Abstract
In spite of their dominance in meltwater environments in the Polar Regions, little is known about conditions that control community structure and production in microbial mats. Microbial mats were sampled at 13 recently separated ponds on the McMurdo Ice Shelf, Antarctica, with the aim to determine microbial mat community response to shifts in deterministic processes. Community structure in ponds of different size classes responded to different environmental variables, with conductivity as a common theme. Biomass and net oxygen exchange did not vary across the pond conditions and may reflect the very slow turnover rates characteristic of Antarctic microbial communities. Microbial mat communities on the MIS appear unresponsive to large intra-annual variability, while the long-term inter-annual physiochemical environment of the overlying appears to be influencing the community dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benthic microbial mat communities dominated by cyanobacteria, and to a lesser extend diatoms, are widespread throughout the meltwater environments, including lakes, ponds and streams in the Arctic (Vincent et al. 2004; Mueller et al. 2005) and Antarctic (Howard-Williams et al. 1989; Howard-Williams and Hawes 2007) regions. These communities dominate the total biomass and biological productivity in these meltwater environments due to their adaptation to the polar environment (Vincent 2000). In Antarctica, one of the largest and most diverse networks of meltwater ponds is on the McMurdo Ice Shelf (MIS), where some 1,500 km of ponds and small lakes lay on the undulating surface of the ice shelf, among widespread patches and bands of marine and moraine debris (Howard-Williams et al. 1990). These ponds represent a unique environment with large variations in physical and chemical conditions even though some of them are only several meters apart (de Mora et al. 1991).
High salinities, which occur in many of these ponds, originated from seawater inundation (de Mora et al. 1991), marine aerosols (Campbell and Claridge 1987) or chemical weathering of sedimentary material such as mirabilite and thernadite (Wait et al. 2006). Major ion concentrations can further increase as a result of freeze concentration, ice ablation and/or summer evaporation (Schmidt et al. 1991; Hawes et al. 1992). Seasonal variation in size, salinity and hydrochemistry therefore occurs as a consequence of freeze thaw cycles (Howard-Williams and Hawes 2007) but inter-annual variability is low in long-established ponds (Hawes et al. 1993). Chemical and thermal stratification are not an uncommon consequence of freeze thaw cycles in the smaller meltwater ponds, as brines, excluded during freezing, remain in the lower layer of the pond (e.g. Schmidt et al. 1991; Webster et al. 1994; Howard-Williams and Hawes 2007), although several of the larger and deeper ponds (>3 m deep) with conductivities nearing freshwater and have permanent ice cover do not appear to form chemical or thermal stratifications (author own data). It is likely that these larger ponds do not undergo such physiochemical extremes, particularly in the deeper communities, as those experienced in the smaller ponds.
Ponds, both large and small, rely on melting snow and ice to replenish water lost through evaporation and ablation ponds. Smaller ponds may desiccate and refill on decadal scales (Howard-Williams and Hawes 2007). In addition to this, ponds can quickly drain away as a result of ice dams cracking or melting out. Often, in the case of larger ponds, a series of small ponds form in the undulating basin of the drained pond lined with microbial mats from the former pond. The remaining pools of water are then subjected to freeze thaw cycles that leads to more extreme fluctuations in the physiochemical environment for the microbial mats than previously experienced in the original pond.
As a consequence of the dynamics seen in the smaller meltwater ponds, the benthic microbial mat communities must endure multiple stressors and possess a wide ecological tolerance. Microbial mats at the bottom of the smaller ponds must tolerate the chemical extremes imposed on them in winter and, for some, throughout most of the summer period when stratification remains (Wait et al. 2006). However, in the larger low conductivity ponds, microbial mats are buffered against such physiochemical extremes. Microbial mats near the surface margins of the ponds undergo constant freeze-drying (winter) and rewetting (summer) cycles (Hawes et al. 1992).
