Abstract
Interpreting the impact of environmental change on food webs requires a clear understanding of predator–prey interactions. Such knowledge is often lacking in the marine environment where the foraging behaviour and prey requirements of some of the major top-predators remains mysterious. For example, very little is known about the underwater foraging behaviour of the little auk, the most numerous seabird in the North Atlantic. In 2004, we used time–depth-recorders at two breeding colonies in East Greenland to examine the diving behaviour of this small, planktivorous seabird during the chick-rearing period. Due to technical difficulties data were only collected for four individuals, but recordings showed that birds dive up to 240 times a day to maximum depths of 27 m (average 10 m), with maximum dive durations of 90 s (average 52 s). In addition, we collected the chick meals from 35 individuals, which were dominated by Calanus copepods (95%), and also determined the field metabolic rates (FMR) of 14 individuals using the doubly labelled water technique, which averaged 609.9 kJ day−1. We integrated information on diving duration with chick diet and FMR to estimate the prey requirements and underwater capture rates of little auks using a Monte Carlo simulation. Chick-rearing little auks needed to catch about 59,800 copepods day−1, which is equivalent to about six copepods caught per second spent underwater. These astonishing results strongly suggest that little auks are, at least partly, filter-feeding, and underline the importance of highly productive, cool marine areas that harbour dense patches of large, energy-rich copepods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the marine environment, food chains are often controlled via bottom-up effects, with hydrographic conditions influencing primary and secondary productivity, and ultimately top-predators (Richardson and Schoeman 2004; Frederiksen et al. 2006). The upper trophic status of marine top-predators such as seabirds consequently makes them sensitive to changes occurring at lower trophic levels (Ainley et al. 1995; Furness and Campuysen 1997), and numerous studies have demonstrated the impact of changes in the marine ecosystem on seabirds (e.g., Cherel and Weimerskirch 1995). Whereas some species of seabird are able to buffer food shortage by adjusting foraging behaviour and time allocation (e.g., Litzow and Piatt 2002), other species, such as those with specialised feeding habits or expensive food searching techniques, have less flexibility and are more prone to breeding failures (e.g., Monaghan et al. 1989). There is also some evidence that planktivorous seabirds (secondary consumers) are more correlated with environmental conditions than fish-eating birds (tertiary consumers) because there are fewer links between predators feeding at lower trophic levels and the ocean physics driving the change (e.g., Kitaysky and Golubova 2000; Lee et al. 2007).
The little auk (Alle alle) is a small planktivorous seabird that breeds in the high Arctic marine zone of the Atlantic. The species composition of zooplankton communities is closely linked to oceanographic conditions, with species of different size and energetic value adapted to different water characteristics (Scott et al. 2000; Beaugrand et al. 2002a). The restructuring of zooplankton communities associated with predicted changes in the North Atlantic current system and water temperature (Hurrell 2000; Beaugrand et al. 2002b) should therefore directly affect food availability to little auks.
The flexibility of foraging behaviour may be an important mechanism for little auks to adjust to changes in food availability. However, although chick-feeding rates, and the size and energy value of chick meals varies with local foraging conditions (e.g., Karnovsky et al. 2003; Jakubas et al. 2007), detailed knowledge of their diving behaviour and at-sea activity is lacking. Miniature data loggers have been successfully used to record the diving behaviour of the larger wing-propelled pursuit diving seabirds such as the penguins (Aptenodytes forsteri) and guillemots (Uria aalge) over the last decades (e.g., Kooyman and Kooyman 1995; Tremblay et al. 2003), but only recently have these devices become light enough to use on the smaller, planktivorous species. In this paper, we present data from a pilot study that used small data loggers (time–depth-recorders; TDRs) to examine the diving behaviour of little auks breeding in East Greenland. Our two aims were to: (1) use TDRs to describe the diving behaviour and time budgets of little auks, and (2) integrate information on diving duration with chick diet and field metabolic rates (FMR) to estimate the prey requirements and underwater capture rates of little auks.
Materials and methods
Study site and species
Little auks have very minimal sexual size dimorphism (Wojczulanis 2006), and annually rear a single chick (Stempniewicz 2001). Both parents share incubation, brooding and chick-provisioning duties, although there is evidence that males deliver more meals than females towards the end of the chick-rearing period (Harding et al. 2004). The single chick is brooded almost continuously by both parents until it attains homeothermy at about 3–4 days of age (Stempniewicz 2001). After this, the chick is usually left alone in the crevice unless it is being fed. Chicks fledge at an average age of 25–27 days (Norderhaug 1980; Stempniewicz 2001; Harding et al. 2004). Little auks have a specialised foraging niche, feeding almost exclusively on planktonic crustaceans, with calanoid copepods (Calanus species) accounting for 84–96% of the energetic content of chick meals across their range (e.g., Pedersen and Falk 2001). These 5–10 mm prey items are caught during wing-propelled dives, and parents carry the fresh, undamaged zooplankton back to their chick at the colony in a specially adapted throat (gular) pouch (Stempniewicz 2001).
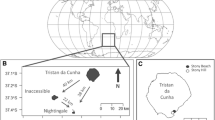
Data were collected in 2004 at two little auk colonies on the east coast of Greenland; from 7 to 17 July at Kap Brewster (70°10′N, 22°09′W) and from 18 July to 1 August at Kap Höegh (70°43′N, 22°38′W; Fig. 1) as part of a pilot season to identify a good colony for future study. The little auk colony at Kap Brewster is situated approximately 300–350 m above sea level, whereas the Kap Höegh colony is located approximately 150 m above sea level.
