Abstract
The lipid storage of the cyclopoid copepod Oithona similis was investigated from spring to late summer 2007 in Kongsfjorden (Svalbard, Norway). The volume of lipid droplets in each individual reflected the amount of stored wax esters. Seasonal changes of lipid storage coupled with informative inter-individual variability were thus obtained. The seasonal pattern showed an increase in lipid store during the spring bloom, starting before the chlorophyll a maximum for both copepodids stage V and females. Those reserves were used during the main reproductive event in June. Individual variability was very high, with a significant proportion of copepods having no droplet, while others were lipid rich. Because of the overlap of generation, females could have different age and feeding history, particularly in September. Consideration of intra-population variability in lipid storage using an optical approach has been shown to be important to understand O. similis’s ecology and life cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Small copepod species (<1 mm) play a key role in the functioning of the pelagic ecosystem. Highly abundant, they represent a link between the ‘classical’ and microbial food webs, as reviewed by Turner (2004). However, their importance and impact are still underestimated, mainly because of inappropriate sampling gears (Gallienne and Robins 2001). Among them, Oithona similis Claus 1866 (Cyclopoida) is a ubiquitous and cosmopolitan copepod, often the most abundant species of the mesozooplankton community from tropical areas to high latitudes. In the Southern Ocean, the importance of small copepods, such as O. similis, has been assessed by Atkinson and Sinclair (2000) from historical data. In this region, its annual production was greater than any of the biomass-dominant copepods (Fransz and Gonzalez 1995). In the same manner, O. similis is one of the most abundant mesozooplankton in the Arctic (Auel and Hagen 2002; Daase and Eiane 2007; Walkusz et al. 2003). Recently, the knowledge about the ecology of this species has increased, especially in the European Arctic region (Castellani et al. 2005a, b; Lischka 2006; Nielsen et al. 2002; Sabatini and Kiørboe 1994). An interesting feature of O. similis is that its life strategy differs from the large Arctic Calanoids, such as Calanus spp. It does not overwinter in diapause, but stays active in the upper layer of the water column (Conover and Huntley 1991; Lischka et al. 2007). This species reproduces year-round (Ashjian et al. 2003; Sabatini and Kiørboe 1994), and shows two major reproductive events at our study site (Lischka and Hagen 2005). Thus, O. similis could play a key role in the Arctic at particular periods of the year, i.e., autumn and winter when other species are undergoing diapause (Lischka and Hagen 2005), and act as stabilization factor of planktonic communities as hypothesized by Paffenhöfer (1993).
The European sector of the Arctic Ocean undergoes a strong influence of Atlantic water masses flowing through the Fram Strait, which is predicted to increase with global climate change (Cottier et al. 2005; Schauer et al. 2004). In this context, the role of smaller taxa, small copepods and other microzooplankton, could become more important (Hansen et al. 2003). Since cyclopoid species show generally less specialization than calanoids (Paffenhöfer 1993), they are able to survive over an extended range of environmental conditions, being thus less affected by water masses modifications even though it has been speculated that O. similis fitness at high latitudes was limited by low temperatures (Ward and Hirst 2007). Therefore, this species could actually benefit from a rising temperature in the Arctic. A clear increase of the relative abundance of O. similis, among other small copepods, has already been underlined in Kongsfjorden, west coast of Spitsbergen, from 1996 to 2002 (Hop et al. 2006).
In arctic regions, zooplankton has adapted to the high seasonality of primary production in storing large amounts of lipids as energy reserves (Falk-Petersen et al. 1990; Hagen and Auel 2001). These reserves ensure the winter survival and the reproductive capacity of populations the next spring (Falk-Petersen et al. 2006; Lee et al. 2006). Hence, arctic zooplankton represents a highly energetic food source, which supports the productivity of the higher trophic levels. At the individual level, looking at lipid stores gives insights into the physiological state of the organism and the population fitness.
Lipid stores in zooplankton are essentially made from neutral lipids, wax esters and triacylglycerols, and can be quantified by several methods (reviewed in Lee et al. 2006). Optical estimation of lipid droplets has been shown to be a useful alternative tool to biochemical methods. Quicker and cheaper, they are non-destructive and give information on the location of the reserves in the organism. Additionally, population features are deduced from individual measurements, which give information on the intra-population variability. First developed for freshwater Cladocerans, the amount of lipid droplets was evaluated by a simple visual index, ranging from 0 to 3 (Goulden and Hornig 1980). Later, Arts and Evans (1991) studied the seasonal changes of lipid reserves in freshwater calanoid copepods and showed that both lipid droplets volume measurements and biochemical analysis gave concordant results. In small marine calanoids, a lipid index calculated as the percentage of the lipid sac area on the prosome area has been proposed by Norrbin (1991). The lipid sac volume measurement was also used to quantify the amount of storage lipids in Calanus finmarchicus (Miller et al. 1998). When lipid storage is rather diffuse, Nile Red staining has been shown to be a useful tool for the relative quantification of neutral lipids, either for the determination of the droplets area (Carman et al. 1991), or using directly the fluorescence intensity of stained lipid droplets (Tankersley 1998). Using Nile Red properties, a spectrofluorometric absolute quantification of neutral and polar lipid has been achieved by Alonzo and Mayzaud (1999).