To date, there are limited studies on the community structure and biomass in relation to physiochemical conditions on the MIS. Microbial communities in long-established ponds are relatively consistent in their morphological diversity over longer time periods (Howard-Williams et al. 1990), and cyanobacterial diversity within these communities appears to be determined by conductivity (Broady and Kibblewhite 1991; Hitzfeld et al. 2000; Nadeau et al. 2001; Jungblut et al. 2005). However, the response of microbial communities from large less extreme ponds to the more extreme physiochemical environment of smaller ponds, when formed through draining, is unknown. This study was conducted to assess the changes in microbial communities in response to recent changes in the pond physiochemical environment as a result of smaller ponds forming from a drained large pond.
Methods
Study site
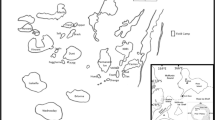
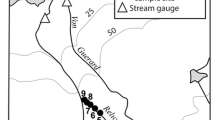
Ponds investigated in this study were located southwest of the Bratina Island Research Station (165°34.205′W, 78°00.885′S; Fig. 1) and were formed as a result of a large pond draining out through a crack in the ice dam approximately two seasons prior. Observations made on a similar neighbouring pond showed that pond shifting from a large pond with low conductivity throughout the water column to smaller pond with increased conductivity in the course of 13 months. The original pond had a surface area of approximately 6,712 m2 with maximum and mean depths of ~10 and ~8 m, respectively. Desiccated microbial mat remains line the dried parts of the basin and along the walls of the drained pond suggesting that it was once lined with microbial mats throughout. Conductivity and temperature profiles and nutrient analyses of similar sized ponds in the vicinity suggest that the original pond was low in conductivity and moderately low in nutrients with no stratification in the lower depths (author own data, unpublished). A series of 13 ponds were formed in the depressions on the undulating basin floor (Fig. 1). Water levels of these ponds were all at the same elevation relative to each other. This suggests that the microbial communities at the edge of the ponds had experienced similar seasonal physiochemical fluctuations when part of the parent pond. They are, therefore, most likely to have been at the same developmental/compositional stage.
Environmental variables
Field sampling was undertaken in December 2006 when all ponds were fully open water. Conductivity, pH and temperature measurements were taken near the surface of microbial mats being sampled, using a TPS portable conductivity-temperature meter. Surface water samples were collected in acid-washed nalgene bottles and returned to the base camp and filtered within a few hours of collecting. Water was sub-sampled and filtered, where appropriate, for subsequent analysis of nutrients, dissolved organic carbon (DOC), and major anions and cations. Samples were kept cool in dark boxes in the ice for periods of up to 1 week before being returned to base where they were deep frozen and transported back to New Zealand. Nitrate (NO3–N), ammonium (NH4–N), dissolved reactive phosphate (DRP), total dissolved nitrogen (TDN) and total dissolved phosphorus (TDP) were determined on a Lachat Quikchem FIA+8000 series Flow injection Analyser, after UV irradiation in alkaline or acidic conditions, respectively (Downes 2001). Major ions (Na, K, Ca, Mg, Cl, SO4) and DOC were analysed according to standardised methods described in APHA (1998).
Microbial mats
Benthic microbial mats were collected approximately 20 cm from the pond edge at a water depth of approximately 30 cm. Samples were taken with a 2.26 cm2 corer from areas of the mats with homeogenous colour, texture and thickness and were considered representative of the pond. Five sites spaced around each pond were sampled and six replicate cores collected at each site. Cores were carefully separated from the underlying sediment layer, transferred into opaque containers and returned to a darkened near-by field laboratory for further processing.
Oxygen exchange
Net photosynthesis was determined by measuring the difference between the rate of evolution and consumption of oxygen. This was determined for 7 of the 13 ponds, across the conductivity range. Microbial mat samples were kept in the cool and dark for 2 h prior to the experiment. Incubations were carried out with two cores of mat per bottle, placed into 30-ml glass serum bottle filled to capacity with unfiltered pond water and sealed with a rubber bung, ensuring air bubbles were eliminated. One bottle from each site was assigned either light or dark treatment (five replicates per pond). Dark treatment bottles were then wrapped in aluminium foil, to eliminate light, and all bottles were placed 20 cm below water surface in a pond (water temperature 1.5°C) and incubated for 4 h under ambient light conditions. Serum bottles containing only unfiltered pond water were incubated as controls. At the end of the incubation, serum bottles were shaken to disperse any oxygen gradients, and oxygen concentrations of the water were determined using a Clark-type O2 microelectrode with an outside tip diameter of 50 μm, with a 90% response time of 1 s (Unisense), attached to a picoammeter (Diamond General). Net exchange in oxygen concentration was calculated as μg O2 evolved cm−2 h−1 by subtracting the dark bottle concentrations from those in the light, correcting for the controls. The microbial mat cores were retained for further analyses (see below).
