Abstract
The annual trend in energy storage in the Antarctic minke whale was examined using catch data from all 18 survey years in the Japanese Whale Research Program (JARPA). Regression analyses clearly showed that blubber thickness, girth and fat weight have been decreasing for nearly 2 decades. The decrease per year is estimated at approximately 0.02 cm for mid-lateral blubber thickness and 17 kg for fat weight, corresponding to 9% for both measurements over the 18-year period. Furthermore, “date”, “extent of diatom adhesion”, “sex”, “body length”, “fetus length”, “latitude”, “age” and “longitude” were all identified as partially independent predictors of blubber thickness. The direct interpretation of this substantial decline in energy storage in terms of food availability is difficult, since no long-term krill abundance series is available. However, an increase in the abundance of krill feeders other than minke whales and a resulting decrease in the krill population must be considered as a likely explanation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Southern Ocean supports a food web with high productivity, and is an important feeding ground for many consumers (see Laws 1985; Hill et al. 2006). The Antarctic krill (Euphausia superba) is the most abundant and main prey for large baleen whales, seals and seabirds. The Antarctic minke whale (Balaenoptera bonaerensis), which is a small baleen whale, also depends largely on E. superba (Ohsumi 1979; Armstrong and Siegfried 1991; Ichii and Kato 1991).
Krill availability in the Antarctic Ocean is generally considered to be the most important limiting factor for population sizes of many krill feeders (Reid and Croxall 2001; Mori and Butterworth 2006). Krill biomass is very large and many animals depend on this species, so that a sudden decrease in certain krill consumers could cause drastic changes in the population sizes of other consumers in Antarctic ecosystem. It has been hypothesized that after more than 50 years of commercial whaling, which started at the beginning of the twentieth century and resulted in the overexploitation of the large baleen whales, the relative abundances of all whale species were totally changed from the situation in the pre-whaling period (Laws 1977; Brown and Lockyer 1984). In parallel with the decline in abundance of the large baleen whales, such as blue (B. musculus), fin (B. physalus) and humpback whales (Megaptera novaeangliae), the minke whale population presumably started to grow (Laws 1977; Mori and Butterworth 2006). This population change hypothesis was recently simulated in a krill predator dynamics model for the Antarctic ecosystem (Mori and Butterworth 2006). According to the “whale reduction” or “krill surplus” hypothesis (Laws 1977), this growth is considered to be a result of abundant food supplies available to the minke whale after the decline of the large baleen whale populations. About 30 years have passed since the large-scale hunting of krill-feeding large baleen whales was stopped, and recent sighting surveys by Southern Ocean Whale and Ecosystem Research (SOWER) and the Japanese Whale Research Program under Special Permit in the Antarctic (JARPA) now suggest that the humpback and fin whales are starting to recover (Matsuoka et al. 2005). Branch (2007) and Branch et al. (2004) also report that the Antarctic blue whale (B. musculus) population has increased, although it is still small.
Whales generally accumulate fat during the summer feeding period at high latitudes and migrate to low latitude areas for reproduction (Næss et al. 1998). The Antarctic minke whale migrates from tropical latitudes around 10–30°S to the Antarctic (Kasamatsu et al. 1995), where it spends the austral summer feeding (Kato and Miyashita 1991; Kasamatsu et al. 1995). Whale blubber serves as energy storage in addition to its thermal function (Parry 1949; Lockyer et al. 1984), and it also gives the whale body a streamlined shape as described by Parry (1949). The fat reserves in blubber have commonly been used as an indication of body condition in whale studies (e.g. Lockyer et al. 1985a, b; Lockyer and Waters 1986; Víkingsson 1995; Koopman 1998; Ichii et al. 1998; Haug et al. 2002). Blubber thicknesses, blubber weight and whale girth have all been found to increase through the feeding season (Lockyer 1987; Víkingsson 1995; Næss et al. 1998). From the measurements presented in Lockyer et al. (1985b), it is reasonable to assume that blubber thickness is positively correlated with lipid content in the whale body.