In this study, we discuss the suitability of an optical-digital method, and the usefulness of Nile Red staining, for the investigation of the lipid reserves in O. similis. The seasonal development of these energetic reserves in an Arctic fjord is presented at the population level, considering the individual variability.
Materials and methods
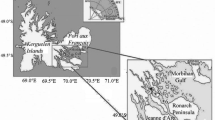
The fieldwork took place from April to September 2007 in Kongsfjorden, a fjord on the west coast of Spitsbergen (Svalbard, Norway). Our sampling station was located in the middle zone of the fjord (78°57.0′N 11°56.4′E), where the bottom depth is of ca.300 m (Fig. 1).
Chlorophyll a and temperature measurements
Once a week from May to September, the chlorophyll a (Chla) concentration was monitored down to the minimum depth of 200 m, with a Seapoint Chlorophyll Fluorometer (Seapoint Sensors, Inc., Exeter, USA). Data acquisition rate was of one measurement per second, corresponding to a vertical accuracy of 0.2–0.7 m. As post-treatment, no smoothing function was applied to the data, but spikes were removed. In April, the chlorophyll a was measured from water samples at discrete depths (surface water, 5, 10, 20 and 30 m). Triplicates of 0.7–1 L of sea-water were filtrated on GF/F filters and stored at −80°C. The extractions have been conducted in 90% acetone in the dark during 5 h and the fluorescence of Chla and phaeopigments in the extracts was measured using a Turner Design 10 Fluorometer (Lorenzen 1966).
Zooplankton sampling
At fifteen dates from the 18th of April to the 24th of September, zooplankton was collected with a 200-μm mesh size WP2 net hauled vertically from 200 m to the surface, at a speed of 0.75 m s−1, and the samples were stored in formalin (4% vol. final concentration). On five separate dates—22 April, 3 May, 29 May, 3 July and 8 September—the WP2 net was towed gently within the 50 upper meters of the water column, at boat drifting speed. The zooplankton caught was kept alive in 10 L of surface seawater, at in situ temperature until sorting for in vivo observation and, at the latest date, Nile Red staining and biochemical analysis.
Lipid droplet observation on formalin-fixed copepods
The fifteen formalin preserved samples were examined under an Olympus SZH10 stereomicroscope with transmitted light. For each sample, 20–30 individuals of both females and copepodites V were picked out and the presence or the lack of lipid droplet was noted. Males were too scarce to be studied over the season.
In vivo lipid droplet observations
Within the day following each of the five catches, successive aliquots of the sample were carefully poured through a sieve of 1-mm mesh size to remove the biggest zooplankton species and then through a 63-μm mesh to retrieve the size class of interest. Different developmental stages from the species O. similis were sorted out alive under stereomicroscope; 27–60 females and 8–37 copepodites V (CV) and additionally in July, 15 CIV and 9 CIII. After a light anesthesia using tricaine methanesulfonate (MS222, Sigma-Aldrich), sorted organisms were individually observed under a microscope; either a Leica DM 2500 microscope or an Olympus IMT-2 inverted microscope. Pictures were taken using a MotiCam 1000 digital camera and its associated software Motic Image Plus (capture resolution 640 × 512 pixels), except in April, when a LEICA camera and its associated software were used instead (capture resolution 2,088 × 1,550 pixels). Specific calibrations were performed for each setting using a micrometric scale (final resolution, 0.73–1.18 μm pixels−1).
The copepods from the September sample were stained alive with the hydrophobic fluorescent dye Nile Red (Sigma-Aldrich) before observation. A stock solution of Nile Red at 0.125 g L−1 in acetone was prepared the week before use and stored in the dark. Several batches of five O. similis were placed in small dishes with filtered seawater (0.45 μm) and Nile Red was added to a final concentration of 2.5 10−3 g L−1. After 5-min staining, the solution was diluted with filtered seawater and the individuals were immediately observed. The concentration of dye and the exposure time for staining have been chosen after testing the fluorescence signal obtained under different conditions: a higher concentration deformed the copepod’s shape and a longer exposure time did not increase fluorescence levels. The stained copepods were viewed for yellow-gold fluorescence of the neutral lipids using an epifluorescence microscope LEICA DM 2500 (excitation wavelength of 450/500 nm, emission >528 nm) according to Greenspan et al. (1985). The image capture was realized with fixed exposure time and gain to allow comparison between the fluorescent levels of different pictures.
