Abstract
Baleen whales and Adelie penguins in the near-shore waters around the Antarctic Peninsula forage principally on Antarctic krill. Given the spatial overlap in the distribution of these krill predators (particularly humpback whales) and their dependence on krill, the goals of this paper are to determine if the inter-annual community structure and relative abundance of baleen whales around Anvers Island is related to krill demography and abundance, and if the potential exists for inter-specific interactions between Adelie penguins and baleen. We use whale sightings and prey data from both net tows and Adelie penguin stomach samples to correlate the abundance of humpback whales with krill demography and abundance from 1993 to 2001. We find significant relationships between whale abundance and the size–frequency distribution of krill targeted by Adelie penguins, as well as the foraging success of Adelie penguins. These findings suggest both krill predators share common prey preferences in the upper portions of the water column around Anvers Island. These findings highlight the need for better knowledge of baleen whale foraging ecology and inter-specific interactions with penguins, as sea ice and krill populations around the Antarctic Peninsula are affected by rapid changes in climate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Baleen whales in the inner shelf waters off the Western Antarctic Peninsula (WAP) are distributed spatially in relation to their prey and secondarily to physical features which may influence prey aggregation (Thiele et al. 2004; Friedlaender et al. 2006). Humpback whales are the most abundant cetaceans in this area, foraging to a large degree on Antarctic krill (Euphausia superba) (Mackintosh 1965; Kawamura 1994). Recent evidence suggests baleen whales may partition resources both vertically within the water column and through species-specific prey patch preference: humpback whales associate with prey aggregations in the upper 100 m of the water column, while minke whales associate with deeper prey (Friedlaender 2006; Friedlaender et al. 2008). While several studies have described the distribution patterns of Antarctic cetaceans over broad spatial scales (Kasamatsu et al. 2000a, b; Nicol et al. 2000; Reid et al. 2000; Murase et al. 2002), cetacean occurrence in coastal areas is poorly known in the Antarctic. Furthermore, there is scant data on how the distribution and relative abundance of cetaceans changes over time (other than from whaling data, e.g. Tynan 1998) or in relation to inter-annual changes in prey availability.
Climate and sea ice conditions are rapidly changing around the Antarctic Peninsula (Smith et al. 1996; King and Turner 1997; Atkinson et al. 2004). Krill cohort strength and demography are tightly coupled to sea ice dynamics, and it has been suggested that krill abundance has decreased by an order of magnitude over the past 20 years in this area (Siegel and Loeb 1995; Loeb et al. 1997; Siegel et al. 1998). In other areas around the Antarctic Peninsula (e.g. South Georgia), diminished prey availability has negative impacts on land-breeding krill-dependent predator populations (Boyd et al. 1994; Croxall et al. 1999; Reid and Croxall 2001). Fraser and Hofmann (2003) postulate that recent trends in sea ice and recruitment events have led to a krill population dominated by a single age-class, affecting the foraging success of Adelie penguins around Anvers Island off the Western Antarctic Peninsula.
While environmental, bottom-up forcing plays a major role in the ecology of Adelie penguins, the opportunity also exists for inter-specific interactions with other krill predators such as baleen whales. Adelie penguins forage in the upper 100 m of the water column concentrating their efforts between 10 and 40 m, typically within 10–15 km (maximally to 96 km) of their nesting sites (Chappell et al. 1993; Fraser and Hofmann 2003). While baleen whales can range over greater spatial scales in search of food, they may be able to diminish the abundance of prey at small spatial scales. Ainley et al. (2006) offer evidence of a trophic cascade where minke whales in the Ross Sea may have helped deplete local prey levels in a small area, leading to prey switching and changes in foraging effort in sympatric Adelie penguins.
Given the spatial overlap in the distribution of Adelie penguins and baleen whales (particularly humpback whales) in the inner shelf waters of the WAP and their dependence on krill, the goals of this paper are to determine if the inter-annual community structure and relative abundance of baleen whales around Anvers Island is related to krill demography and abundance, and if the potential exists for inter-specific interactions between Adelie penguins and baleen whales in this area. We use data collected as part of a long-term ecological research effort (Palmer LTER) to explore and characterize the inter-annual relationships between the relative abundance of baleen whales, krill abundance and demography, and Adelie penguin foraging behavior. We test the null hypotheses that: (1) no relationship exists between the relative abundance and size-class distribution of krill and the relative abundance of baleen whales around Anvers Island over time; and (2) no relationship exists between the relative abundance of baleen whales and the foraging behavior of Adelie penguins.
