Abstract
Viruses play a significant role in nutrient cycling within the world’s oceans and are important agents of horizontal gene transfer, but little is know about their entrainment into sea ice or their temporal dynamics once entrained. Nilas, grease ice, pancake ice, first-year sea ice floes up to 78 cm in thickness, and under-ice seawater were sampled widely across Amundsen Gulf (ca. \(71^\circ \hbox{N}, 125^\circ \hbox{W}\)) for concentrations of viruses and bacteria. Here, we report exceptionally high virus-to-bacteria ratios in seawater (45–340) and sea ice (93–2,820) during the autumn freeze-up. Virus concentrations ranged from 4.8 to 27 × 106 ml−1 in seawater and, scaled to brine volume, 5.5 to 170 × 107 ml−1 in sea ice. Large enrichment indices indicated processes of active entrainment from source seawater, or viral production within the ice, which was observed in 2 of 3 bottle incubations of sea ice brine at a temperature (\(-7^\circ\hbox{C}\)) and salinity (\(110 \permille\)) approximating that in situ. Median predicted virus-to-bacteria contact rates (relative to underlying seawater) were greatest in the top of thick sea ice (66–78 cm: 130×) and lowest in the bottom of medium-thickness ice (33–37 cm: 23×). The great abundance of viruses and more frequent interactions between bacteria and viruses predicted in sea ice relative to underlying seawater suggest that sea ice may be a hot spot for virally mediated horizontal gene transfer in the polar marine environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Horizontal gene transfer (HGT)—the incorporation of genetic material from one organism into another without reproduction—has played a pivotal role in the evolution of extant microbial genomes, as revealed by massive sequencing efforts over the past decade (Brown 2001; Gogarten et al. 2002; Lawrence and Hendrickson 2003; Beiko et al. 2005). This genomic evidence has led to the conclusions that HGT is both widespread among all three Domains of life (Bacteria, Archaea, and Eukarya) and intensive. A number of recent studies have implicated HGT in the transmission of freeze tolerance genes from one Domain of life to another, including ice-binding proteins (Janech et al. 2006; Raymond et al. 2007) and antifreeze proteins (Kiko 2010; Bayer-Giraldi et al. 2010). Sea ice is a very promising candidate site for the occurrence of HGT in these cases, but the environmental locale and gene transfer mechanisms responsible for these apparent transfers are not known, indicating a need to supplement gene sequence-based investigations with laboratory experiments using environmentally relevant microorganisms, and in situ experiments with natural populations.
Viruses can act as agents of HGT in a process called transduction (Zinder and Lederberg 1952). Transducing phage are well known from the marine environment (Baross et al. 1978; Chiura 1997; Jiang and Paul 1998), but have not yet been reported from sea ice. However, high abundances of viruses have been repeatedly observed in sea ice from a variety of sources (Maranger et al. 1994; Gowing et al. 2002, 2004; Wells and Deming 2006b). Transducing phage are often specific in their host requirements (Wommack and Colwell 2000), though some may be generalists capable of infecting a wider range of hosts (Börsheim 1993; Comeau et al. 2006; Holmfeldt et al. 2007). The metabolic state of the host cell and its exposure to stress-inducing factors can affect the virus–host dynamics and may alter the range of phage capable of infecting it (Miller 2001). Microbial activity is known at temperatures down to \(-20^\circ\hbox{C}\) (Junge et al. 2004) in winter sea ice, and the production of viruses in sea ice has been reported in natural brine incubations down to \(-12^\circ\hbox{C}\) (Wells and Deming 2006b). In warmer sea ice, viral production has been reported as an increase in viral abundance over time (Maranger et al. 1994) and by the presence of visibily infected cells (Gowing 2003).
As a first exploration into the cycling of dissolved DNA (including viruses and free extracellular DNA) in sea ice, we sampled several different types of first-year sea ice in late autumn, including frazil, nilas, and pancake ice, as well as floes of varying thicknesses from 33 to 78 cm. At each station, we measured the abundances of bacteria, viruses, and extracellular DNA in the ice and the underlying seawater, as well as various physical and chemical parameters to provide environmental context. Further work pertaining to the physical and chemical conditions within the seawater and sea ice can be found in a companion paper (Collins and Deming, submitted this issue).
Materials and methods
Sample collection and processing
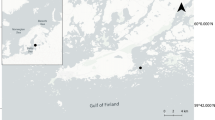
Sampling took place aboard the CCGS Amundsen between November 10, 2007, and December 18, 2007, as part of the Circumpolar Flaw Lead System Study (CFL), a project of the International Polar Year. Sea ice and surface seawater were collected from eight stations in the western Amundsen Gulf, Beaufort Sea, Canada, during freeze-up in late autumn of 2007 (Fig. S1, Table S1). Further details on sampling, sites, and analytical procedures may be found in Collins and Deming (submitted this issue). Briefly, at each station where ice thickness was greater than 10 cm, three "biological" cores were cleanly drilled at the corners of a 1 × 1 m square in conjunction with a "physical" core (used to measure temperature and bulk salinity). The top 10 cm and bottom 10 cm of each core were aseptically removed and transferred to sterile Whirl-Pak bags. The core sections were mechanically crushed and transferred to separate sterile melt jars for isothermal––isohaline melting (Junge et al. 2004). Two volumes of 10 kDa tangential flow filtration (TFF) pre-filtered artificial seawater brine (ASW, made with ASW salts, Sigma Corp.) were added, so that after melting the final salinity was equivalent to the in situ brine salinity, calculated from the ice core temperature (Cox and Weeks 1983; see also Cox and Weeks 1986 and Collins et al. 2008). For ice less than 10 cm thick, ice was scooped into jars and melted directly. For all ice, the volume of meltwater was measured immediately upon melting. Under-ice seawater was sampled using a 2 L Niskin bottle.