Biomass
On return to New Zealand the microbial mat cores from the oxygen exchange study were freeze-dried and ground to a fine powder. Aliquots from each replicate were taken for analysis of total organic matter and chlorophyll a. Aliquots for total organic matter were weighed then combusted at 450°C for 4 h, cooled in a desiccator and organic matter estimated as loss of mass on ignition. Chlorophyll a samples were extracted in 90% acetone at 4°C, in the dark, for 24 h. Samples were then centrifuged at 3,000 rpm for 10 min and the absorbance of the supernatant read on a Shimadzu UV-2550 spectrophotometer before and after acidification (Marker et al. 1980). Chlorophyll a concentrations were estimated using calibration standards prepared with purified Chlorophyll a (Sigma chemicals).
Community composition
Two cores from each site within a pond were kept fresh, stored in the cool and dark and returned to New Zealand were each was used for identification. The cores were drawn through a syringe with distilled water to disperse the mat material and form a homogenous sample. Subsamples of the fresh material were viewed at 1,000× magnification, under oil immersion, on a Leica DMLB microscope. For diatom analysis only valves containing intact chloroplasts were included in the relative abundance counts. Taxa were counted until 300 valves were enumerated. Subsamples were then cleaned of organic content by oxidation in H2O2 and H2SO4 (Barber and Haworth 1981) and settled onto cover glasses, mounted in naphrax mounting medium and used for taxonomic identification. Taxonomic identifications of the diatoms were primarily based on Krammer and Lange-Bertalot (1991, 1997a, b, 2000), and more specialised literature including Cremer et al. (2004), Kellogg and Kellogg (2002), Sabbe et al. (2003), Spaulding et al. (1997, 1999, 2008). While cyanobacteria comprised the majority of the mats, counting trichomes for abundance was not feasible. Instead, relative percent abundance (biovolume), based on observations of 20 fields of view, was used for cyanobacteria estimates. Cyanobacteria taxonomy was based primarily on Komàrek and Anagnostidis (2005), and more specialised literature including Broady and Kibblewhite (1991), Komàrek (2007) and Komàrek et al. (2008).
Statistical and numerical analyses
Square-root transformations were performed on species relative abundance data in order to down-weigh the effects of dominant taxa (Laing and Smol 2000). All environmental variables, except pH, Ca, Mg, K, SO4, DRP, and TDP, had skewed distributions and were log transformed for further analyses. To test for correlations between environmental variables, a Pearson correlation matrix with Bonferroni-adjusted probabilities was performed (SYSTAT, v. 10). The following variables were excluded from further analysis: Mg, Na, K and Cl (correlated with conductivity).
Preliminary differences among diatom and cyanobacterial communities were assessed using Analysis of similarity (ANOSIM). Relationships between the algal communities and environmental variables were explored using the BIO-ENV procedure (Clarke and Warwick 2001). BIO-ENV was considered to be suitable for exploring the present dataset because of the small number of samples from a restricted area. BIO-ENV compares a similarity matrix of the biotic data with a similarity matrix of all combinations of associated environmental variables and computes a Spearman rank correlation coefficient (ρ s) for each combination (Clarke and Ainsworth 1993). Non-metric multi-dimensional scales (NMDS) plots were used to visualise the closest correlations with the biotic data. ANOSIM, BIO-ENV and NMDS analyses were carried out in PRIMER, v. 6.
Biomass and oxygenic photosynthesis data were examined using linear and non-linear regression analysis and Analysis of Variance (ANOVA). ANOVA was followed by posthoc analysis (Tukey’s HSD test) to establish significant differences (STATISTICA v. 8).