The main objective of the present study was to examine trends in body condition of the Antarctic minke whale during a recent 18-year period.
Materials and methods
Sampling and measurements
Blubber thickness and all other variables used in the present investigation were measured in Antarctic minke whales taken by JARPA from 1987/1988 to 2004/2005. JARPA was started in 1987/88 under a special permit issued by the Government of Japan, based on Article VIII of the International Convention for the Regulation of Whaling. The survey period and sample sizes are shown in Table 1. The energetics of baleen whales differ according to their reproductive status and sex (Lockyer 1986; 1987; Aguilar and Borrell 1990; Víkingsson 1995). For instance, pregnant females have more lipid in the blubber than lactating females do (Aguilar and Borrell 1990). To avoid any bias resulting from growth or lactation, we used only mature males (n = 2,890) and pregnant females (n = 1,814), but not lactating females or immature animals, for the present analyses. Besides, mature males and pregnant females accounted for about 70% of all the JARPA samples (Table 1).
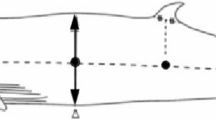
The research area includes the International Whaling Commission (IWC) Management Area III (East), Area IV, Area V and Area VI (West), which together cover the area from 35°E to 145°W, south of 60°S in austral summer seasons (Fig. 1). The two western areas and the two eastern areas, split at 130°E, were surveyed in alternate years so that the entire broad survey area was covered in two years. One or two Antarctic minke whales were randomly sampled from each school observed within three nautical miles of the research track line. The minke whales were captured by three vessels using explosive harpoons and a large-caliber rifle as a secondary weapon in the event that death was not instantaneous (International Whaling Commission 2003). After capture, the animals were placed aboard a research base vessel where they were examined. The sighting positions of the captured animals were recorded by each sighting/sampling vessel. After outer observations were completed, body length (from snout tip to tail fluke notch in a straight line along the deck) and other morphological measurements were taken. Blubber thickness was measured to the nearest mm, by dissecting perpendicularly from skin to muscle without including connective tissue or black surface skin. The measurement positions are shown in Fig. 2. The measurements were usually made on the left side of the animal, but in several cases they were made on the right side, mainly because of damage to the left side caused by the harpoon. Blubber thickness at the lateral position was measured in all whales. The reasons for choosing this particular lateral point for blubber thickness measurements were that skin surface and muscle fascia are parallel in this area, and blubber thickness is close to constant in an area around the measurement site. Any tension in the blubber tissue was released by cutting through the blubber layer down to the muscle fascia around the measurement site, but at a certain distance from this site. In the first animal caught each day, all the blubber including the ventral groove and visceral fat was also removed from the body and weighed to the nearest kilogram. In addition, half girth at the level of the umbilicus was measured in all whales. Sex and maturity were recorded for each whale on the basis of routine observations of reproductive organs during dissection and tissue observations in the laboratory. The age of each whale was determined from the growth layers in the earplug using a stereoscopic microscope. A diatom film is sometimes observed on the surface of the whales and may cover the entire body. The extent of diatom adhesion on the skin is assumed to be a rough indicator of how long a whale has spent in cold waters (Hart 1935; Ohno and Fujino 1952; Nemoto 1980; Best 1982) (Table 2). Fetus length was measured in the same way as adult body length. Biological data including blubber thickness, sex, body length of adult animals, fetus and diatom films were collected routinely by biologists, including some of the authors, on board a research vessel. These data were stored in the database on JARPA held by the Institute of Cetacean Research. Data used in the present study were obtained from this database.
Statistical analysis
Previous studies in balaenopterid whale species have shown that lateral blubber thickness varies seasonally from the middle part of the body towards the tail, while blubber thickness in the anterior part of the whale body is more constant (Næss et al. 1998; Konishi 2006). Visceral fat acts as a secondary energy storage site in the body cavity (Næss et al. 1998). Blubber thickness in the mid-lateral region and “fat weight” (blubber weight + visceral fat) were therefore used as body condition indicators in this study (Fig. 2). A total of 4,704 Antarctic minke whales (mature males and pregnant but not lactating females) were examined on board the mother research vessel during the JARPA program (Tables 1, 2, 3). Blubber thickness measurements at the lateral position shown in Fig. 2 were available from 4,689 of these. The discrepancy is largely explained by the fact that in some whales, the blubber at the measurement points on both sides of the body was damaged. Half girth measurements were available for 4,681 animals. Fat weight was available from only 740 whales.