Picture analysis and estimation of lipid reserves
For both non-stained and stained samples, pictures were analyzed using the Image J 1.40 software (a public domain software developed by W.S. Rasband, US National Institutes of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/, 1997–2008). From each picture, prosome and lipid droplet(s) area were measured in dorsal view by visual adjustment using the polygon selection tool. From the shape of the selected areas, the major (M) and minor (m) axis of the best fitting ellipse were determined. The volume was then calculated both for the droplets and the prosome, assuming an oblate spheroid shape (Arts and Evans 1991), using the following equation:
Lipid content of each individual was computed by adding the volume of all its droplets, and we assumed that staining the September sample did not affect the area measurement.
In addition, for Nile Red stained organisms, pictures were converted to grey scale with pixel values ranging from 0 to 255. The droplet(s) integrated fluorescence was then computed from the intensity values of all pixels contained in the droplet area.
Biochemical analysis
Oithona similis females from the September sample were also sorted out for lipid biochemical analysis. Three samples were pooled depending on the size of the lipid droplet in the copepod: no visible droplet, small droplet (ca. 2,000 μm2) and big droplet (ca. 9,000 μm2)—96, 71 and 63 individuals, respectively. The copepods of each sample were concentrated alive by gentle filtration through small filters made from a 50-μm mesh size net, and immediately frozen at −80°C. The samples remained deep frozen until analysis. The filters were rinsed with water to take the copepods off and the lipid extractions were conducted immediately in chloroform and methanol following Bligh and Dyer (1959). The copepods were homogenized using a Potter homogenizer (glass/teflon) at 0°C. The solvent was separately preserved at 0°C, while the copepod carcasses were extracted two more times following the above protocol. The total lipid samples obtained were kept under non-oxidant atmosphere (N2) at −80°C until further analysis. To investigate the lipid classes composition, two pseudo-replicates from each sample were analyzed by thin-layer chromatography, flame ionization detector using an Iatroscan MK V TH10 (Iatron, Japan; Ackman 1981), solvent systems followed Mayzaud et al. (1988). Calibration was carried out using commercial standards that approximate the lipid composition of the samples. The results presented are the mean values of the two pseudo-replicates (variability <5%), and are shown in percentage of the total lipid weight (sum of all classes). The ratios wax esters/polar lipids and triacylglycerols/polar lipids have been calculated, because the amount of polar lipids is supposed to remain stable within an organism, independently from the lipid reserve metabolism (accumulation or degradation), and thus brings insight on the absolute neutral lipid content.
Statistical analysis
Due to the non-normality of lipid droplets size distributions, we used non-parametric statistics for comparisons. The droplets volume in copepodids V and females over the season was compared with the Friedman’s ANOVA by ranks, whereas the median test was used for date to date comparisons. ANOVAs were performed to investigate the prosome length variability. We calculated Pearson correlations to compare prosome length and droplet area for each date and stage. These analyses were performed using the statistical software STATISTICA 4.3 (Statsoft).
Results
Neutral lipid storage location
For all examined copepods, Nile Red fluorescence was restricted to one or few delimited areas in the copepods prosomes (Fig. 2a) and did not reveal any diffuse storage. Thus, the neutral lipids appeared to be stored most of the time in one ellipsoidal droplet representing up to 24% of the prosome volume. This main droplet was located at the posterior part of the prosome when small (Fig. 2b), and grew towards the front when larger. Additional droplets were observed mainly when the principal droplet was relatively large, and usually located either in the anterior part of the prosome or on each side of the major lipid droplet. All samples taken as a whole: 27% of the copepods had no visible lipid droplet, and 88% of the copepods having a droplet had only one.
Oithona similis females showing lipid droplets inside the prosome. a Nile red stained organism observed using an epifluorescence microscope, picture converted to grey scale. b Live organism observed using a transmitted light microscope. LD main lipid droplet, LD2 additional lipid droplet. Scale bar 100 μm for both pictures
Nile red versus natural light observation
Both integrated fluorescence and area of each droplet were measured on the September organisms, and a linear regression between the two parameters gave a significant correlation coefficient (R 2 = 0.98, P < 0.0001). We can safely assume that the fluorescence measurements provided similar information to the optical ones. Hence, estimation of the neutral lipid content using droplet area is the only valid measurement.
Lipid composition of the droplets
Comparing the neutral lipid composition of copepods with and without droplet, the major difference was the increase of the wax esters proportion when the droplet was larger, from 5.22 to 37.11% of the total lipid weight (Table 1). In addition, the wax esters/polar lipids ratio showed clearly the same increase, from 0.09 to 0.89. Triacylglycerols (TAG) contributed to the total lipid weight to a lesser extent, but their proportion was significant (5.66–8.74%). The highest TAG value occurred when the droplet volume was minimal; however, the TAG/polar lipids ratio remained relatively stable (0.10–0.15) whatever the droplet size and even if there was no droplet. So the decrease observed in TAG relative proportion when the droplet volume was bigger seemed to be an artefact due to the parallel increase in wax ester content. A reasonable assumption is that lipid droplets in O. similis are made from wax esters.