Fourteen nests were found during the end of the incubation period (8–9 July) at Kap Brewster, and checked every 3 days until hatch to provide an index of hatching chronology. The sample of accessible nests was limited by rock size and crevice depth. Out of the 14 nests: ten hatched, two failed to hatch, and two were still in incubation stage when we left the colony on July 17. Median chick hatch date was 11 July (n = 10 nests, min = 10 July, max = 15 July). We arrived at Kap Höegh after chicks hatched and consequently have no measure of chick age, however, the visual comparison of chicks at both colonies suggest a similar hatching chronology.
Diving behaviour and time budgets
Data loggers were attached to a total of 13 birds; seven at Kap Brewster during the end of the incubation period/early chick-rearing period (7–13 July), and six at Kap Höegh during the mid chick-rearing period (20–28 July). Only one adult per nest was used, in order to minimise disturbance. The data loggers (LTD_1110, Lotek Wireless Inc.; 32 mm length, 11 mm diameter, 5 g mass in air) recorded time, water pressure (acc. 0.5 m, down to 50 m) and temperature (acc. 0.3°C, range −5–35°C) at a sampling interval of 5 s. This interval corresponds to 10% of the subsequently recorded average dive duration (see Results), and is therefore considered appropriate (Wilson et al. 1995). A logger memory of 64 KB allowed 45.5 h of data to be stored. Logger mass corresponded to approximately 3.3% of the birds body mass (149 ± 1.7 g, n = 105 birds; this study). Birds were caught in the nest crevice and data loggers were attached to the central back feathers with 10 mm wide strips of Tesa® tape (Wilson et al. 1997). Birds were dyed (picric acid) for quick identification in the field, and released back into the nest after handling. Total handling time was less than 15 min. Regular checks at the nest hole were conducted after 24-h of attachment and until the bird could be caught and the logger retrieved. Dive parameters were calculated using MultiTrace (Jensen Software Systems, Laboe, Germany). The first hour of data after initial release was excluded from the analysis to reduce possible behavioural effects from handling the bird, and the minimum depth for a dive to be included in the analysis was 1 m. A conversion factor of 0.7030695 m PSI−1 was applied to convert the logger pressure unit (PSI) to meters below sea surface. A logger was placed at the colony to record diurnal temperature variation on land and aid in the interpretation of activity.
Only 5 of the 13 loggers were successfully retrieved; 4 at Kap Brewster and 1 at Kap Höegh. Of the remaining eight birds, the logger had fallen off five before recapture and three were not seen again in the colony. The download of one logger at Kap Brewster was unsuccessful for unknown reasons, and we were therefore limited to data from four loggers. The height of the Kap Brewster colony allowed us to distinguish between at-sea and colony pressure, enabling the calculation of activity budgets (time spent foraging, in flight, and at the colony) for these three birds. The lower height of the Kap Höegh colony made these data more difficult to interpret, and the Kap Höegh bird is therefore not included in estimates of time budgets. Except for the standard deviations used in the Monte Carlo simulations (Manly 1997), all values reported are means ± 1 SE and statistical significance was assumed at P < 0.05.
Chick diet
Chick meals were collected at Kap Höegh (19–31 July). Captures were distributed across all hours to avoid potential diurnal differences in provisioning. Parents were caught on rocks in the colony using noose carpets (Pedersen and Falk 2001). Observers sat close to the trap, and ran to hold the bird as soon as it was caught to avoid the individual opening its bill and loosing prey items from the gular pouch. The chick meal was gently scooped out of the gular pouch, and birds were released without harm within 5 min of capture. Diet samples were classified as either complete (no food items lost) or incomplete (determined from the continual observation of caught birds), and were preserved in 4% borax-buffered formaldehyde solution and stored in hard plastic vials to prevent damage to the individual prey samples.
Samples were analysed in the laboratory under a stereomicroscope. Prey items were identified to species level whenever possible, and remaining taxa were identified down to the lowest possible taxonomical level. Calanus species were identified according to Kwaśniewski et al. (2003). Abundant taxa—mainly Copepods and larval forms of Decapoda—were enumerated from subsamples which consisted of 10–30% of the entire sample (more than 200 individuals). Large and/or rare individuals such as Amphipoda, Euphausiacea and adult Decapoda were counted in the whole diet sample. The exact value of dry mass per prey item was used whenever possible, and the appropriate formula was used for organisms which differed in size (Table 2). Energetic values of prey items were calculated using published energetic values (kJ g−1 of dry mass of prey species and developmental stages (Table 2). We calculated the average calorific value per little auk prey item based on the overall relative proportions of the different prey species in the diet.
Adult energy expenditure
Field procedure
We used the two-point doubly labelled water (DLW) technique (Lifson and McClintock 1966) to measure the daily energy expenditure of five little auk adults breeding at Kap Brewster (10–11 July) and 19 adults breeding at Kap Höegh (21–28 July). Out of the five birds measured at Kap Brewster, three were incubating and the remaining two were brooding chicks less than 5 days old. All 19 of the Kap Höegh birds had chicks older than 5 days. Daily energy expenditure at Kap Brewster was measured on four birds that were simultaneously carrying time–depth-recorders, and on one bird without a logger. Loggers were attached just prior to the bird’s release. We were only able to re-catch 14 of the 24 injected birds (58%) because of stormy weather during the periods of recapture. Of these 14 birds, three were from the Kap Brewster colony (one incubating, and two brooding small chicks), and 11 were parents at Kap Höegh.