Regression analysis was therefore first carried out on blubber thickness at the lateral position in 4,689 whales. All other variables except age, body weight, fetus length and total fat weight were available for all 4,704 whales. Fetus lengths were of course only available for the females, and mean fetus lengths were used for fourteen pairs of twins. Age was available for 4,268 whales, with missing whales randomly distributed over the 18 years. Body weights were not measured during the first survey year (1987/1988), because no weighing machine had been installed on the mother research vessel. Regression analyses were therefore carried out first independently for males and females and without body weight as a possible independent variable.
The time spent in the feeding area, and geographical and biological variables that could possibly be related to blubber thickness, should be considered in body condition analyses. To take these factors into account and to possibly exclude some of them, we conducted stepwise multiple linear regression analyses, using average values for the missing values.
At each step of the regression analysis, the next variable was included in the equation if the corresponding P value was below 5%. In an adaptation to a recently developed criteria-based subset selection procedures, we also calculated the Bayesian information criterion (BIC) (Schwarz 1978), formulated as
where L is likelihood and K is the number of parameters. At each step of the regression analysis, the BIC value was calculated. According to this method, the model with the lowest BIC value should be preferred.
In the first set of runs, blubber thickness at the lateral position (in centimeter) was the dependent variable as a body condition indicator, because of the large sample size for this variable. We allowed the following independent variables for separate regression analyses for mature males and pregnant females: “date” (December 1st = day 1), “diatom adhesion” (0–4, Table 2), “latitude (degree)”, “longitude” (in degrees east), “body length” (in meter) and, for females, “fetus length” (in centimeter). “Year” was also included as an independent variable to investigate a possible annual trend (87/88 = 1 88/89 = 2 89/91 = 3…). This regression was also carried out without “fetus length” as a possible independent variable for females to see the effect of this change on the other regression coefficients.
In a second set of runs, the regression analyses were carried out for all 4,689 whales, including “sex” (1: male, 2: female) as a possible explanatory variable, using mean values for the missing values. “Fetus length” is of course only available for females, and assigning mean “fetus length” to all males is likely to reduce the regression coefficient for this variable in the analysis where males and females are combined. To illustrate this effect and to show that the main conclusions from the regression analysis are unaltered, the regression was carried out with and without “fetus length” as a potential explanatory variable. “Body weight” is also considered to be an important variable in studies of energetics of animals, but it is strongly correlated with other variables. To examine how “body weight” affects the results, the regression was carried out both with and without “body weight” as a potential explanatory variable.
In addition, we conducted other stepwise multiple linear regression analyses using either “fat weight” or “half girth” as dependent variables to confirm, using the corresponding sub-samples of the whales, whether other body condition indicators showed a similar decline in these variables over the 18 JARPA years. These analyses were carried out for 740 and 4,681 whales respectively, using average values for the few missing values. We allowed the following independent variables: “date”, “diatom adhesion”, “latitude”, “longitude” (in degrees east), “body length”, “sex”, “age”, and “year”. For runs with “fat weight” as the dependent variable, we calculated “lean body weight” (= “body weight” − “fat weight”) for each animal and used this new variable as a possible predictor variable instead of “body weight”.
Results
Although we used only mature males and pregnant but not lactating females, there was a wide range of values for the variables used (Table 3). Blubber thickness at the lateral point ranged from 1.1 to 7.2 cm in mature males, and from 1.5 to 7.7 cm in pregnant females. Half girth at umbilical position ranged from 162 to 269 cm in mature males, and 175–291 cm in pregnant females. Fat weight ranged from 1.36 to 4.56 t in mature males, and 2.30–5.51 t in pregnant females.