Microscopy
For bacterial abundance, one 39 ml aliquot of meltwater or seawater from each sample was fixed with formaldehyde (2% final concentration) and stored at 2–4\(^\circ\hbox{C}\) until enumeration at the University of Washington within 4 months using epifluorescence microscopy on DAPI-stained samples, as in Collins et al. (2008). The remaining sample was filtered through a 0.22 μm Sterivex filter column to remove bacterial cells; one 14 ml aliquot of the filtrate was collected for viral enumeration and fixed with 0.2 μm-filtered formaldehyde (1.5% final concentration). Fixed aliquots were stored at 2–4\(^\circ\hbox{C}\) until slides (one per aliquot) were prepared within 4 h of fixation (up to 24 h post-sampling in the case of ice samples); some slides were re-prepared from stored samples up to a week old. Viral abundances in this study should be therefore be considered underestimates because significant viral decay can occur within hours of storage in aldehydes (Wen et al. 2004). Aboard ship, 1 mL of each fixed sample was filtered onto a 0.02 μm Anodisc filter and stained with SYBR Gold in accordance with standard protocols (see Wells and Deming 2006b and Patel et al. 2007). Slides were stored at \(-20^\circ\hbox{C}\). At least 10 fields from each slide were photographed with a dedicated CCD camera attached to a Zeiss Axioplan microscope with a ×1000 Plan Apochromat objective (Carl Zeiss) under blue excitation (450–490 nm) and green emission (>515 nm); viruses were counted from the photographs upon return to shore. For each procedure, ASW or distilled water (pre-filtered by 10 kDa TFF) was used as a negative control. We use the term "virus" as a shorthand for "virus-like particle".
Scaling and enrichment index
Concentrations of biological parameters in the ice were measured on individual horizons and were therefore scaled to the volume of liquid brine in which they were presumed to be located in situ, as calculated from the equations of Cox and Weeks (1983, 1986). The assumption that bacteria reside in brine channels within ice (rather than being encased in the solid matrix) is well supported by microscopic observations (Junge et al. 2001); viruses have not been subjected to the same examination. Enrichment indices (E) for bacteria and viruses were calculated using bulk ice concentrations according to Collins and Deming (submitted this issue), whereby E = 0 is equivalent to passive entrainment (i.e., proportionally with sea salts, equivalent to I S = 1 in the enrichment formulation of Gradinger and Ikavalko 1998), E = 1 is equivalent to complete entrainment from seawater, E > 1 indicates active enrichment into the ice, and E < 0 indicates loss relative to seawater. The enrichment index is a bulk measure and is affected by a variety of factors, including frazil ice scavenging, in situ production, degradation, grazing, and any artifacts introduced during sample processing. Statistics were calculated in using the open-source R Project (R Development Core Team 2011). Errors (±) were calculated as standard errors of the mean (SEM).
Contact rate calculations
Calculation of virus––bacteria contact rates (J, contacts cm−3 s−1) proceeded as in Wells and Deming (2006b, following Murray and Jackson 1992), by the equation J = 2π d D v V B Sh, where d is the spherical diameter of the average cell (here, 0.5 × 10−4 cm), D v is the viral diffusivity (cm2 s−1), V and B are the calculated in situ concentrations of viruses and bacteria (cm−3), respectively, and Sh is the Sherwood number, a non-dimensional representation of the enhancement of transport due to fluid flow relative to simple diffusion. Accounting for cell shape and swimming speed, Murray and Jackson (1992) showed that even rapidly swimming bacteria had Sh∼1, indicating that the transport of viruses to bacteria is usually diffusion limited; here, we use Sh = 1. The viral diffusivity is calculated as \(D_v =\frac{kT}{3\pi\mu d_v}\), where k is Boltzmann’s constant (1.38 × 10−9 g cm2 K−1 s−2), T is the temperature (Kelvin), μ the viscosity (g cm−1 s−1), and d v the diameter of the average virus (here, 60 × 10−7 cm). The following least-squares best-fit equation (r 2 = 0.991) for seawater and brine viscosity (μ) at low temperature (T, Kelvin) and high salinity (S, \(\permille\)) was computed with the curve-fitting program ZunZun (http://zunzun.com) using data available from Cox and Weeks (1975): μ = (10.2 − 0.00132S − 0.0835T + 0.000177T 2)−1. Relative contact rates between sea ice and seawater were calculated as \(\frac{J_{ice}}{J_{water}} \). Cell-specific virus contact rates (\(\hbox{J}_{s}\) of Murray and Jackson 1992) were calculated as \(J/B \times 86,400{\frac{s}{d}}\) to yield the daily average virus contacts per bacterium.