All parents were caught in their nest crevice, weighed to the nearest 0.1 g, and immediately given an intra-peritoneal injection of 0.15 mL of a mixture of DLW. The dose was composed of 0.55 g kg−1 estimated total body water (TBW) 96% H 182 O (Rotem Industries Ltd., Israel) and 0.17 g kg−1 estimated TBW 99.9% 2H2O (Cambridge Isotope Laboratories, Andover, MA, USA) that was diluted by 3% NaCl to physiological osmolarity. The doses were calculated to ensure an in vivo enrichment of about 240 and 940‰ for 18-oxygen and deuterium, respectively, [‰(delta per mil) = (Rsample/Rstandard−1) × 1,000 with R being the ratio heavy to light isotope]. Birds were then placed in a dark box for 1 h to allow the DLW to equilibrate in the body. An initial blood sample of approximately 100 μl was collected after equilibrium and flame sealed in microhematocrit tubes. Birds were then replaced back in their nest crevice. Injected birds were recaptured on their nests within 48 h post injection, weighed to the nearest 0.1 g, and a final blood sample of approximately 150 μl taken. We also collected blood samples from 12 additional birds to quantify background isotope levels.
Laboratory procedure
18O and 2H enrichments of body water samples were, respectively, measured by the H2O/CO2 equilibration and Zn reduction techniques after cryodistillation of blood, as routinely performed at the University of Strasbourg (Blanc et al. 2000). Analyses were performed in duplicate for both deuterium and oxygen-18 on an optima isotope ratio mass spectrometer (Fisons UK) and repeated if SD exceeded 2.0 and 0.5‰, respectively. CO2 production (rCO2) was calculated after the single pool equation of Speakman (1997): rCO2 = (No/2.078).(ko-kd)-0.062.kd.No, where No represents the 18-oxygen dilution calculated from Coward (1990) by the plateau method using the 1-h post-dose sample, and corrected for isotope exchange by the factor 1.007 (Racette et al. 1994). The average dilution space ratio Nd/No) was 1.037 ± 0.017 (mean ± SD). ko and kd (day−1) represent the isotope constant elimination rates calculated by linear regression of the natural logarithm of isotope enrichment as a function of elapsed time from day 1 samples. This equation was shown to give the best agreement with indirect calorimetry in rats (Blanc et al. 2000). RCO2 was converted to total energy expenditure (TEE) using the Wier equation (1947) assuming a food quotient of 0.768 estimated from the animal’s diet. TEE is the combined measure of at-sea and at-colony metabolism.
Results
Diving behaviour
The four loggers successfully downloaded provided 158 h of data, including a total of 1,190 dives (see details in Table 1). Depth utilisation by different birds varied considerably (Fig. 2), but the average maximum depth of dives was 10.2 m (±0.2 n = 1,190 dives, range = 1.0–26.8 m), and the duration of an average dive was 52.1 s (±0.4, n = 1,190 dives, range = 9.8–90.0 s). Dive duration was positively and linearly correlated with dive depth (r 2 = 0.94, P < 0.0001; dive duration = 2.0 × dive depth + 32.3) (Fig. 3).
The vast majority (91%) of dives were characterised as “V-shaped”, with the remaining 9% having a “U-shaped” (bell-shaped) profile with a distinct bottom phase to the dive. The average duration of dives without an identified bottom phase was 51.3 s (±0.5, n = 1,084), whereas dives with a bottom phase were significantly shorter (mean = 46.1 s ± 1.2, n = 107, t = 3.727, P = 0.002, df = 1,188). Birds attained similar vertical velocities of up to 1 m s−1 during both the ascent and the descent phases of the dives.
Time budgets
The incubating bird had a very different foraging pattern to the two chick-rearing birds, with only two foraging trips conducted during the 33 h of recording time; one with a duration of approximately 1.5 h (24 dives) while the second lasted more than 26 h and included 300 dives. The two chick-rearing parents made an average of 3.2 and 4.4 foraging trips per 24 h. Trip durations of the two chick-rearing parents ranged from 18 to 304 min (average 189 ± 33 min, n = 10 trips), with an average of 58.9 dives (±1.0, n = 10 trips, min = 13, max = 110) conducted per trip. Overall, the three birds spent an average of 54.4% of their time in the colony and 45.6% at-sea. Of the total amount of time spent at-sea (64 h), 23% was spent diving underwater. Although longer stretches of flight time could be identified, flights of shorter duration were more difficult to interpret, and overall estimation of flight time was therefore impossible. The length of time it took to return to the colony after the final foraging bout was easier to determine, and these flights (n = 15) ranged from 7 to 70 min (average = 20.7 ± 4.0 min).
Birds expressed no obvious diurnal pattern in diving behaviour, and no significant difference (Pearson product moment correlation) in the number of dives or dive depths could be detected across the 24-h cycle.
Chick diet
A total of 35 chick meals were collected, including 26 complete and nine incomplete meals. Average number of prey items per complete meal was 1,553 ± 187.3 items (min = 432, max = 4289). Calanus copepods constituted over 95% of prey items delivered (see Table 2; Calanus hyperboreus = 46.6%, Calanus glacialis = 44.8%, and C. finmarchicus = 4.4% of the total). C. hyperboreus and C. glacialis constituted over 82% of the total energy brought to the chick.
Adult energy expenditure
Average TEE for all birds = 609.9 kJ day−1 (±26.0, n = 14 birds), whereas TEE for all parents with chicks was 602.4 kJ day−1 (±26.8, n = 13 birds). The TEE for non-brooding parents provisioning chicks older than 5 days was 612 kJ day−1 (±30.9, n = 11 birds).
Three of the recaptured DLW birds had loggers, including the one logger that we were unable to download. Sample sizes were too small to examine statistical differences in energy expenditure between birds with and without devices, but the energy expenditure of birds with loggers were lower on average (601.2 ± 53.7 kJ day−1, n = 3 birds) than birds without loggers (612.3 ± 30.9 kJ day−1, n = 11 birds). This difference could, however, also be affected by differences in nest status and chick-age between birds with and without loggers. We were only able to weigh four of the five birds equipped with loggers at recapture. All four birds lost body mass (average = −2.7 ± 0.9 g per 24 h−1; min = −0.7 g, max = −4.6 g) during the period they carried the logger (average = 46.8 h). In contrast, the DLW birds without loggers exhibited a wide range of mass change over a similar period of time (average = 34.7 h). Overall, birds without loggers gained an average of 3.2 g per 24 h (n = 11, SE = 1.9 g; min = −7.2 g, max = 13.0 g).