Results
Environmental variables
Ponds varied along a number of physiochemical properties, with approximate size ranging from 1.2 to 660 m2. The size of pond was not correlated to any other physiochemical gradient. Conductivity ranged from 2.78 to 22.26 mS cm−1, while pH ranged from neutral to biologically high alkaline (7.4–10.5). Total dissolved nitrogen ranged from 1,970 to 17,150 mg m−3, while total dissolved phosphorus ranged from 107 to 716 mg m−3. The inorganic fraction of these major nutrients ranged from 34 to 1,975 mg m−3 for nitrogen (NH4 + NO3) and from 19 to 203 mg m−3 for phosphorus (DRP). For most ponds Na and SO4 were the dominant major ions (Table 1).
Oxygen exchange
Mean net oxygen exchange ranged from 0 to 43 μg cm−2 h−1. ANOVA showed that the highest conductivity pond (pond 13) was significantly lower than all other ponds (P < 0.001), while pond 2 was lower than pond 12 (Fig. 2). There was a general tread of increasing oxygen with increasing conductivity, with the exception of Pond 13. Logarithmic regression analysis between the measured environmental variables and net oxygen exchange found highly significant relationships for a number of variables. Oxygen exchange per unit chlorophyll was positively correlated with ln SO4 and negatively correlated with ln DOC, ln NH4, ln TDN and ln TDP, although correlations with ln TDN and ln DOC were driven primarily by the outlier pond 13 (P < 0.001; Fig. 3).
Biomass
Chlorophyll a values measured in the ponds ranged from 14.8 to 56.6 μg m−2 (Table 2). ANOVA revealed that pond nine was significantly higher than ponds three and one (P < 0.001). Chlorophyll a was not correlated to any of the physiochemical properties. Assimilation rates (net oxygen produced per unit chlorophyll a) ranged from 0.1 to 3.7 μg O2 (μg chla)−1 h−1. The assimilation rates in pond 13 were significantly lower than the other ponds (ANOVA P < 0.005). Organic matter ranged from 11.7 to 23.5 mg cm−2 (Table 2). There were no significant differences between ponds and no correlation with any physiochemical property but there was a significant positive correlation of organic matter with chlorophyll a (R 2 = 0.528; P < 0.001).
Community composition
The microbial mats from all ponds were dominated by filamentous cyanobacteria of the order Oscillatoriales, while diatoms comprised only 10–15% of the community, as determined by relative percent biovolume. A total of 17 cyanobacteria and 19 diatom species were identified over the 13 ponds. Occurrence of species in each pond is shown in Table 3. Total species richness in individual ponds ranged from 19 to 25, with the greatest diversity from diatoms (range 11–17) in all ponds. All species have been previously reported in the Ross Sea sector of Antarctica.
Diatom species composition did not vary greatly between ponds, with at least 60% of diatom species common to all ponds (Fig. 4). No correlation was found between diatom composition and any of the environmental variables measured. In contrast, cyanobacterial communities varied greatly between ponds, with most ponds at least 50% dissimilar to each other (Fig. 5). BIO-ENV analysis for cyanobacterial communities in all ponds and environmental variables produced a poor correlation of ρ s = 0.184 for a combination of conductivity and dissolved inorganic nitrate (DIN = NH4–N + NO3–N).
When ponds were separated into three size classes there were stronger correlations with environmental variables. Ponds were divided into three size classes, size class A were ponds <15 m2 (n = 4), size class B were ponds between 15 and 70 m−2 (n = 4) and size class C were ponds >70 m−2 (n = 5). Cyanobacterial communities in ponds of the largest of the size class (C) were best correlated with conductivity (ρ s = 0.722). For ponds of size class B, the best correlation was found with a combination of conductivity and NO3–N (ρ s = 0.657), while the smallest of the ponds (size class A) were best correlated with DOC (ρ s = 0.794). There were no relationships between diatom communities and environmental variables when compared based on pond size.