Production experiments
At a single site (Station D7b), the production of bacteria and viruses (Wilhelm et al. 2002; Winget et al. 2005) was measured in three bottle incubations of sea ice brine (2 l) collected over 6 h from three sackholes, each drilled to a depth of 45 cm. The brine had a temperature of \(-6^\circ\hbox{C}\) and a salinity of \(110\permille\) as measured by refractometer. Each incubation was conducted in a new, acid-rinsed 2 l polycarbonate bottle to minimize bottle effects. For each, brine was passed across a 0.22 μm TFF unit (Millipore) to concentrate bacteria and reduce viral abundance. The retentate was then diluted with filtrate from 10 kDa TFF of the same brine in an effort to approximate the in situ bacterial concentration and the in situ brine salinity from each sackhole. The bottles were incubated in a freezer at \(-7^\circ\hbox{C}\) for 60 h. Bottles were monitored regularly; the occasional formation of ice crystals required removal to room temperature until the crystals melted within 15 min. At time points of 0, 12, 24, 36, and 60 h, 5 ml aliquots were fixed with formaldehyde (1.5% final concentration) for enumeration of viruses and bacteria by SYBR Gold fluorescence (processed as above). Viral production rates were calculated as the slope of a linear regression calculated for each bottle; rates were corrected by dividing by the fraction of cells recovered after filtration.
Results
Seawater dynamics
In the full depth profile at Station D8 (day 350), the concentrations of bacteria were low (mean: 0.45 × 105 ml−1) and exhibited subsurface maxima at 30 m and at 200 m (Fig. 1); viral abundance decreased through the water column from 14 × 106 ml−1 at the surface to 4.4 × 106 ml−1 at a depth of 441 m (Fig. 1).
Seawater depth profile taken on December 16, 2007 (day 350) at Station D8, showing a temperature (solid line) and salinity (dashed line), and b concentrations of bacteria (filled circles) and viruses (open squares); each point represents a single sample, error bars represent standard errors of the mean microscope counts
Very low concentrations of chlorophyll a were detected in surface seawater (0.042−0.204 ng ml−1), which decreased significantly over the course of the study (ρ = −0.900, n = 9, P < 0.01; Fig. 2a). During the latter half of November, between days 318 and 336, chlorophyll a concentrations decreased at a significant rate of −0.0071 ± 0.0017 ng ml−1 d−1 (R 2 = 0.724, p < 0.01; Fig. 2). The abundance of bacteria in surface seawater was highest on day 316 (12 November; 1.6 × 105 ml−1) and lowest on day 350 (December 16; 0.45 × 105 ml−1); a significant decrease was detected over the course of the study (ρ = −0.839, n = 10, P < 0.01). Between days 318 and 333 bacterial abundance decreased at a significant rate of −0.041 ± 0.003 × 105 ml−1 d−1 (R 2 = 0.972, p ≪ 0.001; Fig. 2b). The abundance of viruses ranged from 4.8 to 17 × 107 ml−1, excluding a possible outlier at Station 437 (day 326; 27 × 106 ml−1). Viral abundance was not significantly different over the course of the study (ρ = 0.425, n = 10, P > 0.1), though a significant increase in viral abundance was observed between days 318 and 333 (from 4.8 to 14 × 106 ml−1) at a rate of 0.57 ± 0.16 × 106 d−1 (R 2 = 0.733, P = 0.041; Fig. 2c).
Concentrations of a chlorophyll a, b bacteria, and c viruses within surface seawater (≤10 m depth) of the Amundsen Gulf by sampling day during autumn freeze-up in 2007. Each point represents a single sample, error bars represent standard errors of the mean microscope counts. Lines indicate linear regression rate estimations over subsets of the data; a possible outlier was excluded from the rate estimation of viral abundance (circle with ‘×’). Significant negative slopes were detected over the subsets of concentrations of chlorophyll a (slope = − 0.0071 ± 0.0017 ng ml−1 d−1, R 2 = 0.724, P < 0.01) and bacteria (slope = −0.041 ± 0.0031 × 105 d−1, R 2 = 0.972, P ≪ 0.001); a significant positive slope was detected over the subset of virus concentrations (slope = 0.57 ± 0.16 × 106 d−1, R 2 = 0.733, P = 0.041)
Sea ice dynamics
Sea ice samples were separated into three groups by their thickness: thin ice (4–9 cm), medium ice (33–37 cm), and thick ice (66–78 cm). Temperatures within the ice ranged from −2.2 to \(-11.3^\circ\hbox{C}\), with calculated brine salinities of 41 to \(153\permille\) (Table 1). There was little snow on the ice, usually less than 2.5 cm. Further details on physical and chemical characterization of sea ice and seawater samples may be found in Table S1 and Collins and Deming (submitted this issue).
Virus concentrations in sea ice (scaled to brine volume) ranged from 5.5 to 170 × 107 ml−1; bacteria concentrations in sea ice (scaled to brine volume) were 0.96 to 9.2 × 105 ml−1 (Table 1; Fig. 3). Virus-to-bacteria ratios (VBR) observed in sea ice (846 ± 169; Fig. 3) were significantly higher (two-tailed t-test, p < 0.001) than those in seawater (160 ± 38). The highest mean VBRs were found in the bottom 10 cm of thick ice (1,654 ± 267), but the maximum VBR (2,820) was observed in the bottom 10 cm of medium-thickness landfast ice collected within 500 m of the shore in Summers Harbor (Station R; Fig. S1).