Prey requirements and underwater capture rates
Calanus copepods constituted over 95% of prey items delivered to chicks (see Table 2). Using our estimate of FMR for non-brooding provisioning parents (612 kJ d−1 ± 102.5 SD), an assimilation efficiency of 0.8 (Taylor and Konarzewski 1992), and an average calorific value per prey item based on the relative proportion of prey species in the diet (Table 2; 24.3 kJ g−1), we estimated that little auk parents needed to consume 30 g dry mass of food a day. Using 0.59 mg as the average dry mass of an individual prey item (Table 2), we then calculated that 30 g of food is the equivalent to 50,847 individual prey items. Parents also have to catch food for their chick in addition to personal consumption. The average number of prey items per whole chick meal in this study was 1,553 prey items (±955 SD, n = 26 whole chick meals). Using an average chick provisioning rate of 4.9 meals a day ± 1.8 SD (Welcker et al. 2009 Kap Höegh data, n = 15 birds), each parent should provide an average of 7,610 prey items to the chick. Using these means and standard deviations (±10% of mean when SD unknown) in a Monte Carlo simulation (10,000 runs; see Manly 1997), we estimated that provisioning little auks need to catch a total of 59,771 prey items per day (±14,031 SD, 5–95% confidence limits 38,904–86,255).
Individuals spent an average of 2.7 h (9,600 s ± 1,482 SD) underwater per day. Using Monte Carlo simulations with our estimates of daily prey requirements and foraging behaviour, we estimated that little auks were catching an average 6.4 (±1.8 SD, 5–95% confidence limits = 3.9–10.1) copepods s−1 while diving. Taking an average diving speed of 0.5 m s−1 ± 0.1 SD, little auks covered an average total of 3,744 m during daily dives. Using Monte Carlo simulations, little auks had an average prey capture rate of 13.5 prey items m−1 (±12.7 SD, 5–95% confidence limits = 6.7–24.7).
Discussion
To our knowledge, this is the first study to examine the diving behaviour and at-sea activity of little auks using time–depth-recorders. Due to technical difficulties data were only collected for four individuals, but recordings showed that birds dive up to 240 times a day at maximum depths of 27 m (average 10 m), with maximum dive durations of 90 s (average 52 s). In addition, we collected the food loads of 35 individuals and determined the FMR of 14 individuals using the DLW technique. In this discussion we (a) describe the diving behaviour and time budgets of little auks, (b) integrate information on diving duration, chick diet and FMR to estimate the prey requirements and underwater capture rates of little auks, and (c) discuss the potential effects of the time–depth-recorders.
Diving behaviour
Maximum dive depths recorded in this study are at the lower end of values previously measured using maximum depth gauges (MDGs) that record the deepest dive conducted during the total deployment interval (Falk et al. 2000). The majority of dives recorded by the MDGs in NW Greenland were within the 20–32 m range (average = 27.3 ± 4.1 m, n = 33 dives; Falk et al. 2000). In contrast, about half of the dives recorded in this study were between 1 and 10 m deep, and only 8% were deeper than 20 m. The majority of dives recorded in our study were also much shallower than theoretical estimates of maximum dive depths for little auks (30–35 m; Bradstreet and Brown 1985; Burger 1991). These rather shallow diving depths may be explained by the high abundance of prey in the upper layers of the water column. Rysgaard et al. (1999) found maximum photosynthesis in NE Greenland to appear in a subsurface layer at 15–20 m deep. Tightly coupled to this phytoplankton bloom were large numbers of grazing copepods (especially C. glacialis). Previous direct observations of diving little auks have recorded average dive durations of 20–30 s with a maximum duration of 68 s (review in Bradstreet and Brown 1985; Stempniewicz 2001). Dive durations from our study were considerably longer (average duration = 52 s; longest = 90 s), and this difference may be explained by the difficulty of observing undisturbed offshore diving behaviour.
Most dives made by little auks in this study were V-shaped. V-shaped dives have also been recorded in the razorbill (Alca torda; Benvenuti et al. 2001; Dall’Antonia et al. 2001), whereas most dives performed by the common and Brünnich’s guillemot are U-shaped dives (Croll et al. 1992; Tremblay et al. 2003). The prevalence of V-shaped dives and the fact that little auk underwater speeds were similar during descent and ascent suggests that they may also feed on the way back to the ocean surface. However, higher resolution data is needed to fully qualify the shape of little auk dives and compares these shapes with those of other species (Wilson et al. 1995).
Time activity budgets
The two chick-rearing birds were each feeding their chick an average of 3–4 meals a day. There is often an uneven contribution of meal provisioning within little auk pairs (e.g., Norderhaug 1980), so it is hard to equate these values to the total number of meals received by the chick. However, chick-feeding rates recorded in this study are within the range previously recorded, with chicks generally receiving between five and nine meals a day (Norderhaug 1980; Evans 1981; Stempniewicz 2001; Harding et al. 2004; Jakubas et al. 2007). Foraging trip durations for the two chick-rearing parents ranged from 18 to 304 min. These values are slightly shorter than trips recorded at Hornsund, Spitsbergen, in 2002 (Harding et al. 2004; range = 31–591 min, n = 51 trips), and may indicate that birds in East Greenland were either able to forage nearer the colony, find more fertile feeding grounds, have quicker prey capture rates, or were acquiring a lower number of prey items per foraging trip.