Phormidium autumale ((Adardh) Trevisan ex Gomont) was the most variable among cyanobacteria species (0–83%), while Craspedostauros laevissima ((West & West) Sabbe in Sabbe) was the most variable among the diatoms (2–84%). While there were significant differences between ponds for seven cyanobacteria and four diatom species (with relative abundance >5%) there were no strong correlations with any of the environmental variables for any of the species. The highest relationships were found between Pseudoanabaena frigida and NO3–N (ρs = 0.513), Nodularia cf harveyana and DIN (ρs = 0.481) Phormidium pseudopristleyi and TDN (ρs = 0.479), and Leptolyngbya fritschiana and DRP (ρs = 0.467).
Evaporative changes
Evaporative concentration changes in conductivity were predicted for volumetric changes in the ponds based on a 15 cm decrease in water level as a result of evaporation over the summer period (see Hawes et al. 1993 for details). Final concentrations (C f) were calculated by
where C i is the initial concentration, and V i and V f are the initial and final volumes, respectively. Predicted conductivities at the point of sampling are shown in Table 4. BIO-ENV analysis on all ponds produced a moderate rank correlation between the cyanobacterial community and the predicted changes in conductivity of ρ s = 0.449 for a combination of conductivity and DIN.
Discussion
Biomass, as determined by spectrophotometric chlorophyll a and by organic matter, as well as photosynthesis, as measured by net oxygen exchange, did not vary significantly along the gradient of environmental variables in this study, with the exception of pond 13 where oxygen exchange was negative. Studies of microbial mats in other aquatic systems have shown enhancement of primary production when osmotic stress is released (Bebout 1992; Pinckney et al. 1995). As this was not evident in the current study, it is likely that the microbial communities within the study ponds were able to overcome osmotic stress without compromising photosynthesis (determined as oxygen exchange). The exception was pond 13 but while it had the highest conductivity it was still well within the conductivity range where measureable productivity has been detected in ponds on the MIS (Howard-Williams et al. 1989). Another plausible explanation is that the measurements of biomass are not sensitive enough to moderately short-term changes in biomass (e.g. <3 years). While there is no published data on biomass accrual rates over time on the MIS, measured growth rates (as determined by assimilation rates) are low with respiration/photosynthesis ratio in mature shallow-water communities close to zero throughout much of the growth season (Vincent 2000; Quesada et al. 2008). Measureable biomass changes to in response to a changed physiochemical environment may occur over decades.
Differences between cyanobacterial communities and the physiochemical environment were not evident across the entire range of ponds. However, strong correlations were evident when ponds were classified by size, with different size classes responding to different environmental variables.
In the case of the smallest ponds (size class A), evaporative losses throughout the summer season would result in either the formation of a hypersaline waters or lead to complete desiccation. In this study, based on published evaporative losses from MIS ponds (see Hawes et al. 1992), three out four of the smallest ponds would have completely dried out by the end of summer, assuming no significant snow drift blown in during this period. Species diversity in these ponds was shown to be responsive to changes in DOC but this may in fact be an artefact of stress response during periods of desiccation. Increased damage to the microbial communities would likely occur during desiccation resulting in increased DOC released from the microbes.
Conductivity was the main determining factor for the overall community structure in large ponds, and together with NO3–N in medium ponds. Improvements in relationships between community structure and both conductivity and DIN across all ponds were greatly improved under predictions of decreased summer water levels. This was mainly driven by the loss of the smaller ponds and an increase in the range and spread of conductivities among the remaining ponds. Conductivity and light intensity have been shown to play a role in the community composition in microbial mats in Antarctica (Jungblut et al. 2005; Taton et al. 2006). In this study, ponds with a greater relationship with conductivity may indicate communities that are coming into equilibrium with their physiochemical environment faster than ponds undergoing more extreme regimes.
Physiochemical predictors of individual cyanobacteria species were more variable than for whole communities, although changes in nitrogen and phosphorus appear to be an over-riding theme. While detailed morphological analysis was used to distinguish cyanobacteria species, plastic phenotypes responding to changes in the physiochemical environment may have limited the information on the community changes to some degree. Uses of molecular analysis would both aide and compliment this study.