Concentrations of bacteria and viruses measured in surface seawater (≤10 m; open circles), brine from sackholes (45 cm from surface; inverted triangles), and scaled to brine volume: thin ice (<10 cm thick; triangles), medium-thickness ice (33–37 cm; squares), and thick ice (66–78 cm; diamonds). For medium and thick ice, two horizons were sampled: bottom 10 cm (open) and top 10 cm (filled). Parallel angled lines indicate lines of constant virus-to-bacteria ratio (VBR). Additional data were included from three other studies of bacterial and viral abundance in sea ice. Data collected during an Arctic spring algal bloom (+, Maranger et al. 1994) were replotted from the authors’ Figs. 1 and 6, using a conversion factor of 4 × 104 ml m−2 and an estimated brine volume fraction of 17% for bottom ice at \(-2.2^\circ\hbox{C}\). Data from Antarctic summer pack ice (•, Gowing et al. 2004) were replotted from the authors’ Fig. 2, also using an estimated brine volume fraction of 17%. Data from Arctic winter sea ice (×, Wells and Deming 2006b) were replotted from the authors’ Table 1
Differences in enrichment index were observed among ice types for bacteria (Kruskal-Wallis U = 10.09, df = 2, P = 0.0064) but not for viruses (U = 0.98, df = 2, P = 0.61). Bacteria were more highly enriched in thin sea ice than salts (1.4 ± 0.3, n = 5); in contrast, the observed enrichments of bacteria in the bottom 10 cm of medium and thick ice (0.03 ± 0.03, n = 12) were in agreement with those expected due to passive entrainment (Fig. 4). Viruses were highly enriched in all sea ice types relative to salts (Fig. 4); the observed median enrichment (1.05, range 0.13−8.0, n = 19) was consistent with either active entrainment of the entire seawater virus community or viral production within the ice following entrainment.
Enrichment indices of bacteria and viruses in newly formed sea ice (for details on calculation of index, see Collins and Deming submitted this issue). An enrichment of E = 0 (solid line) indicates enrichment equivalent to passive entrainment into sea ice (proportional with sea salts), E = 1 (dashed line) indicates an enrichment equivalent to complete entrainment from underlying seawater. All indices are plotted (bullet); in addition, lower and upper limits of boxplots indicate 25 and 75% quartiles, respectively; center line indicates median. Asterisks indicate significant (*P < 0.05) or highly significant (**P < 0.001) differences from E = 0 as given by the Mann–Whitney test; asterisks above bracket indicate significance level for all samples combined, asterisks below bracket indicate significance levels for each ice group
Calculated cell-specific daily contact rates between bacteria and viruses were higher in sea ice than in underlying seawater in all cases (W = 231, n = 41, P ≪ 0.001; Figs. 5, S2). Over the course of one day, the average seawater bacterium would have been expected to contact 14 ± 1 viruses per day. The rates were several times higher in thin ice (46 ± 4), but the highest rates were calculated for medium and thick ice (233 ± 49), accounting for a statistically significant difference among ice types (U = 10.7, df = 2, P < 0.005). Contact rates were not correlated with temperature in the ice (ρ = −0.036, df = 33, P > 0.1; Fig. 5).
Cell-specific viral contact rate (d−1) calculated using the diffusion-based model of Wells and Deming (2006b) in seawater (≤10 m; open circles), brine from sackholes (45 cm from surface; inverted triangles), thin ice (<10 cm thick; triangles), medium-thickness ice (33–37 cm; squares), and thick ice (66–78 cm; diamonds). For medium and thick ice, two horizons were sampled: bottom 10 cm (open) and top 10 cm (filled)
Production experiments were performed on virally reduced samples of brine collected from sackholes, but some bacteria were also lost during TFF. Bacterial recoveries in bottles A, B, and C were 36, 10, and 54%, respectively; viral abundance was reduced by 90, 95, and 91% respectively. Statistically significant increases in viral abundance were detected in bottles A and C (Fig. 6), at rates (corrected for initial losses of bacteria) of 5.1 × 106 ml−1 d−1 (r = 0.97, n = 4, P < 0.05) and 2.4 × 106 ml−1 d−1 (r = 0.91, n = 5, P < 0.05), respectively. Suggestive (but not statistically significant at α < 0.05) increases in bacterial abundance were observed in bottles A and B (Fig. 6), with corrected estimated rates of 1.9 × 105 ml−1 d−1 (r = 0.82, n = 5, P < 0.1) and 5.8 × 105 ml−1 d−1 (r = 0.87, n = 5, P < 0.1), respectively.
Concentrations of a viruses and b bacteria in parallel bottle incubations of sea ice brine at \(-7^\circ\hbox{C}\) and a salinity of \(110\permille\). Solid lines in a indicate significant increases in viral abundance (at P < 0.05); dashed lines in b indicate suggestive, but not statistically significant (0.05 < P < 0.1), increases in bacterial abundance. Each point represents a single sample; error bars indicate standard error of the mean microscopic counts
Discussion
Viruses and bacteria in seawater
Based on the full depth profile of the water column taken at Station D8 on day 350 (16 December), the water column was stratified, as is typical for the Amundsen Gulf, with shallow Polar Mixed Layer water separated from the deeper, isothermal Halocline Arctic Layer by an intrusion of warm Pacific water at 20–40 m (Fig. 1). A peak in bacterial abundance was observed in the Pacific water layer at 30 m, but the maximum viral concentrations were found in the surface layers. This discrepancy, and the generally low concentrations of bacteria in surface seawater discussed below, may indicate non-equilibrium processes occurring in the surface layer of seawater during ice formation, e.g., viral production outpacing bacterial production. Alternatively, repeated rounds of sea ice formation followed by ice advection could draw down the concentrations of bacteria in surface waters if bacteria had higher enrichment indices into thin ice, but although the median enrichment index for bacteria into thin ice was almost twice that of viruses (1.72 and 0.92, respectively), no significant difference in enrichment index was detected between them.