Seasonal patterns
The chlorophyll a (Chla) concentration (Fig. 3) revealed a spring bloom of moderate intensity (4 μg Chla L−1). Chla accumulation, as a proxy of phytoplankton biomass, started around 25 m depth from the 5 May and sank, with a later maximum around 80 m the 15 May. Later on, several smaller summer blooms (2–2.5 μg Chla L−1) occurred from the end of July onwards, in the 25 first meters of the water column. Those later blooms did not sink because of the established pycnocline (unpublished data).
Seasonal variation of lipid droplet occurrence in O. similis CV and females is presented in Fig. 4. The percentage of individuals having droplets followed the same pattern in fixed organisms than for in vivo observations for the two stages; except at one date for CV, when only eight individuals were observed in vivo. An abrupt increase, starting before the peak of phytoplankton biomass, was seen both in CV and females. This percentage notably decreased during the Chla maximum in CV (Fig. 4a), then increased and remained relatively constant from July to September (87–100%). In females, the decrease occurred right after the Chla maximum and this trend continued during the summer, except one high value in August, with only half of females containing a lipid droplet in September (Fig. 4b).
Oithona similis. Seasonal changes of the percentage of individuals with lipid droplet from April to September 2007. a Copepodids stage V, b females. Black circles correspond to formalin-fixed samples, white circles to in vivo observations. Grey undergrounds represent periods of high chlorophyll a concentration
Although the percentage of CV having droplets showed a seasonal trend, the median volume of this lipid droplet (Fig. 5a) remained stable throughout the season around an overall median of 0.35 mm3ind−1. The maximal values, however, were twice as high in July and September as they were earlier in the season (ca. 1.2 instead of 0.6 mm3 ind−1). The droplet volume distribution around the median was rather symmetric except at the end of May. At this date, the distribution was shifted towards higher values, whereas the percentage of CV having a droplet showed a decrease. Concerning females having droplets (Fig. 5b), all medians but one were homogeneous (overall median, 0.05 mm3 ind−1). At the end of May, the median was significantly higher than the others (median test, P < 0.001), meaning that the median volume increased in spring and decreased in summer. The distributions around the medians were asymmetric in favor of low values, especially in September although the maximal value was the highest.
Oithona similis. Seasonal changes of the lipid droplet volume, in vivo observations from April to September 2007. a Copepodids stage V, b females. Boxes limits represent the 25 and 75 percentiles around the median; whiskers extend from the maximal to the minimal values. Asterisk significantly higher median (P < 0.001). Copepods devoid of lipid droplet were excluded
Interstage variability
Data obtained in July showed that copepodids stored neutral lipid in a droplet at least from stage III (Fig. 6). The ratio between lipid droplet volume and prosome volume was not significantly different in CIII, CIV and CV (overall median, 5.20%), meaning that these copepodids accumulated lipid reserves in proportion to their growth. Conversely, females stored lower amount lipid than the three other stages (median test, P < 0.0001). Considering the whole period of study (Fig. 5), the difference between females and CV was confirmed, lipid droplets being significantly larger in the latest (Friedman ANOVA on ranks, P = 0.02).
Oithona similis. Percentage of lipid droplet volume/prosome volume in different copepodid developmental stages, in vivo observations in July 2007. C III to V copepodids stage III to V, fem females. Boxes limits represent the 25 and 75 percentiles around the median; whiskers extend from the maximal to the minimal values. Asterisk significantly lower median (P < 0.001). Copepods devoid of lipid droplet were excluded
Prosome length variability
The prosome length of the copepods was not correlated to the droplet area, except for females in September (Pearson correlation, P < 0.01). The prosome lengths in samples from different seasons were homogeneous in CV. In females, a significant variation was found (ANOVA, P < 0.01) due to lower values in April. The lipid content did not seem to be driven by the size of the copepods; thus, lipid droplets volume values are given per individual.
Discussion
Optical estimation of lipid reserves
In previous studies on marine planktonic organisms, Nile Red staining coupled with image analysis have been used successfully to estimate the total amount of neutral lipids (Carman et al. 1991; Tankersley 1998). In those cases, the neutral lipids were allocated in small droplets throughout the body and Nile Red fluorescence signal from one focal distance was a good estimate of the total amount of neutral lipids, rather than the area measurement of all droplets. We observed that O. similis stored neutral lipids in one or a few big droplets, already observed using transmitted light. Moreover, we showed that the integrated fluorescence intensity was proportional to the area of the droplet and was not affected by its thickness. The main reason is probably that the depth of focus associated with the magnification required to observe O. similis is very narrow compared to the thickness of the droplet. The contamination of the fluorescence signal at one focal distance from the vertical diffusion of fluorescence emitted from other levels was not sufficient to reflect the thickness of the droplet. With respect to O. similis, this study showed that image analysis of pictures taken under transmitted light brought as much information as analysis of pictures obtained after fluorescent staining.