As mentioned earlier, microbial mats in the bottom waters in small ponds on the MIS must endure multiple stressors resulting from the strong physiochemical gradients imposed through stratification. The microbial mat consortia may play a role in protecting those species more sensitive to these extremes through organisation of the community. Most microbial mats on the MIS are stratified with an oxygen maximum in the deep chlorophyll range in the subsurface layers (Howard-Williams et al. 1989; Hawes et al. 1993). Different species are organised in different layers throughout the microbial mat (de los Ríos et al. 2004) and this may serve as a mechanism of avoidance by sensitive species. Communities may be able to maintain their level of biodiversity within a certain range of physiochemical variables by means of species stratification. Ranges for individual species would ultimately determine the level of biodiversity across fluctuating ponds thus leading to the differences seen in long-established ponds (Jungblut et al. 2005; Taton et al. 2006, author’s unpublished data).
In this study, the diatoms constituted only a small proportion of the entire community and did not correlated to any environmental variable measured. Diatom sensitivity to small scale changes in environmental variables, such as pH (Battarbee et al. 1999), conductivity (Fritz et al. 1999) and DOC (Fallu et al. 2000) is well documented and robust prediction models have been produced as a result. However, such responses were not clear in this study as intra-pond variability exceeded inter-pond variability. A possible explanation for this is that the distribution of diatoms was patchy throughout the mat samples and such patchiness did not truly reflect the diatom community as a whole.
While microbial mat communities are, in part, influenced by the physiochemical environment of the water column, it is apparent that microbial mat communities are capable of withstanding large intra-annual variability, while the long-term inter-annual water column properties appear to be one of the main influencing drivers in the community dynamics. Development of community models, in long-established ponds, across a broad range of environmental variables is essential to better understand evolutional and functional dynamics and how these communities will ultimately respond to large-scale, long-term changes.
References
APHA (1998) Standard methods for the analysis of water and wastewater, 20th edn. American Public Health Association, Washington
Barber HG, Haworth EY (1981) A guide to the morphology of the diatom frustule. Freshwater Biological Association
Battarbee RW, Charles DF, Dixit SS, Renberg I (1999) Diatoms as indicators of surface water acidity. In: Stoermer EF, Smol J (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, London, pp 85–127
Bebout B (1992) Interactions of nitrogen and carbon cycling in microbial mats and stromatolites. Ph.D. dissertation. Curriculum in Marine Sciences, University of North Carolina, Chapel Hill
Broady P, Kibblewhite A (1991) Morphological characterization of Oscillatoria (Cyanobacteria) from Ross Island and southern Victoria Land, Antarctica. Antarct Sci 3:35–45. doi:10.1017/S095410209100007X
Campbell IB, Claridge GGC (1987) Antarctica: soils, weathering processes and environment. Developments in soil science, vol 16. Elsevier, New York
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219. doi:10.3354/meps092205
Clarke KR, Warwick RM (2001) Changes in marine communities. An approach to statistical analysis and interpretation, 2nd edn. Plymouth Marine Laboratory, UK
Cremer H, Gore D, Hultzsch N, Melles M, Wagner B (2004) The diatom flora and limnology of lakes in the Amery Oasis, East Antarctica. Polar Biol 27:513–531. doi:10.1007/s00300-004-0624-2