Materials and methods
Cetacean sightings data
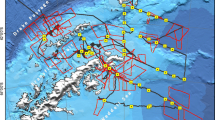
Cetacean sightings were made during January–February from 1993 to 2001 concurrent with bird census work conducted on Palmer LTER cruises. Data were collected from the MV Polar Duke from 1993 to 1997, and from the ARSV Laurence M. Gould from 1998 to 2001. Observations were made from the starboard corner of the bridge within a 90° arc from the bow of the ship to the beam. Surveys were only done when the ship was traveling faster than four knots. A standardized high density grid (10 × 20 km area) was established in the vicinity of Palmer Station, extending to the south and west of Anvers Island (Fig. 1), and sampled generally at the same time during each summer cruise. This area is broken into 10 km east/west running legs, and 2.5 km north/south running legs. Each 10 km leg is broken into 2.5 km transects. Sightings made in the vicinity of Anvers Island were used in our analyses.
Krill sample data
Krill samples collected as part of a long-term Adelie penguin foraging and monitoring study near Anvers Island were used as a measure of penguin diets (see Fraser et al. 1992; Fraser and Hofmann 2003). Several studies have shown seabird diets to be an appropriate proxy or indicator of the spatio-temporal variance associated with the structure of their prey populations (e.g. Ainley et al. 1993; Croxall et al. 1999; Fraser and Hofmann 2003). Adelie penguins dive to and forage between 3 and 98 m (Chappell et al. 1993), and diet samples from these birds are considered a proxy for prey availability in the upper 100 m of the water column. Penguin diet samples (4–5 samples for each of approximately 10 weeks) were obtained during January and February from 1993 to 2001 during the chick-feeding phase of the reproductive cycle using a water off-loading method (forced regurgitation through stomach lavage, Wilson 1984) as described in Fraser and Hofmann (2003). Typically, 50–100 krill were measured for standard lengths from the eye to the tip of the telson, and binned into 5 mm classes to resolve changes in population size-class structure between years (Fraser and Hofmann 2003). We then pooled all samples for each season to generate a length–frequency distribution which was further binned into 4 size-classes (<30, 31–40, 41–50, >50 mm).
In order to estimate both the overall krill density as well as the length–frequency (age) distribution of the krill population around Anvers Island and the LTER study area, we use data from Quetin and Ross (2003). These estimates come from predetermined stations where standardized net tows were conducted. Data from Quetin and Ross (2003) are also used to express the persistence of krill cohorts as percentages of the total krill sampled from the two large recruitment events (1990–1991, 1995–1996) which occurred during the period of the study. Lastly, annual anomalies of estimated krill density across the study area were determined by comparing individual years to the average density calculated from 1993 to 2001 (R. M. Ross and L. B. Quetin, personal communication).
Data analysis
In order to test our hypotheses and quantify the relationships between the annual relative abundance of baleen whales, Adelie penguin foraging behavior, and krill abundance and demography around Anvers Island, we conducted linear regression analyses.
Using the relative abundance from best estimates of humpback whale sightings around Anvers Island for each year (1993–2001), we ran a stepwise Poisson AIC generalized linear model (GLM) to determine relationships between whale numbers and Adelie penguin foraging behavior based on the length–frequency of size/age classes of krill consumed. Regression models are a commonly used technique to elucidate the relationship between cetacean distributions and one or more habitat predictor variables (Redfern et al. 2006). GLMs assume a parametric (linear or quadratic) relationship between response and predictor variables, but have flexibility to handle non-constant variances. An extension of GLM is the Poisson regression which can be used when the response variable is a count, with large outcomes being rare events (Redfern et al. 2006). We then used Akaike’s Information Criterion (AIC) to evaluate which combination of predictor variables provided the best-fit to the observed data. AIC attempts to select the model providing the best-fit to the data as measured by a decrease in variance, while minimizing the number of variables included in the model, essentially optimizing the trade-off between bias in the model from the number of predictor variables and variance (Hilborn and Mangel 1997; Redfern et al. 2006).