Although field measurements of little auk flight velocity are not available, it is reasonable to assume a velocity similar to other alcids (60–70 km h−1; Bradstreet and Brown 1985). If birds are taking an average of 20.7 min to fly from the location of their last dive back to the colony, and we assume a straight line of flight, these data suggest that the feeding grounds of little auks breeding in the Scoresbysund area of East Greenland are located on average about 21–24 km from the colony. The range (7–70 min) of flight time from feeding grounds to the colony corresponds to a foraging distance of approximately 8–76 km from the colony. Little auks have previously been observed to feed up to 100–150 km away from the colony (summarised in Stempniewicz 2001), and as close as 25 km (Bradstreet and Brown 1985; Evans 1981).
Previous studies on the activity budgets of guillemots and razorbills at-sea have recorded a range of 12.2–33.8% time spent diving (summarised in Tremblay et al. 2003). Little auks in our study were spending 23% of their time at-sea diving. Parental time allocation can provide a useful indication of foraging effort and local food availability (e.g., Harding et al. 2007). A detailed inter-colony comparison of little auk parental time budgets and food availability would help identify the flexibility of their time budgets, and determine which parameter would provide the best indication of local foraging conditions.
There has been much speculation about the diurnal and seasonal pattern of little auk colony attendance (e.g., Evans 1981; Stempniewicz 2001). Although little auks at lower latitudes may capitalise on the vertical migration of zooplankton by foraging more at night (van Franeker et al. 1998), the regular daily vertical movements of zooplankton may be less pronounced or absent in the high Arctic (Digby 1961; Blachowiak-Samolyk et al. 2006) and could explain the lack of any distinct diurnal pattern of little auk foraging in East Greenland.
Diet
The distribution and composition of zooplankton communities are linked to oceanographic conditions, with different copepod species adapted to different water characteristics (Scott et al. 2000; Beaugrand et al. 2002b). Arctic zooplankton communities are generally dominated by large, cold water species that store more lipids and are more energy rich that their smaller counterparts in warmer Atlantic waters (Węsławski et al. 1994; Węsławski et al. 1999b). Little auks foraging in water that originates from the Arctic have access to such larger, energy-rich copepod species (C. hyperboreus and C. glacialis), whereas birds breeding in areas influenced by warmer Atlantic water may be forced to forage on smaller, less energy-rich C. finmarchicus (e.g., Karnovsky et al. 2003; Jakubas et al. 2007). Little Auks in this study are foraging in cold water from the Arctic Ocean that flows southward along the coast of East Greenland (the East Greenland Current). This is the first study to examine the diet of little auks breeding in East Greenland, and the data show that they are foraging on a higher percentage of C. hyperboreus and C. glacialis than little auks foraging off the West coast of Spitsbergen in water influenced to varying degrees by the inflow of warm Atlantic water (e.g., Karnovsky et al. 2003; Jakubas et al. 2007).
Energy expenditure
Little auks are expected to have particularly high FMR because of their small size and high wing-loading (ratio of body mass to wing area; Gabrielsen et al. 1991). Results of an energetic study on little auks in Spitsbergen support this assumption (Gabrielsen et al. 1991, average = 696.1 ± 28.8 kJ day−1, mean body mass = 164.3 ± 9.5 g; n = 13 birds), showing that the FMR of little auks, corrected for body size, is higher than other small cold water wing-propelled pursuit diving seabirds such as the least auklet (Aethia pusilla) and the black guillemot (Cepphus gryle; summarised in Gabrielsen et al. 1991). Results from our study show that little auks breeding in East Greenland also have very high FMR (619.9 ± 22.1 kJ day−1, or 47.2 W kg−1).
Despite the very limited sample size of two birds with combined TDR and DLW data, it is interesting to note that the incubating bird had higher TEE than the chick-rearing bird (707 vs. 562 kJ day−1, respectively). Although the chick-rearing bird made over twice as many trips to the colony than the incubating bird, the incubating bird made nearly 40% more dives than the chick-rearing bird. Knowledge of the energetic costs of diving and flight (e.g., Jodice et al. 2003) is important to understand whether it is more profitable for little auk parents to fly longer distances to high food densities versus forage in lower food densities closer to the colony.
Food requirements and prey capture rates
By integrating measures of dive behaviour and energy expenditure we estimate that little auks catch about 6 copepods s−1 underwater. It is very unlikely little auks catch any prey from the surface of the water whilst swimming. Our capture rates may be overestimated because very short dives are not detected with the 5 s sampling interval, and higher resolution dive data are needed for greater accuracy. However, these values may also be underestimated because they assume that birds feed at a constant rate whilst underwater, and it is more likely that dive times include underwater travel to a high density prey patch. These values are surprisingly high for a bird which is typically assumed to be catching prey items one by one (Stempniewicz 2001), and it seems likely that little auks are filter-feeding for at least some of the time spent foraging. Although little auks have large palatal papillae that may aid this feeding mechanism (Lovvorn et al. 2001) and have been observed to expel water out from the back of their bill, underwater observations are needed to confirm their feeding strategy. Our estimates of daily prey capture rates may be artificially inflated by the assumption that adults are self-feeding on the same prey as they feed their chick. Study of the energy metabolism of little auk chicks found that the energy delivered to the chick by one parent was 15% of the total energy gathered by the parent (Konarzewski et al. 1993). Our estimates of prey capture suggest that chicks receive approximately 13% of the total number of prey items that a parent needs to catch (for its own and the chick’s needs). This slight discrepancy may be explained by adults feeding on fewer, larger prey items than they provide to their chick, and that our estimates of total prey capture rates are a little high. This theory is supported by evidence of larval fish in the diet of some adult little auk (Bradstreet 1982). However, even if our estimates of total prey capture rates are slightly high, these data demonstrate the benefit of high prey densities for profitable little auk foraging.