Despite covering thousands of square kilometers of area in the Amundsen Gulf during the course of sampling, our results are highly suggestive of temporal changes in concentrations of biological parameters occurred there during the latter half of November 2007 (Fig. 2). In regression analyses, day of year explained 72, 97, and 73% of the variance in concentrations of chlorophyll a, bacteria, and viruses, respectively. No systematic biases were present in the ship’s location with respect to time (e.g., latitude, longitude, or distance from shore) that may have driven the correlation. Concentrations of bacteria in seawater decreased dramatically during late autumn, until about day 333 (November 29), while viral abundance increased over the same period (Fig. 2). At the estimated rates of change, the observed bacterial mortality could be wholly explained by viral production with a burst size of 160 viruses cell−1. While this value is similar to the mean of 185 viruses cell−1 compiled by Börsheim (1993) across a range of cultivated marine bacteriophage, it is much greater than the mean of 24 viruses cell−1 compiled by Wommack and Colwell (2000) for a range of aquatic studies, suggesting viruses may have made a more moderate contribution to bacterial declines of perhaps 15%. Additionally, the possible induction of lysogens could have contributed to decreasing bacterial abundances in the water column (Payet and Suttle 2008). Limited data in December suggested that concentrations of chlorophyll a, bacteria, and viruses all leveled off through the middle of the month.
The concentrations of bacteria observed in the late autumn of 2007 in Amundsen Gulf seawater (0.45−1.6 × 105 ml−1) were lower than expected based on other studies of Arctic coastal seawater (e.g., 2.1−21 × 105 ml−1 in the Bering and Chukchi Seas, Steward et al. 1996; 2.0−9.5 × 105 ml−1 in the Chuckchi and Beaufort Seas, Yager et al. 2001; 1.6−25 × 105 ml−1 in the Beaufort Sea and Amundsen Gulf, Payet and Suttle 2008). Most similar to our study site, season, and concentrations, Alonso-Sáez et al. (2008) found bacterial abundances of 2–4 × 105 ml−1 in winter seawater. A strong storm traveled through the region in early November and might have mixed low-cell-concentration deeper waters into the surface, but the water column was stratified by the middle of December (Fig. 1). Several lines of evidence preclude methodological or computational errors, including reproducibility by independent researchers and congruently low counts between surface seawater and sea ice. No decrease in bacterial abundance due to decay during storage was observed in sea ice samples; abundances in seawater increased over time. A combination of limited primary productivity, grazing, and viral lysis could explain this phenomenon, which appears to have been widespread over the Amundsen Gulf during the cruise.
In contrast to the seemingly atypical bacterial abundances in Amundsen Gulf seawater, the concentrations of viruses (4.8−27 × 106 ml−1) were comparable with those previously found from Arctic seawater (e.g., 2.5−36.0 × 106 ml−1 in the Bering and Chukchi Seas, Steward et al. 1996; 0.8−7.9 × 106 ml−1 in the Chuckchi and Beaufort Seas, Yager et al. 2001; and 0.1−23 × 106 ml−1 in the Beaufort Sea and Amundsen Gulf, Payet and Suttle 2008). The virus-to-bacteria ratios (VBRs) observed in seawater were thus surprisingly high (45–340), considering that most reported VBRs in seawater fall close to 10 (Maranger and Bird 1995), with VBRs up to 20 reported in Arctic seawater at the height of a spring algal bloom (Yager et al. 2001).
Viruses and bacteria in sea ice
Prior to our sampling in Amundsen Gulf in the autumn of 2007, seawater freeze-up had been delayed by several weeks relative to previous years due to the massive decline in sea ice during the summer of 2007 (Comiso et al. 2008). Of the sea ice we sampled, with thicknesses from 4 to 78 cm, thin ice (≤10 cm), almost certainly grew from the underlying seawater that we collected simultaneously with the ice. Older ice may have drifted from the site of its origin but due to the spatial boundaries of our sampling it is unlikely to have derived from a separate water mass.
High abundances of viruses have been repeatedly observed in sea ice from a variety of sources (Maranger et al. 1994; Gowing et al. 2002; Gowing et al. 2004; Wells and Deming 2006b), including the sea ice described here, which contained 4−150× more viruses (when scaled to the volume of brine) than the underlying seawater (Table 1). A disparity was observed between calculated brine concentrations (more viruses, fewer bacteria) and sackhole brine concentrations (fewer viruses, more bacteria), indicating the need for further methods development to optimize the recovery of viruses, and bacteria, from sea ice. The isothermal–isohaline melting approach used here (and by Wells and Deming 2006b) was designed to minimize the effects of osmotic shock on sea ice bacteria and increase recoveries (Junge et al. 2004), but the same optimization has not yet been performed for viruses. The differences between calculated and observed in situ concentrations in brine might arise from lysis of cells and production of viruses during melting (e.g., from induction of lysogens), entrainment of viruses into the solid (rather than brine) fraction of sea ice, or vagaries of the sackhole collection method that bias it towards the collection of bacteria. If the differences reflect artifacts of sample processing, the effects of these differences would be overestimation of VBRs, underestimation of bacterial enrichment, overestimation of viral enrichment, and overestimation of cell-specific virus–bacteria contact rates.