The results from the regression analyses are presented in Tables 4, 5, 6 and 7. The format of the tables is as follows: the first line in each table represents the constant in the regression equation. Each of the next lines represents one explanatory variable. The variables are listed from the top of the table in the order in which they were entered into the equation during the stepwise procedure. Variables that were not included in the regression equation at the 5% level are listed below the table. The numbers in the column captioned “R square” show the value of R square after the corresponding variable has been entered into the equation (in addition to all variables above it). The values in the column captioned “F value” show the value of F for inclusion of this variable before its inclusion. The values in the other columns show values of the corresponding parameters for the final model.
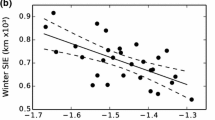
In the first three runs the two sexes were analysed separately, and females both with and without “fetus length” were used as a possible explanatory variable (Table 4). “Date” in mature males and “fetus length” in pregnant females were the best predictors of blubber thickness at the lateral point. “Date” was an important predictor variable in the other two runs as well. “Year” was included as a predictor of blubber thickness at the 5% level in all three runs and gave a similar regression coefficient. In the next set of runs, where the two sexes were pooled and “sex” was included as a possible predictor variable (Table 5), all independent variables were included as predictors at the 5% level In the first run, “date” was the best predictor of blubber thickness, followed by “diatom”, “sex”, “longitude”, “year”, “age”, “latitude” and “body length”. The results of the other runs show very similar results, although “age” was not included as a predictor variable in the second run at the 5% level (Table 5b). In all these analyses (Tables 4, 5), the point estimates of the coefficients for “year” ranged from −0.0180 to −0.0280 cm per year, showing that the blubber thickness of the Antarctic minke whale at the lateral point has decreased substantially over the years (Tables 4, 5, Fig. 3a). The results further indicate that blubber thickness increased with time spent feeding, and from west to east and north to south. Blubber thickness also increased with diatom adhesion level and fetus length. The blubber layer was found to be approximately 0.33 cm thinner in males than in females. The order of inclusion of independent variables in the regression equations did not differ between the procedure using a P value based criterion and that using BIC except for a small and unimportant difference in Table 5a. In all regression runs, the minimum BIC model included “date”, “diatom” and “year” as predictor variables.
Temporal trends in body condition indicators for the Antarctic minke whale in its feeding season, from the results of stepwise multiple regressions. Regression lines were drawn using the population mean for all the other predictor variables than “year”. Open circles represent residuals from the regression line. The three figures are based on the following tables: a Table 5a, b Table 6, c Table 7
The runs using “fat weight” and “half girth” as dependent variables also gave similar results (Tables 6, 7), showing “year” to be a significant independent variable. “Fat weight” was found to decrease by approximately 17 kg/year and “half girth” by approximately 0.46 cm/year (Fig. 3b, c), corresponding to 0.92 cm/year for the total girth and 17 cm (4%) for the 18-year JARPA period. The results were similar when the two sexes were analysed separately.
Discussion
The results of the stepwise regression analyses demonstrate that many factors affect the body condition of the Antarctic minke whale. Not unexpectedly, there is a significant increase in blubber thickness and fat weight during the feeding season (“date”). This result clearly confirms that the fattening is caused by intensive feeding in the Antarctic Ocean after long-distance migration from the reproductive areas. Similar seasonal fattening has been documented in fin whales (Lockyer 1987; Víkingsson 1995) and in common minke whales (Næss et al. 1998) in the North Atlantic. Fattening and diatom adhesion are also positively correlated, confirming that the extent of this adhesion can be used as a measure of how long a whale has spent in cold water. The results also show that blubber thickness and total fat weight increase from west to east and with increasing length of the fetus. Fetus length was one of the most important predictors of blubber thickness in the pregnant females in the JARPA data set, suggesting that departure from the reproductive area and the timing of conception in the minke whale are related.