As discussed, zooplankton community composition is closely linked to oceanographic conditions, and the availability of the different Calanus species will directly affect the number of prey items that little auks need to consume to balance their energy budget. Little auks foraging in cold Arctic waters on larger, energy richer species will need to catch fewer prey items than little auks foraging in warmer Atlantic water on smaller prey items.
Effect of device
Although the use of data loggers has become an integral part of modern seabird research, there is evidence that devices may affect the feeding behaviour and reproductive performance of birds (e.g., Wilson et al. 2002; Ackerman et al. 2004). Data obtained from loggers may therefore not represent the natural behaviour of the species, and these concerns are especially relevant for the alcids which have high wing-loading (Croll et al. 1992). Although the long, relatively shallow dives recorded in this study could reflect the distribution of zooplankton, they could also be the result of the additional weight and drag of the TDR. Further study is required to examine this possibility; the simultaneous deployment of both MDGs and TDRs and TDRs and radio-transmitters could help determine whether TDRs are affecting the dive depth and dive duration of little auks.
The limited sample sizes in this study made it impossible to draw firm conclusions about the effect of loggers on energy expenditure and mass change, and our results are further complicated by individual differences in nest status and chick age. Our results nonetheless suggest that birds with loggers had similar or slightly lower FMR but lost more mass on average than birds without loggers. Although a larger sample size is required to interpret these data, the additional cost of the logger likely resulted in a reduction of foraging efficiency and loss of body mass. Three of the 13 birds equipped with loggers were not seen in the few days following deployment. Again, however, it is hard to interpret whether these absences were due to the logger or bad weather. Stormy weather at both colonies post logger deployment affected the colony attendance of birds in general, and relatively few birds were observed at the surface of the colony during these days. Despite these uncertainties, our estimates of chick-feeding rates and forage trip durations are not incomparable to previous studies (summarised in Stempniewicz 2001), and suggest that birds with loggers were functioning at least fairly normally. More detailed study on the physiological costs of external loggers (e.g., Tremblay et al. 2003), combined with the simultaneous observation of birds with and without loggers is needed to determine any adverse effect of these devices.
Conclusions
Despite limited sample sizes, this study has provided initial baseline knowledge of the underwater foraging behaviour of little auks. These data should be qualified with data derived from lighter devices, and we expect conclusions to change as more data are collected. We expect that the use of data loggers will play an increasingly large role in the study of little auk foraging behaviour, and a thorough investigation of the effect of devices on behaviour is therefore crucial.
With increasing evidence of climate change in Arctic marine ecosystems (Beaugrand et al. 2002b; Clark and Harris 2003), it is essential to understand the capacity of individual species to adjust to changes in their environment. The breeding range of little auks is largely concentrated in the high Arctic marine zone of the Atlantic (Stempniewicz 2001). This region is crucial for large-scale oceanographic circulation (Clark et al. 2002), and climate models are forecasting drastic changes (Bryden et al. 2005; Arctic Climate Impact Assessment 2004). Although recent study has shown that little auk parents were able to compensate for suboptimal foraging conditions by increasing their chick-feeding rates (Jakubas et al. 2007), little is known about their flexibility in dive behaviour and time and energy budget. The simultaneous study of energetic expenditure alongside detailed measures of foraging behaviour and time activity budgets of little auks in different oceanographic regions is therefore vital to understand their plasticity of foraging effort and ability to adjust to changing food availabilities.
References
Ackerman JT, Adams J, Takekawa JY, Carter HR, Whitworth DL, Newman SH, Golightly RT, Orthmeyer DL (2004) Effects of radiotransmitters on the reproductive performance of Cassin’s auklets. Wildlife Soc Bull 43(4):1–13
Ainley DG, Sydeman WJ, Norton J (1995) Upper trophic level predators indicate interannual negative and positive anomalies in the California current food web. Mar Ecol Prog Ser 118:69–79
Arctic Climate Impact Assessment (ACIA) (2004) Impacts of a warming Arctic: Arctic Climate Impact Assessment. Cambridge University Press, Cambridge
Båmstedt U (1986) Chemical composition and energy content. In: Corner EDS, O’Hara SCM (eds) The biological chemistry of marine copepods. Clarendon, Oxford, pp 1–58
Båmstedt U, Eilertsen H-C, Tande K, Slagstad D, Skjoldal HR (1991) Copepod grazing potential and its potential impact on the phytoplankton development in the Barents Sea. Polar Res 10:339–353
Beaugrand G, Reid PC, Ibañez F, Lindley JA (2002a) Diversity of calanoid copepods in the North Atlantic and adjacent seas: species associations and biogeography. Mar Ecol Prog Ser 232:179–195
