004) Culturable bacteria in subglacial sediments and ice from two southern hemisphere glaciers. Microb Ecol 47:329–340
Gostinĉar C, Urŝiĉ V, De Hoog S, Gunde-Cimerman N (2006) Local evolution of black yeast A. pullulans in sub glacial Arctic ice. In: Proceedings of international conference on alpine and polar microbiology, Innsbruck, Austria, p 19
Hagen JO, Kohler J, Melvold K, Winther JG (2003) Glaciers in Svalbard: mass balance, runoff and freshwater flux. Polar Res 22:145–159
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron 4:1–9
Hankin L, Anagnostakis SL (1975) The use of solid media for detraction of enzyme production by fungi. Mycologia 67:97–607
Hodson A, Anesio AM, Ng F, Watson R, Quirk J Irvine-Fynn T et al. (2007) A glacier respires: quantifying the distribution and respiration CO2 flux of cryoconite across an entire Arctic supraglacial ecosystem. J Geophys Res doi:10.1029/2007JG000452
Hodson AJ, Anesio AM, Tranter M, Fountain AG, Osborn M, Priscu J, Laybourn-parry J, Sattler B (2008) Glacial ecosystems. Ecol Monogr 78:41–67
Kastovska K, Elster J, Stibal M, Santruckova H (2005) Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (High Arctic). Microb Ecol 50:396–407
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Ainsworth and Bisby’s dictionary of the fungi, 10th edn. CABI Publishing, UK
Libkind D, Brizzio S, Ruffini A, Gadanho M, Van Broock M, Paulo SJ (2003) Molecular characterization of carotenogenic yeasts from aquatic environments in Patagonia, Argentina. Antonie Leeuwenhoek 84:313–322
Mindl B, Anesio AM, Meirer K, Hodson AJ, Laybourn-Parry J, Sommaruga R, Sattler B (2007) Factors influencing bacterial dynamics along a transect from supralgacial runoff to proglacial lakes of a high Arctic glacier. FEMS Microbiol Ecol 59:307–317
Mueller DR, Vincent WF, Pollard WH, Fristen CH (2001) Glacial cryconite ecosystems: a bipolar comparison of algal communities and habitats. Nova Hedwigia 123:173–197
Säwström C, Mumford P, Marshall W, Hodson A, Laybourn-parry J (2002) The microbial communities and primary productivity of cryoconite holes in Arctic glacier (Svalbard 79N). Polar Biol 25:591–596
Säwström C, Grane′li W, Laybourn-Parry J, Anesio AM (2007) High viral infection rates in Antarctic and Arctic bacterioplankton. Environ Microbiol 9:250–255
Sharp M, Parkes J, Cragg B, Fairchild IJ, Lamb H, Tranter M (1999) Widespread bacterial populations at glacier beds and their relationships to rock weathering and carbon cycling. Geology 27:107–110
Skidmore ML, Foght JM, Sharp MJ (2000) Microbial life beneath a High Arctic glacier. Appl Environ Microbiol 66:3214–3220
Skidmore ML, Anderson SP, Sharp MJ, Foght JM, Lanoil BD (2005) Comparison of microbial community composition of two subglacial environments reveals a possible role for microbes in chemical weathering processes. Appl Environ Microb 71:6986–6997
Skowronek M, Kuszewska J, Fiedurek J, Gromada A (2003) Invertase activity of psychrotrophic fungi. Annales Universitatis Mariae Curie-Skłodowska Lublin-Polonia 58:1–9
Stibal M, Anesio AM, Blues CJD, Tranter M (2009) Phosphatase activity and organic phosphorus turnover on a high Arctic glacier. Biogeosciences 6:913–922
Stonehouse B (1989) Polar ecology. Chapman and Hall, New York
Subbiah BH, Asija GL (1956) A rapid procedure for determination of available nitrogen in soils. Curr Sci 25:259–260
Takeuchi N, Kohshima S, Seko K (2001) Structure, formation, and darkening process of albedo-reducing material (cryoconite) on a Himalayan glacier: a granular algal mat growing on the glacier. Arct Antarct Alp Res 33:115–122
Turchetti B, Buzzini P, Goretti M, Branda E, Diolaiuti G, D’Agata C, Smiraglia C, Vaughan-Martini A (2008) Psychrophilic yeasts in glacial environments of Alpine glaciers. FEMS Microbiol Ecol 63:73–83
Waksman SA (1916) Do fungi live and produce mycelium in the soil? Science NS 44:320–322
Walkley A, Black CA (1934) An examination of Degtjareff methods for determining soil organic matter and a proposed modification of chromic acid titration method. Soil Sci 37:29–38
Wharton RA, McKay CP, Simmons GM, Parker BC (1985) Cryoconite holes on glaciers. Bioscience 35:499–503
Yarrow D (1998) Methods for the isolation, maintenance and identification of yeasts. The Yeasts. In: Kurtzman CP, Fell JW (eds) A taxonomic study. Elsevier, Amsterdam, pp 77–100
Acknowledgments
Author (PS1) is highly indebted to Department of Science & Technology (DST), New Delhi for financial support. Authors are thankful to Dr Rasik Ravindra, Director NCAOR, Director BITS and Dr Jagdev Sharma, NRCG for encouragement and facilities. Thanks are due to Dr C. T. Achuthankutty for improving the English language of the manuscript, Chief Editor and anonymous reviewers for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, P., Singh, S.M. Characterization of yeast and filamentous fungi isolated from cryoconite holes of Svalbard, Arctic. Polar Biol 35, 575–583 (2012). https://doi.org/10.1007/s00300-011-1103-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1103-1