The results of the statistical analyses clearly show that blubber thickness, fat weight and girth in Antarctic minke whales have decreased during the 18-year JARPA research period. Analyses of residuals show that there has been a linear decline in blubber thickness at the lateral position, fat weight and girth over this period. The total magnitude of the decline over these 18 years is 9% for blubber thickness in the lateral position, 9% for fat weight and 4% for girth measurements. The inclusion or exclusion of other independent variables in the regression equations resulted in only small changes in these results, showing that the finding of a substantial decline in fat storage is a robust result. This is the first time a long-term decline in energy storage in minke whales has been demonstrated, although Ohsumi et al. (1997) found some non-significant indications of a decline in blubber thickness in the lateral position starting in the early 1980s. Our result shows a decrease of 0.02 cm/year in blubber thickness at the lateral position over a period of 18 years. This is roughly equivalent to 36 intensive summer feeding days, indicating that the overall decrease in energy storage in Antarctic minke whale must be large.
The results primarily indicate an increasing shortage of food for the Antarctic minke whale over the last two decades and perhaps even longer. The Antarctic minke whale depends largely on the Antarctic krill (Ohsumi 1979; Armstrong and Siegfried 1991; Ichii and Kato 1991), so the amount of krill available for the minke whales must have declined in its feeding areas. Kawamura (1978) mentioned that there was some surplus of krill after populations of the large baleen whales had been depleted by hunting and up to the 1970s. And Clapham and Brownell (1996) noted that there is no evidence that baleen whales are resource-limited. However, our results clearly show that baleen whales are resource-limited animals, indicating that the minke whale population may be affected by krill availability.
Environmental change could perhaps have been an important causal factor, since this is known to affect krill abundance. The abundance of krill around the Antarctic Peninsula is known to have declined since the 1970s, because high temperatures have resulted in a decrease in the extent of the sea ice (Loeb et al. 1997; Atkinson et al. 2004). But no such environmental trend has been observed in austral summer in the JARPA survey area (Watanabe et al. 2006). Of course, other environmental factors could have been responsible. Unfortunately, information about long-term trends in krill abundance is not available from this area.
Interspecies competition for krill is another possible explanation for the decline in body condition of the Antarctic minke whale. There is overlap between the habitats of different whale species (Kasamatsu et al. 2000), suggesting the possibility of interference between the different species in Antarctic waters. Sighting surveys carried out by the Southern Ocean Whale and Ecosystem Research (SOWER) program and JARPA have demonstrated the recent recovery of humpback (M. novaeangliae) and fin whales (B. physalus) (Matsuoka et al. 2005). Branch (2007) and Branch et al. (2004) also report that the Antarctic blue whale (B. musculus) population has increased, although it is still small. These large whales also feed on the Antarctic krill and thus share the same food niche as the minke whale (Nemoto 1962; Kawamura 1980). Interestingly, humpback whales have recently shifted their distribution further south to areas where minke whales now also occur (Matsuoka et al. 2005; see Nishiwaki et al. 2006). Friedlaender et al. (2006) showed niche separation between humpback and Antarctic minke whales on the western side of the Antarctic Peninsula. Ballance et al. (2006) also mentioned that in the Southern Ocean, the bottom-up effect is more likely to occur than the top-down effect, because of physical and biological factors such as ice extent that cause year-to-year variations in krill abundance. However, krill abundance varies in a roughly 5-year cycle (see Ballance et al. 2006), which is considerably shorter than the period covered by this study. Moreover, a recent population trend model, which includes krill, four baleen whale species and two seal species in the Antarctic ecosystem, gives evidence that these predator species interact (Mori and Butterworth 2006). Another example of competition for food was reported between Antarctic fur seals Arctocephalus gazella and Macaroni penguins Eudyptes chrysolophus, although in this case it was regional (Ballance et al. 2006). These results suggest that competition for food may affect Antarctic minke whales, in addition to other factors.