Lipid droplet volume was extrapolated from droplet area in dorsal view assuming a perfect ellipsoidal shape. This latter assumption could lead to a slight overestimation of lipid content in some particular cases. Indeed, thickness and width were very similar in small droplet but when droplet size increased, thickness seemed to reach a maximum before length and width probably because droplet growth in that direction was limited by the gut. However, this potential overestimation concerned a limited number of copepods.
The copepods have to be observed alive for an accurate measurement of the droplets size. Though formalin fixation is not likely to affect the size of the lipid sac, the prosome of fixed copepods is often less transparent and its shape can be damaged. However, good results were obtained when considering only the presence or absence of droplet inside the prosome.
Droplet location and composition
A histological study described two types of lipid deposits in calanoid copepods (Blades-Eckelbarger 1991). The copepods presented either a large, thin-walled lipid sac containing a single large deposit of intracellular lipid enclosed by a very thin rim of cytoplasm, or discrete lipid deposits as cytoplasmic droplets in cells near the anterior part of the mid-gut. Although we showed no evidence of any structure for accumulation of lipids, the lipid deposits observed in O. similis could be described as oil sacs with respect to these definitions.
Our results from September suggested that O. similis’s lipid sacs were made up of wax esters, which are known as the major storage lipids in high latitude species (Lee et al. 2006). Although, lipid classes present in O. similis have not been totally described yet, wax esters are known to be part of the lipid storages since Lischka and Hagen (2007) found significant seasonal variations of the total fatty alcohols amount, as wax esters estimate, in Kongsfjorden. In Antarctica, high amounts of fatty alcohols were found in O. similis males, but relatively lower in females (Kattner et al. 2003). In the present study, we showed that O. similis also contained triacylglycerols (TAG) in lower proportion, but this compound did not seem to be linked to the lipid sac. Similarly, Miller et al. (1998) showed that Calanus finmarchicus stores only wax esters inside the oil sac and concluded that TAG storage takes place elsewhere. Since we only investigated lipid sacs, we were not able to gather information about inter-individual variability or seasonal variability of TAG throughout the body, leaving open the question of their role as energy reserves in O. similis.
Accumulation of lipids at the onset of the spring bloom
We observed that the proportion of both females and CV with visible lipid reserves increased rapidly between 22 April and 3 May, with at least 90% of copepods containing lipid reserves at the later date (Fig. 4). Considering individuals having droplet, the median droplet volume did not change significantly during this period (although the maximal size did, Fig. 5), implying that this increase of lipid reserves occurred at the population level. Conversely, during the maximum of phytoplankton biomass around mid May, the increase of lipid storage in O. similis was observed at the individual level both in CV and females. The median size of the droplets increased while fewer copepods contained droplets, reflecting individuals with positive and negative energetic budget.
Strikingly, we showed an increase of lipid content starting even before the accumulation of phytoplankton biomass in the water column (from 5 May). This apparent mismatch supports the hypothesis that at least those two stages of O. similis took advantage of other food sources than the blooming algae to accumulate lipids. O. similis is known to have an omnivorous feeding regime, eating preferentially ciliates and other nano- and micro-zooplankton (Castellani et al. 2005a; Lonsdale et al. 2000), but also fecal pellets and detritus, although this matter is still under discussion (Reigstad et al. 2005; Svensen and Nejstgaard 2003). Moreover, in Kongsfjorden, the highest food quality in particulate organic matter (i.e., high proportion of polyunsaturated fatty acids) has been encountered before the maximum biomass in spring (Leu et al. 2006). This observation, supported by other in situ and experimental studies (respectively, Hayakawa et al. 1996; Parrish et al. 2005), indicates that during the onset of the bloom the algae prioritize polar lipid production for rapid growth. Thus, O. similis could have benefited from this high nutritional value and high production at the beginning of the bloom to build up neutral lipid reserves. Similariy, females of Calanus glacialis and Calanus finmarchicus, seem to profit of the high nutritional quality in particulate matter prior to the peak of the bloom (Leu et al. 2006; Wold et al. 2007). But in these cases, the authors suggested that phospholipids rather than neutral lipids are synthesized first, in order to support egg production.
Our results underlined an increase of neutral lipids in spring, whereas Lischka and Hagen (2007), who conducted a seasonal survey in the same fjord in 1998–1999, concluded that O. similis does not take advantage of the spring bloom. After a decrease during winter, they noted the lowest wax ester content in spring (first half of May) for both CV and females. These differences could come from the different investigation methods used. We benefited from a better time resolution and looked into individual variability, allowing us to follow the lipid dynamics in greater details. We could also relate these differences to the contrasting environmental conditions during the two studies. The winter was cold and the fjord filled with sea ice when the previous study took place, while the temperatures were milder and the fjord ice-free during ours. O. similis could have thus exhibited two different life strategies, underlining its high adaptive capacity to various food conditions.