Beaugrand G, Reid PC, Ibañez F, Lindley JA, Edwards M (2002b) Reorganization of North Atlantic marine copepod biodiversity and climate. Science 296:1692–1694
Benvenuti S, Dall’Antonia L, Lyngs P (2001) Foraging behaviour and time allocation of chick-rearing razorbills Alca torda at Graesholmen, central Baltic Sea. Ibis 143:402–412
Berestovskii EG, Anisimova NA, Denisenko CG, Luppova EN, Savinov VM, Timofeev CF (1989) Relationships between size and body mass of some invertebrates and fish of the North-East Atlantic. Academy of Sciences of the USSR. Murman Marine Biological Institute, Apatity, pp 1–24
Błachowiak-Samołyk K, Kwaśniewski S, Richardson K, Dmoch K, Hansen E, Hop H, Falk-Petersen S, Mouritsen LT (2006) Arctic zooplankton do not perform diel vertical migration (DVM) during periods of midnight sun. Mar Ecol Prog Ser 308:101–116
Blanc S, Geloen A, Pachiaudi C, Gharib C, Normand S (2000) Validation of the doubly labeled water technique in rats during isolation and simulated microgravity. Am J Physiol Regul Integr Comp Physiol 279:R1964–R1979
Bradstreet MSW (1982) Pelagic feeding ecology of dovekies Alle alle in Lancaster Sound and Western Baffin Bay. Arctic 35:126–140
Bradstreet MSW, Brown RGB (1985) Feeding ecology of the Atlantic Alcidae. In: Nettleship DN, Birkhead TR (eds) The Atlantic Alcidae. Academic Press, London-Toronto, pp 264–313
Bryden HL, Longworth HR, Cunningham SA (2005) Slowing of the Atlantic meridional overturning circulation at 25°N. Nature 438:655–657
Burger AE (1991) Maximum diving depths and underwater foraging in alcids and penguins. Can Wildl Occasion Paper 68:9–15
Cherel Y, Weimerskirch H (1995) Seabirds as indicators of marine resources: black-browed albatrosses feeding on ommastrephid squids in Kerguelen waters. Mar Ecol Prog Ser 129:295–300
Clark A, Harris CM (2003) Polar marine ecosystems: major threats and future change. Environ Conserv 30:1–25
Clark PU, Pisias NG, Stocker TF, Weaver AJ (2002) The role of the thermohaline circulation in abrupt climate change. Nature 415:863–869
Coward WA (1990) Calculation of pool sizes and flux rates. In: Prentice AM (ed) The doubly labelled water method: technical recommendations for use in humans. Report of an IDECG Expert Working Group, AERA, Vienna, Austria, pp 48–68
Croll DA, Gaston AJ, Burger AE, Konnoff D (1992) Foraging behavior and physiological adaptation for diving in thick-billed murres. Ecology 73:344–356
Dall’Antonia L, Gudmundsson GA, Benvenuti S (2001) Time allocation and foraging pattern of chick-rearing razorbills in northwest Iceland. Condor 103:469–480
Digby PSB (1961) The vertical migration and movements of marine plankton under midnight-sun conditions in Spitsbergen. J Anim Ecol 30:9–25
Evans PGH (1981) Ecology and behaviour of the little auk Alle alle in West Greenland. Ibis 123:1–18
Falk K, Pedersen CE, Kampp K (2000) Measurements of diving depths in dovekies (Alle alle). Auk 117:522–524
Frederiksen M, Edwards M, Richardson AJ, Halliday C, Wanless S (2006) From plankton to top-predators: bottom-up control of a marine food web across four trophic levels. J Anim Ecol 75:1259–1268
Furness RW, Campuysen CJ (1997) Seabirds as monitors of the marine environment. ICES J Mar Sci 54:726–737
Gabrielsen GW, Taylor JRE, Konarzewski M, Mehlum F (1991) Field and laboratory metabolism and thermoregulation in dovekies (Alle alle). Auk 108:71–78
Harding AMA, Van Pelt TI, Lifjeld JT, Mehlum F (2004) Sex differences in little auk Alle alle parental care: transition from biparental to paternal-only care. Ibis 146:642–651
Harding AM, Piatt JF, Schmutz JA, Shultz MT, Van Pelt TI, Kettle AB, Speckman SG (2007) Prey density and the behavioral flexibility of a marine predator: the common murre (Uria aalge). Ecology 88:2024–2033
Hirche H-J (1997) Life cycle of the copepod Calanus hyperboreus in the Greenland Sea. Mar Biol 128:607–618
Hurrell JW (2000) Climate: Northern Atlantic and Arctic Oscillation (NAO/AO). In: Holton J, Pyle J, Curry J (eds) Encyclopedia or atmospheric sciences. Academic Press, New York
Jakubas D, Wojczulanis-Jakubas K, Walkusz W (2007) Response of dovekie to changes in food availability. Waterbirds 30:421–428
Jodice PGR, Roby DD, Suryan RM, Irons DB, Kaufman AM, Turco KR, Visser GH (2003) Variation in energy expenditure among black-legged kittiwakes: Effects of activity-specific metabolic rates and activity budgets. Physiol Biochem Zool 76:375–388
Karnovsky NJ, Kwaśniewski S, Węsławski JM, Walkusz W, Beszczyńska-Mőller A (2003) Foraging behaviour of little auks in a heterogeneous environment. Mar Ecol Prog Ser 253:289–303
Kitaysky AS, Golubova EGl (2000) Climate change causes contrasting trends in reproductive performance of planktivorous and piscivorous alcids. J Anim Ecol 69:248–262
Konarzewski M, Taylor JRE, Gabrielsen GW (1993) Chick energy requirements and adult energy expenditures of dovekies (Alle alle). Auk 110:343–353
Kooyman GL, Kooyman TG (1995) Diving behavior of emperor penguins nurturing chicks at Coulman Island, Antarctica. Condor 97:536–649
Kosobokova KN (1980) Caloric value of some zooplankton representatives from the Central Arctic Basin and the White Sea. Oceanology 20:84–89
Kwaśniewski S, Hop H, Falk-Petersen S, Pedersen G (2003) Distribution of Calanus species in Kongsfjorden, a glacial fjord in Svalbard. J Plankton Res 25:1–20
Lee DA, Nur N, Sydeman WJ (2007) Climate and demography of the planktivorous cassin’s auklet Ptychoramphus aleuticus off northern California: implications for population change. J Anim Ecol 76:337–347
Lifson N, McClintock R (1966) Theory of the use of the turnover rates of body water for measuring energy and material balance. J Theor Biol 12:46–74