Competition with krill predators other than baleen whales may be important. Reilly et al. (2004) estimated that consumption by whales corresponds to 4–6% of the estimated krill biomass in the South Atlantic sector around the Antarctic Peninsula. Although our study area was in another part of the Antarctic Ocean, interactions between baleen whales and other krill consumers must be considered. Much information is available on other krill predators, such as penguins and seals, in Antarctic waters. However, unlike baleen whales, these animals live, feed and breed in or near Antarctic waters throughout the year, and are mainly affected by climate and the extent of the sea ice in the winter season. For example, the Adélie penguin (Pygoscelis adeliae) population is primarily affected by the extent of the sea ice in winter (Wilson et al. 2001). Populations of phocid seals such as crabeater seals, Weddell seals (Leptonychotes weddellii) and leopard seals (Hydrurga leptonyx) also show periodic fluctuations every 5 years, which are related to the El Niño-Southern Oscillation (ENSO) (Testa et al. 1991). Thus, we do not have enough data to understand the interactions between whales and other krill consumers, and need further studies so that all possible explanations for the decline in body condition of the Antarctic minke whale can be considered.
Global warming and climate change could cause changes in the Southern Ocean ecosystem. Population changes that are considered to be caused by warming have already been reported in penguins (Fraser et al. 1992), pinnipeds (Reid and Croxall 2001, Weimerskirch et al. 2003) and invertebrates (Loeb et al. 1997) around the Antarctic Peninsula, where rapid regional warming was been observed (Vaughan et al. 2001). Although there are no obvious signs of warming in the present study area, this process may have serious effects on krill-feeding animals in the near future.
Understanding how krill demography is affected by changes in physical environmental factors and by predator consumption, and how, in turn, this influences predator reproduction and survival, is an essential basis for predicting future change in the Antarctic marine ecosystem (Reid and Croxall 2001). However, investigating and understanding the dynamics of the widely distributed krill population is quite difficult, so that monitoring energy storage by a krill consumer, such as the minke whale, can be most useful. Recent studies of upper trophic-level predators, such as seabirds, penguins and pinnipeds, indicate Antarctic ecosystem changes (Reid and Croxall 2001; Weimerskirch et al. 2003). Understanding the status of the Antarctic minke whale is one of the keys to predicting changes in the populations of krill consumers, and also of crucial importance in detecting food web interactions and changes in the krill population itself. Standardized krill abundance surveys are needed throughout the area where Antarctic minke whales and large krill-eating large baleen whales occur. The JARPA survey has been carrying out krill abundance surveys for several years, and the results are useful in interpreting the interactions identified in the analyses.
References
Aguilar A, Borrell A (1990) Patterns of lipid content and stratification in the blubber of fin whales (Balaenoptera physalus). J Mamm 71:544–554
Armstrong AJ, Siegfried WR (1991) Consumption of Antarctic krill by minke whales. Ant Sci 3:13–18
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Ballance LT, Pitman RL, Hewitt RP, Siniff DB, Trivelpiece WZ, Clapham PJ, Brownell LB Jr (2006) In: Estes A et al. (eds) Whales, whaling and ocean ecosystems. University of California Press, pp 215–230
Best PB (1982) Seasonal abundance, feeding, reproduction, age and growth in minke whales off Durban (with incidental observations from the Antarctic). Rep Int Whale Comm 32:759–786
Branch TA (2007) Abundance of Antarctic blue whales south of 60° S from three complete circumpolar sets of surveys. J Cetacean Res Manage 9:253–262
Branch T, Matsuoka K, Miyashita T (2004) Evidence for increases in Antarctic blue whales based on Bayesian modelling. Mar Mamm Sci 20:726–754