de los Ríos A, Ascaso C, Wierzchos J, Fernández-Valiente E (2004) Microstructural characterization of cyanobacterial mats from the McMurdo Ice Shelf, Antarctica. Appl Environ Microbiol 70:569–580. doi:10.1128/AEM.70.1.569-580.2004
De Mora SJ, Whitehead RF, Gregory M (1991) The chemical composition of glacial melt water ponds and steams on the McMurdo Ice Shelf, Antarctica. Antarct Sci 6:17–27
Downes M (2001) NIWA Christchurch auto-analyser methods. NIWA internal report no. 103. NIWA, Christchurch
Fallu M, Allaire N, Pienitz R (2000) Freshwater diatoms from northern Quebec and Labrador (Canada). Species-environment relationships in lakes of boreal forest, forest-tundra and tundra regions. Bibl Diatomol 45:1–200
Fritz SC, Cumming BF, Gasse F, Laird KR (1999) Diatoms as indicators of hydrologic and climatic change in saline lakes. In: Stoermer EF, Smol J (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, London, pp 41–72
Hawes I, Howard-Williams C, Vincent WF (1992) Desiccation and recovery of Antarctic cyanobacterial mats. Polar Biol 12:587–594. doi:10.1007/BF00236981
Hawes I, Howard-Williams C, Pridmore RD (1993) Environmental control of microbial biomass in the ponds of the McMurdo Ice Shelf, Antarctica. Arch Hydrobiol 127:271–287
Hitzfeld B, Lampert C, Späth N, Mountfort D, Kaspar H, Dietrich D (2000) Toxin production in cyanobacterial mats from ponds on the McMurdo Ice Shelf, Antarctica. Toxicon 38:1731–1748. doi:10.1016/S0041-0101(00)00103-3
Howard-Williams C, Hawes I (2007) Ecological processes in Antarctic inland waters: interactions between physical processes and the nitrogen cycle. Antarct Sci 19:205–217. doi:10.1017/S0954102007000284
Howard-Williams C, Pridmore R, Downes MT, Vincent WF (1989) Microbial biomass, photosynthesis and chlorophyll a related pigments in the ponds of the McMurdo Ice Shelf, Antarctica. Antarct Sci 1:125–131. doi:10.1017/S0954102089000192
Howard-Williams C, Pridmore RD, Broady PA, Vincent WF (1990) Environmental and biological variability in the McMurdo Ice Shelf ecosystem. In: Kerry KR, Hempel G (eds) Antarctic ecosystems: ecological change and conservation. Springer, Berlin, pp 23–31
Jungblut A-D, Hawes I, Mountfort D, Hitzfeld B, Dietrich DR, Burns BP, Neilan BA (2005) Diversity within cyanobacterial mat communities in variable salinity meltwater ponds of McMurdo Ice Shelf, Antarctica. Environ Microbiol 7:519–529. doi:10.1111/j.1462-2920.2005.00717.x
Kellogg TB, Kellogg DE (2002) Non-marine and littoral diatoms from Antarctic and subantarctic regions––distribution and updated taxonomy. In: A. Witkowski (ed) Diatom monographs. ARG. Gantner Verlag KG
Komàrek J (2007) Phenotype diversity of the cyanobacteria genus Leptolyngbya in maritime Antarctica. Polar Res 28:211–231
Komàrek J, Anagnostidis K (2005) Cyanoprokaryota 2. Teil/2nd part: Oscillatoriales. In: Büdel B, Krienitz L, Gärtner G, Schagerl M (eds) Süsswasserflora von Mitteleuropa, Band 19/2. Elsevier, Munchen, pp 1–759
Komàrek J, Elster J, Komàrek O (2008) Diversity of the cyanobacteria microflora of the northern part of James Ross Island, NW Weddell Sea, Antarctica. Polar Biol 31:853–865. doi:10.1007/s00300-008-0424-1
Krammer K, Lange-Bertalot H (1991) Bacillariophyceae. 4. Teil: Achanathaceae, Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In: Ettl H, Gäntner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/4. Gustav Fisher Verlag, Stuttgart, pp 1–437
Krammer K, Lange-Bertalot H (1997a) Bacillariophyceae. 1. Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/1. Spektrum Akademischer Verlag, Heidelberg, pp 1–876
Krammer K, Lange-Bertalot H (1997b) Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/1. Spektrum Akademischer Verlag, Heidelberg, pp 1–611
Krammer K, Lange-Bertalot H (2000) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/3. Gustav Fisher Verlag, Stuttgart, pp 1–599
Laing TE, Smol JP (2000) Factors influencing diatom distributions in circumpolar treeline lake of northern Russia. J Phycol 36:1035–1048. doi:10.1046/j.1529-8817.2000.99229.x