Lipid reserves and reproduction
Between the end of May and the beginning of July, we observed a decrease of both median droplet size and droplet occurrence in females (Figs. 4b, 5b). This high lipid consumption was most probably associated with an energetic demand from egg production, since June corresponds to the main reproduction peak of O. similis at this location (Lischka and Hagen 2005). It seems, from our study, that reproduction in June relied on newly accumulated lipids rather than last autumn’s reserve as Lischka and Hagen (2007) concluded.
From this earlier study, the second reproduction peak (August–September and presumably October) is supported directly by the summer phytoplankton blooms and wax esters are stored at the same time, reaching maximal values in August–September (Lischka 2006). If we looked at our data in terms of average droplet volume, we would observe a clear increase between July and September as well. But from a median point of view, the lipids reserves were stable and the proportion of copepods having droplets was decreasing during the summer. Our individual-based study revealed huge intra-population variability in September. The low droplet median volume in females was hiding a high inter-individual variability due to a limited number of individuals with very big droplets, while almost half of the population had no droplet at all. This observation could be interpreted as some females stopping reproduction or taking a better advantage of the summer blooms than others or, more probably, as a significant input of newly molted females from the lipid rich CV stock. In other words, this could be associated with the overlap of two generations: rich CV that just developed into females and the “old” females that are still reproducing. Moreover, Sabatini and Kiørboe (1994) showed that O. similis females are rather long-lived (up to at least 50 days). The lipid rich females could be the youngest ones and could reproduce in autumn, when this species is known to undergo a major reproduction event (Lischka and Hagen 2005). Even if this copepod is known to remain active during winter, the food is supposed to be limiting and this huge lipid reserve could also help to survive the winter and ensure next year generations (Lischka et al. 2007).
Lipid reserves as life-cycle strategy or short-term adaptation?
Comparably, a study on Calanus finmarchicus from the Georges Bank showed that this copepod is either lipid poor or lipid rich at the end of the productive season. In this case, individuals with large lipid reserves are ready to overwinter in deep layers, whereas individuals that are not rich enough will stay active in the upper layer (Miller et al. 2000). In the case of O. similis, the cohorts are not synchronous due to the permanent production of eggs throughout the year (Ashjian et al. 2003; Lischka and Hagen 2005). Thus, the identified stage of a copepod does not necessarily indicate its age, and this is especially true for females which may live longer. The extreme intra-population variability could therefore be linked to the year-round reproduction of this species rather than to two different overwintering strategies. Similarly, Norrbin (1991) noticed less seasonality in visible lipid storage of Microcalanus than in Pseudocalanus, and hypothesized that it could be related to its smaller size. Because small copepods have higher weight-specific metabolic rates, they need a constant food supply, leading to a longer period of activity. The presence of wax esters, as long-term energy reserves, is typical in species which show a drastic seasonality in their life cycle (Falk-Petersen et al. 2006; Lee et al. 2006). However, O. similis has the ability to store wax esters although it feeds omnivorously and continuously, as identified by Lischka and Hagen (2007). The lipid stores observed in the present study might be an adaptation to short-term food variability rather than a seasonal pattern.
Importance of individual variability in ecological studies
Using classical biochemical methods to study small copepods like O. similis, the need of sufficient amount of material leads to a loss of information about individual variability, tens to hundreds of animals being pooled for one analysis. Here, using image analysis, we observed not only seasonal variation in O. similis lipid content but also got informative individual variability.
Overall values of lipid content are meaningful when looking from a food supply point of view for upper trophic levels, as it represents the average energetic value of the ingested food, although the heterogeneity of the preys is surely significant. But investigating the lipid reserves of a population at the individual level brings new insights on the life strategy of the species and allows a better understanding of the pelagic ecosystem (Båmstedt 1988).
Conclusion
In this study, we underlined new features on Oithona similis’s ecology, opening questions on its management of lipid reserves, which seemed to differ from the traditional seasonal pattern described in Calanus spp. The image analysis method used here appeared to be a very good tool especially for the small sized copepods, because questions at this level of variability cannot be answered using the traditional biochemical analysis.