Extremely high VBRs were observed in the autumn sea ice we sampled (mean 846, maximum 2,820; Fig. 3) values up to two orders of magnitude greater than previously reported from the marine environment. Even under a scenario where the observed bacterial abundances were 10× too low (regarding prior discussion of seawater abundances), these VBRs would be considerably higher than expected based upon previous observations of VBRs in sea ice of different seasons, which also tend toward higher maxima than in seawater (Fig. 3): VBRs of 3 to 18 were observed in Arctic winter ice (Wells and Deming 2006b); 10–72 in an Arctic spring algal bloom (Maranger et al. 1994); and 0.7–119 in Antarctic summer pack ice (Gowing et al. 2004). Freshwater and saline lakes frequently have VBRs greater than in marine environments, including several Arctic and Antarctic lakes (Maranger and Bird 1995; Laybourn-Parry et al. 2007; Säwström et al. 2008), one of which had VBRs up to 128 (Madan et al. 2005). Large VBRs were shown to increase the apparent transduction rate in a series of experiments with freshwater Pseudomonas aeruginosa microcosms (Saye et al. 1987; Replicon et al. 1995). Extremely high VBRs may also increase the likelihood of abrupt cell lysis due to high multiplicities of infection (‘lysis from without’), or the induction of lysogeny (Kokjohn 1989), both of which would limit the production of new viruses in the system. Polylysogeny could increase the likelihood of high frequency of transduction lysates (Kokjohn 1989) in sea ice, increasing the potential for transduction there.
Bacteria were more highly enriched into newly formed nilas and pancake ice than salts, as previously observed by Riedel et al. (2007) and Gradinger and Ikavalko (1998), but were likely passively incorporated from seawater into the columnar ice at the base of medium and thick ice. In contrast, the extremely high enrichment indices calculated for viruses in autumn sea ice suggested highly preferential entrainment of viruses or the active production of viruses within the ice in combination with low viral decay rates (Fig. 4). To explain the high concentrations of viruses in some upper sea ice samples without in situ production, at least 10× the mean seawater concentration of viruses would have had to have been completely entrained during ice formation. No abiotic mechanisms of entrainment into sea ice have been measured for viruses, but we cannot exclude the possibility that this occurred, for example by high-affinity binding of viruses to frazil ice crystals which then become entrained into the growing ice sheet. The viruses we observed in brine that drained into sackholes demonstrates that at least some viruses reside in the liquid fraction of the brine matrix. The mechanisms of entrainment and the residence sites of viruses within the sea ice matrix are both important topics that should be addressed in future studies.
Predicted contact rates between bacterial cells and viruses in autumn sea ice were much higher (7−844×) than in the underlying seawater, using the diffusion-based model of Wells and Deming (2006b), who found similarly high increases in contact rates within sea ice. A comparison of the absolute virus–bacteria contact rates between sea ice and seawater highlights the ecological importance of these differences. Over the course of one day, the average Amundsen Gulf seawater bacterium would have been expected to contact about 14 viruses (Fig. 5), while the average sea ice bacterium would have beeen expected to contact about 190 viruses, regardless of temperature within the ice. Even at −2°C, predicted contact rates were much greater in sea ice. Despite the higher contact rates in sea ice, an assumption of equal infectivities may not hold between sea ice and seawater: during a 3 weeks incubation in briny conditions (\(161\permille\) salinity and \(-12^\circ\hbox{C}\)), about 50% inactivation was observed relative to seawater conditions (\(36\permille\) salinity and \(-1^\circ\hbox{C}\)) for the cold-active bacteriophage 9A (Wells and Deming 2006a), potentially due to the denaturation of capsid proteins at high salinity. Phenotypic changes in bacterial hosts under extreme conditions might also affect the infectivity of viruses entrained from seawater. While metabolic activity by the host cell is required for viral production, cells have been shown to be active in sea ice at temperatures down to \(-20^\circ\hbox{C}\) (Junge et al. 2004), and metabolism is not required for either adsorption or injection of DNA into the host cell (Kokjohn 1989). Injected DNA can persist intracellularly until the phage is induced into either the lytic or lysogenic pathways (called pseudolysogeny, Miller 2001), meaning that latent infections may persist until the return of favorable host growth conditions. Alternatively, lysogeny requires a minimum of energetic expense so it may be favored in overwintering conditions in the seawater (Payet and Suttle 2008) or in sea ice.
The viral production rates we observed over the course of 60 h in 2 (of 3) bottle incubations of brine at \(-7^\circ\hbox{C}\) (2.4−5.1 × 106 ml−1 d−1; Fig. 6) were somewhat greater than the rates observed over the first 45 h of incubations of isothermal- and isohaline-melted sea ice at \(-12^\circ\hbox{C}\) from the same region (1.4−2.6 × 106 ml−1 d−1; Wells and Deming 2006b) using similar techniques. While the use of brine from sackholes in lieu of melted sea ice reduced the processing required for analysis, these rates may still not be reflective of instantaneous in situ production due to the possibility of secondary infections during the 2.5 days incubations. Nevertheless, the rates we measured in sea ice were similar to previous estimates of viral production in seawater at high latitudes by this and other methods, for example in the North Sea (0.07−12 × 106 ml−1 d−1; Winter et al. 2004), the Bering and Chuckchi Seas (0.02−14 × 106 ml−1 d−1; Steward et al. 1996), and the North Water polynya (0.11−1.3 × 10 6 ml−1 d−1; Middelboe et al. 2002). These rates of production support the claims of Wells and Deming (2006b) that bacteria and viruses can remain active in sea ice at in situ temperatures; at the calculated rates of increase, bacteria were predicted to have turnover times of 17–20 days and viruses 24–33 days, assuming steady state concentrations within the ice. The combination of an active microbial community in sea ice brine down to at least \(-7^\circ\hbox{C}\), the high VBRs observed relative to seawater, and the increased virus–bacteria contact rates in sea ice relative to seawater suggest the potential for greater gene flow in sea ice relative to underlying seawater. Further experiments necessary to assess the potential for HGT by transduction in sea ice include placing better constraints on the rates of production and decay of viruses in sea ice, their infectivities at low temperature and high salinity, and the frequency of occurrence of transducing phage.