Brown SG, Lockyer CH (1984) Whales. In: Laws RM (ed) Antarct Ecol, vol 2. Academic Press, Cambridge, pp 717–781
Clapham PJ, Brownell RL Jr (1996) The potential for interspecific competition in baleen whales. In: Report of international whaling commission SC/48/SH27 46: 361–367
Fraser W, Trivelpiece W, Ainley D, Trivelpiece S (1992) Increases in Antarctic penguin populations: reduced competition with whales or a loss of sea ice due to environmental warming? Polar Biol 11:525–531
Friedlaender AS, Lawson GL, Haplin PN (2006) Evidence of resource partitioning and niche separation between humpback and minke whales in Antarctica: implications for interspecific competition. In: International whaling commission scientific committee document SC/58/E32, 37 pp
Hart TJ (1935) On the diatoms of the skin film of whales, and their possible bearing on problems of whale movement. Discov Rep 10:247–282
Haug T, Lindstøm U, Nilssen KT (2002) Variation in minke whale (Balaenoptera acutorostrata) diet and body condition in response to ecosystem changes in the Barents Sea. Sarsia 87:409–422
Hill SL, Murphy EJ, Reid K, Trathan PN, Constable AJ (2006) Modelling Southern Ocean ecosystems: krill, the food-web, and the impacts of harvesting. Biol Rev 81:581–608
Ichii T, Kato H (1991) Food and daily food consumption of southern minke whales in the Antarctic. Polar Biol 11:479–487
Ichii T, Shinohara N, Fujise Y, Nishiwaki S, Matsuoka K (1998) Interannual changes in body fat condition index of minke whales in the Antarctic. Mar Ecol Prog Ser 175:1–12
International Whaling Commission (2003) Annex E report of the workshop on whaling killing methods and associated welfare issues. In: Annual report of the International Whaling Commission
Kasamatsu F, Nishiwaki S, Ishikawa H (1995) Breeding areas and southbound migrations of southern minke whales Balaenoptera acutorostrata. Mar Ecol Prog Ser 119:1–10
Kasamatsu F, Matsuoka K, Hakamada T (2000) Interspecific relationships in density among the whale community in the Antarctic. Polar Biol 23:466–473
Kato H, Miyashita T (1991) Migration strategy of southern minke whales in relation to reproductive cycle estimated from foetal length. Rep Int Whale Comm 41:363–369
Kawamura A (1978) An interim consideration on a possible interspecific relation in southern baleen whales from the viewpoint of their food habits. Rep Int Whal Commun 28:411–420
Kawamura A (1980) A review of food of Balaenopterid whales. Sci Rep Whales Res Inst 32:155–197
Koopman HN (1998) Topographical distribution of the blubber of harbor porpoises (Phocoena phocoena). J Mamm 79:260–270
Konishi K (2006) Characteristics of the blubber and body condition indicator for the Antarctic minke whales (Balaenoptera bonaerensis). Mamm Study 31:14–22
Laws RM (1977) Seals and whales of the southern ocean. Philos Trans R Soc Lond B 279:81–96
Laws RM (1985) The ecology of the Southern Ocean. Am Sci 73:26–40
Lockyer CH, McConnell LC, Waters TD (1985a) Body condition in terms of anatomical and biochemical assessment of body fat in North Atlantic fin and sei whales. Can J Zool 63:2328–2338
Lockyer C (1986) Body fat condition in Northeast Atlantic fin whales, Balaenoptera physalus, and its relationship with reproduction and food resource. Can J Fish Aquat Sci 43:142–147
Lockyer CH, McConnell LC, Waters TD (1984) The biochemical composition of fin whale blubber. Can J Zool 62:2553–2562
Lockyer CH, McConnell LC, Waters TD (1985b) Body condition in terms of anatomical and biochemical assessment of body fat in North Atlantic fin and sei whales. Can J Zool 63:2328–2338
Lockyer C, Waters T (1986) Weight and anatomical measurements of Northeastern Atlantic fin (Balaenoptera physalus, Linnaeus) and sei (B. borealis, Lesson) whales. Mar Mamm Sci 2:169–185
Lockyer C (1987) The relationship between body fat, food resource and reproductive energy costs in north Atlantic fin whales (Balaenoptera physalus). Symp Zool Soc Lond 57:343–361
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900
Matsuoka K, Hakamada T, Kiwada H, Murase H, Nishiwaki S (2005) Abundance increases of large baleen whales in the Antarctic based on the sighting survey during Japanese whale research program (JARPA). Global Environ Res 9:105–115