References
Ackman RG (1981) Application of flame ionization detectors to thin layer chromatography on coated quartz rods. Method Enzymol 72:205–252
Alonzo F, Mayzaud P (1999) Spectrofluorometric quantification of neutral and polar lipids in zooplankton using Nile red. Mar Chem 67:289–301
Arts MT, Evans MS (1991) Optical-digital measurements of energy reserves in calanoid copepods: intersegmental distribution and seasonal patterns. Limnol Oceanogr 36:289–298
Ashjian CJ, Campbell RG, Welch HE, Butler M, Van Keuren D (2003) Annual cycle in abundance, distribution, and size in relation to hydrography of important copepod species in the western Arctic Ocean. Deep-Sea Res I 50:1235–1261
Atkinson A, Sinclair JD (2000) Zonal distribution and seasonal vertical migration of copepod assemblages in the Scotia Sea. Polar Biol 23:46–58
Auel H, Hagen W (2002) Mesozooplankton community structure, abundance and biomass in the central Arctic Ocean. Mar Biol 140:1013–1021
Båmstedt U (1988) Ecological significance of individual variability in copepods bioenergetics. Hydrobiologia 167(168):43–49
Blades-Eckelbarger PI (1991) Comparative ultrastructure of lipid storage sites in female Euchaeta marina and Pleuromamma xiphias (Copepoda: Calanoida). Mar Biol 108:49–58
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Carman KR, Thistle D, Ertman SC, Foy M (1991) Nile red as a probe for lipid-storage products in benthic copepods. Mar Ecol Prog Ser 74:307–311
Castellani C, Irigoien X, Harris RP, Lampitt RS (2005a) Feeding and egg production of Oithona similis in the North Atlantic. Mar Ecol Prog Ser 288:173–182
Castellani C, Robinson C, Smith T, Lampitt RS (2005b) Temperature affects respiration rate of Oithona similis. Mar Ecol Prog Ser 285:129–135
Conover RJ, Huntley M (1991) Copepods in ice-covered seas—distribution, adaptations to seasonally limited food, metabolism, growth patterns and life cycle strategies in polar seas. J Mar Syst 2:1–41
Cottier F, MacLachlan S, Howe J (2005) Rapid Shifts in Arctic marine climate. Ocean Challenge 14:16–22
Daase M, Eiane K (2007) Mesozooplankton distribution in northern Svalbard waters in relation to hydrography. Polar Biol 30:969–981
Falk-Petersen S, Hopkins CCE, Sargent JR (1990) Trophic relationships in the pelagic, Arctic food web. In: Barnes MaG, R. N. (Eds) Trophic relationships in the marine environment. Proc. 24th Europ. Mar. Biol. Symp. Aberdeen University Press, pp 315–333
Falk-Petersen S, Timofeev S, Pavlov V, Sargent JR (2006) Climate variability and possible effects on arctic food chains: The role of Calanus. In: Ørbæk JB, Tombre T, Kallenborn R, Hegseth E, Falk-Petersen S, Hoel AH (eds) Arctic Alpine Ecosystems and People in a Changing Environment. Springer Verlag, Berlin, pp 147–166
Fransz HG, Gonzalez SR (1995) The production of Oithona similis (Copepoda: Cyclopoida) in the Southern Ocean. ICES J Mar Sci 52:549–555
Gallienne CP, Robins DB (2001) Is Oithona the most important copepod in the world oceans? J Plankton Res 23:1421–1432
Goulden CE, Hornig LL (1980) Population oscillations and energy reserves in planktonic Cladocera and their consequences to competition. Proc Natl Acad Sci 77:1716–1720
Greenspan P, Mayer EP, Fowler SD (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100:965–973
Hagen W, Auel H (2001) Seasonal adaptations and the role of lipids in oceanic zooplankton. Zoology 104:313–326
Hansen AS, Nielsen TG, Levinsen H, Madsen SD, Thingstad TF, Hansen BW (2003) Impact on changing ice cover on pelagic productivity and food web structure in Disko Bay, West Greenland: a dynamic model approach. Deep Sea Res I 50:171–187
Hayakawa K, Handa N, Kawanobe K, Wong CS (1996) Factors controlling the temporal variation of fatty acids in particulate matter during a phytoplankton bloom in a marine mesocosm. Mar Chem 52:233–244
Hop H, Falk-Petersen S, Svendsen H, Kwasniewski S, Pavlov V, Pavlova O, Søreide JE (2006) Physical and biological characteristics of the pelagic system across Fram Strait to Kongsfjorden. Prog Oceanogr 71:182–231
Kattner G, Albers C, Graeve M, Schnack-Schiel SB (2003) Fatty acid and alcohol composition of the small polar copepods, Oithona and Oncaea: indication on feeding modes. Polar Biol 26:666–671
Lee RF, Hagen W, Kattner G (2006) Lipid storage in marine zooplankton. Mar Ecol Prog Ser 307:273–306
Leu E, Falk-Petersen S, Kwasniewski S, Wulff A, Edvardsen K, Hessen DO (2006) Fatty acid dynamics during the spring bloom in a high Arctic fjord: importance of abiotic factors versus community changes. Can J Fish Aquat Sci 63:2660–2779
Lischka S (2006) Life-history traits of the copepods Pseudocalanus minutus (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjorden (Svalbard) with particular emphasis on seasonality. Dissertation, Universität Bremen