Litzow MA, Piatt JF (2002) Variance in prey abundance influences time budgets of breeding seabirds: evidence from pigeon guillemots. J Avian Biol 34:54–64
Lovvorn JR, Beduini CL, Hunt GL (2001) Modeling underwater visual and filter feeding by planktivorous shearwaters in unusual sea conditions. Ecology 82:2343–2356
Manly BFJ (1997) Randomization, Bootstrap and Monte Carlo methods in biology. Chapman & Hall, London
Monaghan P, Uttley JD, Burns MD, Thaine C, Blackwood J (1989) The relationship between food supply, reproductive effort and breeding success in arctic terns Sterna paradisaea. J Anim Ecol 58:261–274
Mumm N (1991) Zur sommerlichen Verteilung des Mesozooplanktons im Nansen-Becken, Nordpolarmeer. Ber Polarforsch 92:1–173
Norderhaug M (1980) Breeding biology of the little auk (Plautus alle) in Svalbard. Norsk Polarinstitutt 104:1–119
Pedersen CE, Falk K (2001) Chick diet of dovekies Alle alle in Northwest Greenland. Polar Biol 24:53–58
Poltermann M (1997) Biology and ecology of cryopelagic amphipods from Arctic Sea ice. Ber Polarf 225:1–170
Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF (1994) Relative dilution spaces of 2H and 18O-labelled water in humans. Am J Physiol 30:E585–E590
Richardson AJ, Schoeman DS (2004) Climate impact on plankton ecosystems in the Northeast Atlantic. Science 305:1609–1612
Richter C (1994) Regional and seasonal variability in the vertical distribution of mesozooplankton in the Greenland Sea. Ber Polarforsch 154:1–90
Rysgaard S, Nielsen TG, Hansen B (1999) Seasonal variation in nutrients, pelagic primary production and grazing in a high-Arctic coastal marine ecosystem, Young Sound, Northeast Greenland. Mar Ecol Prog Ser 179:13–25
Scott CL, Kwasniewski S, Falk-Petersen S, Sargent JR (2000) Lipids and life strategies of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus in late autumn, Konsgsfjorden, Svalbard. Polar Biol 23:510–516
Speakman JR (1997) Doubly labelled water: theory and practice. Chapman and Hall, New York
Stempniewicz L (2001) Alle alle Little Auk. The journal of the birds of the western palearctic. BWP Update, vol. 3. Oxford University Press, Oxford, pp 175–201
Taylor JRE, Konarzewski M (1992) Budget of elements in little auk (Alle alle) chicks. Funct Ecol 6:137–144
Tremblay Y, Cherel Y, Oremus M, Tveraa T, Chastel O (2003) Unconventional ventral attachment of time–depth recorders as a new method for investigating time budget and diving behaviour of seabirds. J Exp Biol 206:1929–1940
Van Franeker JA, Camphuysen CJ, Mehlum F (1998) The birds of Jan Mayen. Circumpolar J 13:28–43
Welcker J, Harding AMA, Karnovsky NJ, Steen H, Strøm H, Gabrielsen GW (2009) Flexibility in the bimodal foraging strategy of a high Arctic alcid, the little auk Alle alle. J Avian Biol (in press)
Węsławski JM, Kwaśniewski S (1990) The consequences of climatic fluctuations for the food web in Svalbard coastal waters. In: Barnes M, Gibson RM (eds) Trophic relationships in the marine environment. Proceedings of 24th European marine biology symposium. Aberdeen University Press, Aberdeen, pp 281–295
Węsławski JM, Stempniewicz L, Galaktionov K (1994) Summer diet of seabirds from the Frans Josef Land archipelago, Russian Arctic. Polar research 13:173–181
Węsławski JM, Koszteyn J, Kwaśniewski S, Stempniewicz L, Malinga M (1999a) Summer food resources of the little auk, Alle alle (L.) in the European Arctic seas. Pol Polar Res 20:387–403
Węsławski JM, Stempniewicz L, Mehlum F, Kwaśniewski S (1999b) Summer feeding strategy of the little auk (Alle alle) from Bjørnoya, Barents Sea. Polar Biol 21:21–134
Wilson RP, Pütz K, Charrassin J-B, Lage J (1995) Artifacts arising from sampling interval in dive depth studies of marine endotherms. Polar Biol 15:575–581
Wilson RP, Pütz K, Peters G, Culik BM, Scolaro JA, Charrassin J-B, Ropert-Coudert Y (1997) Long-term attachment of transmitting and recording devices to penguins and others seabirds. Wild Soc Bull 25:101–106
Wilson RP, Grémillet D, Syder J, Kierspel MAM, Garthe S, Weimerskirch H, Schäfer-Neth C, Scolara JA, Bost C-A, Plötz J, Nel D (2002) Remote-sensing systems and seabirds; their use, abuse and potential for measuring marine environmental variables. Mar Ecol Prog Ser 228:241–261
Wojczulanis K, Jakubas D, Walkusz W, Wennerberg L (2006) Differences in food delivered to chicks by males and females of little auks (Alle alle) on South Spitsbergen. J Ornithol 147:543–548
Acknowledgments
We thank K. Dmoch and the Institute of Oceanology for help with prey biomass and calorific values, A. Zahariev (Centre national de la recherche scientifique, Strasbourg) for help with DLW analysis, P. Jodice and D. Roby for invaluable DLW advice, and NANU Travel for logistical support in East Greenland. Earlier versions of this manuscript benefited from thoughtful reviews and feedback by J. Welcker, L. Stempniewicz, and J.M. Węsławski. This project was funded by the Greenland Institute of Natural Resources and the French Polar Institute Paul-Emile Victor (Grant 388 to D.G.). All field work was conducted with the permission of the Greenland Home Rule, Ministry of Environment and Nature.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harding, A.M.A., Egevang, C., Walkusz, W. et al. Estimating prey capture rates of a planktivorous seabird, the little auk (Alle alle), using diet, diving behaviour, and energy consumption. Polar Biol 32, 785–796 (2009). https://doi.org/10.1007/s00300-009-0581-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0581-x