Mori M, Butterworth DS (2006) A first step towards modelling the krill-predator dynamics of the Antarctic ecosystem. CCAMLAR Sci 13:217–277
Næss A, Haug T, Nilssen M (1998) Seasonal variation in body condition and muscular lipid contents in Northeast Atlantic minke whale Balaenoptera acutorostrata. Sarsia 83:211–218
Nemoto T (1962) Food of baleen whales collected in recent Japanese Antarctic whaling expeditions. Sci Rep Whales Res Inst 16:89–103
Nemoto T (1980) Diatom films on whales in South African Waters. Sci Rep Whales Res Inst 32:97–103
Nishiwaki S, Ishikawa H, Fujise Y (2006) Review of general methodology and survey procedure under the JARPA. In: Report of the intersessional workshop to review data and results from special permit research on Minke Whales in the Antarctic, Tokyo, 4–8 December 2006, SC/D06/J2
Ohno M, Fujino K (1952) Biological investigation on the whales caught by the Japanese Antarctic whaling fleets, season 1950/51. Sci Rep Whales Res Inst 7:125–188
Ohsumi S (1979) Feeding habits of the minke whale in the Antarctic. Rep Int Whale Comm 29:473–476
Ohsumi S, Fujise Y, Ishikawa H, Hatanaka T, Zenitani R, Matsuoka K (1997) The fattyness of the Antarctic minke whale and its yearly change. Paper SC/M97/18 presented to the JARPA review meeting, May 1997
Parry DA (1949) The structure of whale blubber, and a discussion fits thermal properties. Quarterly J. Microscopical Sci s3–90:13–25
Reid K, Croxall JP (2001) Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proc R Soc Lond B 268:377–384
Reilly S, Hedley S, Borberg J, Hewitt R, Thiele D, Watkins J, Naganobu M (2004) Biomass and energy transfer to baleen whales in the South Atlantic sector of the Southern Ocean. Deep-Sea Res II 51:1397–1409
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464
Testa JW, Oehlert G, Ainley DG, Bengtson JL, Siniff DB, Laws RM, Rounsevell D (1991) Temporal variability in Antarctic marine ecosystems: periodic fluctuations in the Phocid Seals. Can J Fish Aquat Sci 48:631–639
Vaughan DG, Marshall GJ, Connolley WM, King JC, Mulvaney M (2001) Devil in the detail. Science 293:1777–1779
Víkingsson GA (1995) Body condition of fin whales during summer off Iceland. In: Blix AS, Walløe L, Ulltang Ø (eds) Whales, seals, fish and man. Elsevier, Amsterdam, pp 361–369
Watanabe T, Yabuki T, Suga T, Hanawa K, Matsuoka K, Kiwada H (2006) Results of oceanographic analyses conducted under JARPA and possible evidence of environmental changes. In: Report of the intersessional workshop to teview data and results from special permit research on Minke Whales in the Antarctic, Tokyo, 4–8 December 2006, SC/D06/J30
Weimerskirch H, Inchausti P, Guinet C, Barbraud C (2003) Trends in bird and seal population as indicators of a system shift in the Southern Ocean. Ant Sci 15:249–256
Wilson PR, Ainley DG, Nur N, Jacobs SS, Barton KJ, Ballard G, Comiso JC (2001) Adélie penguin population change in the pacific sector of Antarctica: relation to sea-ice extent and the Antarctic circumpolar current. Mar Ecol Prog Ser 213:301–309
Acknowledgments
We would like to thank all the captains and crews, especially for K. Makita and Y. Murai, of the ships that took part and the scientists who were involved in JARPA surveys. Thanks are also due to Y. Fujise for providing us with small fetus length datasets of the minke whales, to Y. Hosone for helping us to organize the data set, and to Alison Coulthard for correcting the English. We would also like to thank D. Butterworth, S. Ohsumi, H. Hatanaka, T. Ichii, T. Hakamada and other people who have helped with and made valuable comments on this paper. The JARPA program was conducted with permission from the Japanese fisheries agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konishi, K., Tamura, T., Zenitani, R. et al. Decline in energy storage in the Antarctic minke whale (Balaenoptera bonaerensis) in the Southern Ocean. Polar Biol 31, 1509–1520 (2008). https://doi.org/10.1007/s00300-008-0491-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-008-0491-3