Lischka S, Hagen W (2005) Life history and seasonal variability in abundances of Pseudocalanus minutus (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjord (Svalbard). Polar Biol 28:910–921
Lischka S, Hagen W (2007) Seasonal lipid dynamics of the copepods Pseudocalanus minutus (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjorden (Svalbard). Mar Biol 150:443–454
Lischka S, Giménez L, Hagen W, Ueberschär B (2007) Seasonal changes in digestive enzyme (trypsin) activity of the copepods Pseudocalanus minutus (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjorden (Svalbard). Polar Biol 30:1331–1341
Lonsdale DJ, Caron DA, Dennett MR, Schaffner R (2000) Predation by Oithona spp. on protozooplankton in the Ross Sea, Antarctica. Deep Sea Res II 47:3273–3283
Lorenzen CJ (1966) A method for a continuous measurement of in vivo chlorophyll concentration. Deep Sea Res 13:223–227
Mayzaud P, Albessard E, Cuzin-Roudy J (1988) Changes in lipid composition of the Antarctic krill Euphausia superba in the indian sector of the Antarctic Ocean. Distribution among organs and sexual maturity stage. Mar Ecol Prog Ser 173:149–162
Miller CB, Morgan CA, Prahl FG, Sparrow MA (1998) Storage lipids of the copepod Calanus finmarchicus from Georges Bank and the Gulf of Maine. Limnol Oceanogr 43:488–497
Miller CB, Crain JA, Morgan CA (2000) Oil storage in Calanus finmarchicus. ICES J Mar Sci 57:1786–1799
Nielsen TG, Møller EF, Satapoomin S, Ringuette M, Hopcroft RR (2002) Egg hatching rate of the cyclopoid copepod Oithona similis in arctic and temperate waters. Mar Ecol Prog Ser 236:301–306
Norrbin MF (1991) Gonad maturation as an indication of seasonal cycles for several species of small copepods in the Barents Sea. Polar Res 10:421–432
Paffenhöfer GA (1993) On the ecology of marine cyclopoid copepods (Crustacea, Copepoda). J Plankton Res 15:37–55
Parrish CC, Thompson RJ, Deibel D (2005) Lipid classes and fatty acids in plankton and settling matter during the spring bloom in a cold ocean coastal environment. Mar Ecol Prog Ser 286:57–68
Reigstad M, Wexels Riser C, Svensen C (2005) Fate of copepod faecal pellets and the role of Oithona spp. Mar Ecol Prog Ser 304:265–270
Sabatini M, Kiørboe T (1994) Egg production, growth and development of the cyclopoid copepod Oithona similis. J Plankton Res 16:1329–1351
Schauer U, Fahrbach E, Osterhus S, Rohardt G (2004) Arctic warming through the Fram Strait: oceanic heat transport from 3 years of measurements. J Geophys Res (Oceans) 109:C06026. doi:10.1029/2003JC001823
Svensen C, Nejstgaard JG (2003) Is sedimentation of copepod faecal pellets determined by cyclopoids? Evidence from enclosed ecosystems. J Plankton Res 25:917–926
Tankersley RA (1998) Fluorescence techniques for evaluating the lipid content of larval and juvenile freshwater mussels. In: Survey OB (eds) Proceedings of the conservation, captive care, and propagation of freshwater mussels symposium. pp 115–125
Turner JT (2004) The importance of small planktonic copepods and their roles in pelagic marine food web. Zool Stud 43:255–266
Walkusz W, Storemark K, Skau T, Gannefors C, Lundberg M (2003) Zooplankton community structure; a comparison of fjords, open water and stations in the Svalbard area. Pol Polar Res 24:149–165
Ward P, Hirst AG (2007) Oithona similis in a high latitude ecosystem: abundance, distribution and temperature limitation of fecundity rates in a sac spawning copepod. Mar Biol 151:1099–1110
Wold A, Leu E, Walkusz W, Falk-Petersen S (2007) Lipids in copepodite stages of Calanus glacialis. Polar Biol 30:655–658
Acknowledgments
The authors wish to thank Janine Cuzin-Roudy, Haakon Hop and three anonymous reviewers for constructive comments on the manuscript. The sampling logistic was assisted by Kings Bay AS, the French Polar Institute-Institut Paul Emile Victor (IPEV), the Norwegian Polar Institute (NPI) and Margaux Noyon. Marc Boutoute performed the Chla fluorometric measurements. The fieldwork season was funded by IPEV through the PRACEAL project, and NPI through an Arktisstipend scholarship. This study was supported by the ARCTOS Research Network. This is a contribution to the EUR-OCEANS Network of Excellence funded by the European Commission (contract WP4-SYSAN-1058). F. Narcy is supported by a EUR-OCEANS scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narcy, F., Gasparini, S., Falk-Petersen, S. et al. Seasonal and individual variability of lipid reserves in Oithona similis (Cyclopoida) in an Arctic fjord. Polar Biol 32, 233–242 (2009). https://doi.org/10.1007/s00300-008-0524-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-008-0524-y