References
Alonso-Sáez L, Sánchez O, Gasol JM, Balagué V, Pedrós-Alió C (2008) Winter-to-summer changes in the composition and single-cell activity of near-surface Arctic prokaryotes. Environ Microbiol 10:2444–2454
Baross J, Liston J, Morita R (1978) Incidence of Vibrio parahaemolyticus bacteriophages and other Vibrio bacteriophages in marine samples. Appl Environ Microbiol 36:492–499
Bayer-Giraldi M, Uhlig C, John U, Mock T, Valentin K (2010) Antifreeze proteins in polar sea ice diatoms: diversity and gene expression in the genus Fragilariopsis. Environ Microbiol 12:1041–1052
Beiko RG, Harlow TJ, Ragan MA (2005) Highways of gene sharing in prokaryotes. PNAS 102:14332–14337
Börsheim K (1993) Native marine bacteriophages. FEMS Microbiol Ecol 102:141–159
Brown JR (2001) Genomic and phylogenetic perspectives on the evolution of prokaryotes. Syst Biol 50:497–512
Chiura HX (1997) Generalized gene transfer by virus-like particles from marine bacteria. Aquat Microb Ecol 13:75–83
Collins RE, Deming JW (submitted this issue) Abundant dissolved genetic material in Arctic sea ice, part I: extracellular DNA. Polar Biol
Collins RE, Carpenter SD, Deming JW (2008) Spatial heterogeneity and temporal dynamics of particles, bacteria, and pEPS in Arctic winter sea ice. J Mar Sys 74:902–917
Comeau AM, Chan AM, Suttle CA (2006) Genetic richness of vibriophages isolated in a coastal environment. Environ Microbiol 8:1164–1176
Comiso JC, Parkinson CL, Gersten R, Stock L (2008) Accelerated decline in the Arctic sea ice cover. Geophys Res Lett 35:L01,703
Cox G, Weeks W (1975) Brine drainage and initial salt entrapment in sodium chloride ice. CRREL Res Rep 345:1–46
Cox GFN, Weeks WF (1983) Equations for determining the gas and brine volumes in sea-ice samples. J Glaciol 29:306–316
Cox GFN, Weeks WF (1986) Changes in the salinity and porosity of sea-ice samples during shipping and storage. J Glaciol 32:371–375
Gogarten JP, Doolittle WF, Lawrence JG (2002) Prokaryotic evolution in light of gene transfer. Mol Biol Evol 19:2226–2238
Gowing MM (2003) Large viruses and infected microeukaryotes in Ross Sea summer pack ice habitats. Mar Biol 142:1029–1040
Gowing MM, Riggs BE, Garrison DL, Gibson AH, Jeffries MO (2002) Large viruses in Ross Sea late autumn pack ice habitats. Mar Ecol Prog Ser 241:1–11
Gowing MM, Garrison DL, Gibson AH, Krupp JM, Jeffries MO, Fritsen CH (2004) Bacterial and viral abundance in Ross Sea summer pack ice communities. Mar Ecol Prog Ser 279:3–12
Gradinger R, Ikavalko J (1998) Organism incorporation into newly forming Arctic sea ice in the Greenland Sea. J Plankton Res 20:871–886
Holmfeldt K, Middelboe M, Nybroe O, Riemann L (2007) Large variabilities in host strain susceptibility and phage host range govern interactions between lytic marine phages and their Flavobacterium hosts. Appl Environ Microbiol 73:6730–6739
Janech MG, Krell A, Mock T, Kang JS, Raymond JA (2006) Ice-binding proteins from sea ice diatoms (Bacillariophyceae). J Phycol 42:410–416
Jiang SC, Paul JH (1998) Gene transfer by transduction in the marine environment. Appl Environ Microbiol 64:2780–2787
Junge K, Krembs C, Deming J, Stierle A, Eicken H (2001) A microscopic approach to investigate bacteria under in situ conditions in sea-ice samples. Annal Glaciol 33:304–310
Junge K, Eicken H, Deming JW (2004) Bacterial activity at –2 to –20 degrees C in Arctic wintertime sea ice. Appl Environ Microbiol 70:550–557
Kiko R (2010) Acquisition of freeze protection in a sea-ice crustacean through horizontal gene transfer? Polar Biol 33:543–556
Kokjohn T (1989) Transduction: mechanism and potential for gene transfer in the environment. In: Levy S, Miller R (eds) Gene transfer in the environment. McGraw-Hill Publishing Co., New York, pp 73–97
Lawrence JG, Hendrickson H (2003) Lateral gene transfer: when will adolescence end? Mol Microbiol 50:739–749
Laybourn-Parry J, Marshall W, Madan N (2007) Viral dynamics and patterns of lysogeny in saline Antarctic lakes. Polar Biol 30:351–358
Madan NJ, Marshall WA, Laybourn-Parry J (2005) Virus and microbial loop dynamics over an annual cycle in three contrasting Antarctic lakes. Freshwater Biol 50:1291–1300
Maranger R, Bird D (1995) Viral abundance in aquatic systems: a comparison between marine and fresh waters. Mar Ecol Prog Ser 121:217–226
Maranger R, Bird DF, Juniper SK (1994) Viral and bacterial dynamics in Arctic sea-ice during the spring algal bloom near Resolute, NWT, Canada. Mar Ecol Prog Ser 111:121–127