We also tested for relationships between the relative abundance of baleen whales and several other metrics of foraging behavior and efficiency in Adelie penguins. Fraser and Hofmann (2003) find that Adelie penguin foraging may be limited by their ability to find krill, rather than by their ability to efficiently exploit krill once located. Thus, we can also refer to the number of krill in each stomach sample as another indicator for the overall prey availability in their foraging habitat. We further tested for a relationship between the relative abundance of whales and the seasonal, average number of krill per penguin sampled. A final analysis of the relationship between whale abundance and penguin foraging used the average annual foraging trip duration of Adelie penguins (from Fraser and Hofmann 2003). As a central-place forager, foraging trip duration in Adelie penguins can serve as an indicator of the time it takes to find food; presumably longer foraging trip durations indicate fewer prey in an immediate area, and subsequently either taking more time to find sufficient prey or having to range farther to find adequate prey resources.
We then tested for relationships between baleen whale relative abundance and several krill population metrics sampled independent of penguin foraging. Using net tow data, we ran regression models against the annual density of krill and also within each year-class present in the population. We then ran a similar analysis of whale abundance against the overall krill density anomaly (density of each year compared to the mean from 1993 to 2001). Finally, we tested for a relationship between whale abundance and the persistence of the two major krill cohorts from recruitment episodes (1990–1991, 1995–1996) during the period of the study by regressing the percentage of the overall krill catches associated with each recruitment cohort over time.
Results
Cetacean sightings
Between 1993 and 2001 humpback whales were seen in all years except one (Fig. 2, Table 1). Humpback numbers ranged from 0 to 58 individuals annually. The highest number of whales was seen in 1997, while no whales were seen the previous year. The mean annual abundance of humpback whales was 14.3 (standard deviation = 17.6).
Krill samples
Krill samples collected from both net tows and Adelie penguins around Anvers Island are summarized in Table 1. The number of penguins sampled annually ranged between 37 and 50. The total number of krill measured from stomach samples ranged between 1,580 in 2001 and 3,619 in 1997. Typically, the greatest number of krill was between 30 and 50 mm (in 2000 the greatest number was >50 mm). Krill < 30 mm averaged 5.4% of the total length–frequency of krill ranging between 0.4% in 1993 and 16% in 1996. Krill between 30 and 40 mm ranged between 72 (1997) and 10% (1999) of the total annual sample of krill, averaging 35.6%. Krill between 40 and 50 mm were on average the most represented length class, averaging 46.5% of the total sample (between 15% in 1996 and 75.8% in 1994). The largest size-class of krill > 50 mm averaged 12.4% of the total sample, was variable, ranging between 0.4 and 43.2%. The number of krill per penguin stomach ranged from 42.7 in 2001 to 72.4 in 1997.
The greatest density of krill occurred in net samples around the episodic recruitment events of 1990–1991 and 1995–1996 as those cohorts grow and enter the adult krill population (Table 1). The highest densities of krill were found in the ac2 age class in 1993 and the ac4 age class in 1998. Krill from the 1991 to 1992 recruitment dominate the overall population, accounting for 100 and 92% of the overall catch in 1993 and 1994. This cohort then diminished and was replaced by the 1995–1996 cohort which dominated the catches from 1996 to 2001 (70–90%).
The krill density anomalies correspond to these years as well, with the largest positive anomaly occurring in 1993. Over the period of the study the mean krill density appeared to be very high, and then dropped well below the mean for 2 years, becoming stable around the mean and increasing slightly before dropping once again in 1999–2001.
Whales and penguin analysis
The generalized linear models to test the associations between humpback whales with the size–frequency distribution of krill consumed by Adelie penguins around Anvers Island yield significant results (Table 2). A total of 129 humpback whales was sighted from 1993 to 2001 around Anvers Island. The best-fit GLM model included all four of the krill length classes used as predictor variables. A significant and negative association was found with krill < 30 mm (P < 0.001). Stronger positive correlations were found with krill 30–40 mm and >50 mm (P < 0.0001), and a significant relationship was also found with krill 40–50 mm (P < 0.01). The AIC value for this model was 63.91 with a residual deviance of 20.162.
A significant positive relationship also exists between the relative abundance of humpback whales and the number of krill per penguin stomach sampled (P = 0.003). While a linear relationship was significant (R 2 = 0.72), we found a better fit and more significant relationship expressed as an exponential function (P < 0.001, R 2 = 0.94) (Fig. 3). There was no significant relationship between humpback whale relative abundance and Adelie penguin foraging trip duration (P = 0.18). The results of these regression analyses are shown in Table 3.