Marker AFH, Crowther CA, Gunn RJM (1980) Methanol and acetone as solvents for estimating chlorophyll a and phaeopigments by spectrophotometery. Arch Hydrobiol Ergeb Limnol 14:52–69
Mueller DR, Vincent WF, Bonilla S, Laurion I (2005) Extremotrophs, extremeophiles and broadband pigmentation strategies in a high arctic ice shelf ecosystem. FEMS Microbiol Ecol 53:73–87. doi:10.1016/j.femsec.2004.11.001
Nadeau TL, Milbrandt EC, Castenholz RW (2001) Evolutionary relationships of cultivated Antarctic Oscillatorians (Cyanobacteria). J Phycol 37:650–654. doi:10.1046/j.1529-8817.2001.037004650.x
Pinckney J, Paerl HW, Bebout BM (1995) Salinity control of benthic microbial mat community production in a Bahamian hypersaline lagoon. J Exp Mar Biol Ecol 187:223–237. doi:10.1016/0022-0981(94)00185-G
Quesada A, Fernández-Valiente E, Hawes I, Howard-Williams C (2008) Benthic primary production in polar lakes and rivers. In: Vincent WF, Laybourn-Parry J (eds) Polar lakes and rivers. Oxford University Press, USA, pp 179–196
Sabbe K, Verleyen E, Hodgson D, Vanhoutte K, Vyverman W (2003) Benthic diatom flora of freshwater and saline lakes in the Larsemann Hills and Rauer Islands, East Antarctica. Antarct Sci 15:227–248. doi:10.1017/S095410200300124X
Schmidt SW, Moskal W, De Mora S-J, Howard-Williams C, Vincent WF (1991) Limnological properties of Antarctic ponds during winter freezing. Antarct Sci 3:379–388. doi:10.1017/S0954102091000482
Spaulding SA, McKnight DM, Stoerner EF, Doran PT (1997) Diatoms in sediments of perennially ice-covered Lake Hoare, and implications for interpreting lake history in the McMurdo Dry Valleys of Antarctica. J Paleolimnol 17:404–420
Spaulding SA, Kociolek JP, Wong D (1999) A taxonomic and systematic revision of the genus Muelleria (Bacillariophyta). Phycologia 38:314–341
Spaulding S, Esposito R, Lubinski D, Horn S, Cox M, McKnight D, Alger A, Hall B, Mayernick M, Whittaker T, Yang C (2008) Antarctic freshwater diatoms web site, McMurdo Dry Valleys LTER. http://huey.colorado.edu/diatoms/
Taton A, Grubisic S, Balthasart P, Hodgson DA, Laybourn-Parry J, Wilmotte A (2006) Biogeographical distribution and ecological ranges of benthic cyanobacteria in East Antarctic lakes. FEMS Microbiol Ecol 57:272–289. doi:10.1111/j.1574-6941.2006.00110.x
Vincent WF (2000) Cyanobacterial dominance in the polar regions. In: Whitton BA, Potts M (eds) Ecology of the cyanobacteria: their diversity in space and time. Kluwer, Dordrecht, pp 321–340
Vincent WF, Mueller DR, Bonilla S (2004) Ecosystems on ice: the microbial ecology of Markham Ice Shelf in the high Arctic. Cryobiology 48:103–112. doi:10.1016/j.cryobiol.2004.01.006
Wait BR, Webster-Brown JG, Brown KL, Healy M, Hawes I (2006) PChemistry and stratification of Antarctic meltwater ponds 1: coastal ponds near Bratina Island, McMurdo Ice Shelf. Antarct Sci 18:515–524. doi:10.1017/S0954102006000563
Webster JG, Brown KL, Vincent WF (1994) Geochemical processes affecting meltwater chemistry and the formation of saline ponds in the Victoria Valley and Bull Pass region, Antarctica. Hydrobiologia 281:171–186
Acknowledgments
This study was funded and supported by the New Zealand Foundation for Research, Science and Technology (CO1605) and Antarctica New Zealand. Ian Hawes is thanked for his assistance in the field. Map and pond diagram was prepared by Greg Kelly. Dr. Clive Howard-Williams and three anonymous reviews provided useful comments that helped strengthen this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sutherland, D.L. Microbial mat communities in response to recent changes in the physiochemical environment of the meltwater ponds on the McMurdo Ice Shelf, Antarctica. Polar Biol 32, 1023–1032 (2009). https://doi.org/10.1007/s00300-009-0601-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0601-x