Middelboe M, Nielsen TG, Bjørnsen PK (2002) Viral and bacterial production in the North Water: in situ measurements, batch-culture experiments and characterization and distribution of a virus-host system. Deep-Sea Res Pt II 49:5063–5079
Miller R (2001) Environmental bacteriophage-host interactions: factors contribution to natural transduction. Antonie van Leeuwenhoek 79:141–147
Murray AG, Jackson GA (1992) Viral dynamics: a model of the effects of size shape, motion and abundance of single-celled planktonic organisms and other particles. Mar Ecol Prog Ser 89:103–116
Patel A, Noble RT, Steele JA, Schwalbach MS, Hewson I, Fuhrman JA (2007) Virus and prokaryote enumeration from planktonic aquatic environments by epifluorescence microscopy with SYBR Green I. Nat Protoc 2:269–276
Payet JP, Suttle CA (2008) Physical and biological correlates of virus dynamics in the southern Beaufort Sea and Amundsen Gulf. J Mar Sys 74:933–945
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, ISBN 3-900051-07-0
Raymond JA, Fritsen C, Shen K (2007) An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiol Ecol 61:214–221
Replicon J, Frankfater A, Miller RV (1995) A continuous culture model to examine factors that affect transduction among Pseudomonas aeruginosa strains in freshwater environments. Appl Environ Microbiol 61:3359–3366
Riedel A, Michel C, Gosselin M, LeBlanc B (2007) Enrichment of nutrients, exopolymeric substances and microorganisms in newly formed sea ice on the Mackenzie shelf. Mar Ecol Prog Ser 342:55–67
Säwström C, Lisle J, Anesio A, Priscu J, Laybourn-Parry J (2008) Bacteriophage in polar inland waters. Extremophiles 12:167–175
Saye DJ, Ogunseitan O, Sayler GS, Miller RV (1987) Potential for transduction of plasmids in a natural freshwater environment: effect of plasmid donor concentration and a natural microbial community on transduction in Pseudomonas aeruginosa. Appl Environ Microbiol 53:987–995
Steward G, Smith D, Azam F (1996) Abundance and production of bacteria and viruses in the Bering and Chukchi Seas. Mar Ecol Prog Ser 131:287–300
Wells LE, Deming JW (2006a) Effects of temperature, salinity and clay particles on inactivation and decay of cold-active marine Bacteriophage 9A. Aquat Microb Ecol 45:31–39
Wells LE, Deming JW (2006b) Modelled and measured dynamics of viruses in Arctic winter sea-ice brines. Environ Microbiol 8:1115–1121
Wen K, Ortmann AC, Suttle CA (2004) Accurate estimation of viral abundance by epifluorescence microscopy. Appl Environ Microbiol 70:3862–3867
Wilhelm S, Brigden S, Suttle C (2002) A dilution technique for the direct measurement of viral production: a comparison in stratified and tidally mixed coastal waters. Microb Ecol 43:168–173
Winget DM, Williamson KE, Helton RR, Wommack KE (2005) Tangential flow diafiltration: an improved technique for estimation of virioplankton production. Aquat Microb Ecol 41:221–232
Winter C, Herndl GJ, Weinbauer MG (2004) Diel cycles in viral infection of bacterioplankton in the North Sea. Aquat Microb Ecol 35:207–216
Wommack KE, Colwell RR (2000) Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64:69–114
Yager PL, Connelly TL, Mortazavi B, Wommack KE, Bano N, Bauer JE, Opsahl S, Hollibaugh JT (2001) Dynamic bacterial and viral response to an algal bloom at subzero temperatures. Limnol Oceanogr 46:790–801
Zinder ND, Lederberg J (1952) Genetic exchange in Salmonella. J Bacteriol 64:679–699
Acknowledgments
We thank the captain, crew, and scientific party of the CCGS Amundsen for a successful cruise. We gratefully acknowledge M. Pucko, W. Walkusz, P. Galand, B. Else, N. Sutherland, and M. Gupta for field assistance, C. Marrasé for assistance with chlorophyll a measurements, J. Islefson, D. Barber and CFL Team 2 for ice microstructure information and the use of ice-coring equipment, and S. Carpenter for help with laboratory analyses. The input of three reviewers helped to improve the manuscript, we thank them for their efforts.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article belongs to the special issue “Circumpolar Flaw Lead Study (CFL)”, coordinated by J. Deming and L. Fortier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Collins, R.E., Deming, J.W. Abundant dissolved genetic material in Arctic sea ice Part II: Viral dynamics during autumn freeze-up. Polar Biol 34, 1831–1841 (2011). https://doi.org/10.1007/s00300-011-1008-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1008-z