Whale and krill analysis
Regression analysis testing the relationship between humpback whale abundance and the density of krill and age/length classes from net tows was not significant for the overall model (P = 0.26) or for any individual age/size class (Table 3). Similarly, there was no significant relationship between whale abundance and annual krill density expressed as an anomaly around the mean (P = 0.63). We find no significant relationship between humpback whale relative abundance and the percentage of the 1990–1991 (P = 0.37) or 1995–1996 (P = 0.30) krill cohort in the overall population.
Discussion
Over a 9-year period from 1993 to 2001, we find no significant relationships between the availability and population structure of krill (as measured from net tow data) and the relative abundance of humpback whales around Anvers Island. Based on these results, our data do not reject the first null hypothesis that no relationships exist between krill and whales in this area. However, we do find evidence that differences in the size–frequency distribution of krill present in the upper portion of the water column around Anvers Island targeted by Adelie penguins relate to the annual relative abundance of humpback whales. We also find evidence of a strong relationship between the foraging success of Adelie penguins and the annual relative abundance of humpback whales. We do not find evidence of a direct relationship between Adelie penguin foraging effort, based on foraging trip duration, and the relative abundance of humpback whales. This information suggests rejecting our second null hypothesis. Thus, humpback whales and Adelie penguins appear to target and share similar spatio-temporal prey resources in the upper portions of the near-shore waters around Anvers Island.
While the data presented here offer insights into the distribution and ecology of baleen whales in the Antarctic, we are limited in our confidence and ability to quantify relationships due to sampling circumstances. Whale sightings were collected somewhat opportunistically concurrent to bird surveys. Effective effort was limited to a single forward quadrant of the visible sighting environment, and search effort was primarily restricted to 1 km from the ship. Thus, we consider our sample size limited from a search area perspective, and present it as only a relative index of humpback whale abundance. We are also limited by the spatial and temporal scales at which data were collected. Whale sightings and penguin diet data come from concurrent spatial ranges across a similar temporal scale. However, the krill density data from net tows represent a somewhat larger spatial range than sightings. They were, however, collected within the same time period as the sightings.
The krill data used for the GLM of length–frequency classes were obtained from diet samples of Adelie penguins. While noted earlier that these samples have been shown to be an appropriate proxy for the structure of their prey populations, it is possible that penguins and whales target prey in different ways. We assume that the krill being eaten by penguins is reflective of the overall krill community structure in the portion of the water column where they are foraging (as suggested by Fraser and Hofmann 2003), and that the thresholds below which is it not profitable for penguins to forage on certain sizes of prey may be similar to that of whales.
A large gap exists in our basic understanding of the foraging behavior of baleen whales in the Antarctic. Friedlaender et al. (2008) suggest vertical partitioning of krill in the water column between humpback and minke whales, and where the two species do associate with prey in a similar part of the water column they may associate with krill in different sized patches and with differing densities. We find more evidence of species-specific prey preferences in how the relative abundance of humpback whales around Anvers Island is influenced by different size-classes of krill present in the upper portion of the water column. Our analysis offers novel insights by comparisons with specific age/length classes of krill. The GLMs suggests a significant, but not statistically robust (due to sample size), connection. Humpback whale relative abundance is positively associated with all krill size-classes >30 mm, and negatively with krill < 30 mm. One potential explanation may be that the vast majority of krill in the sample are >30 mm, and humpback whales have energetic demands which require a greater energetic threshold via density or abundance of prey in order to make foraging profitable (see Piatt and Methven 1992). However, this relationship only exists with krill consumed by penguins, and not with the frequency distribution of krill from the area at large.
The relationship between humpback whale abundance and krill size-classes from penguin stomachs may indicate that these two krill predators have similar prey preferences. Both humpbacks and Adelie penguin appear to target larger krill when they are available, avoiding smaller krill. Additionally, the relationship between whale abundance and the number of krill per penguin stomach sample adds further evidence to this concept. The shape of the best-fit function for this relationship is exponential with respect to whale abundance, and toward an asymptote for the number of krill per penguin stomach. This function may indicate the upper limits of how many krill an Adelie penguin can consume on a single foraging trip. To our knowledge, there is no data published on stomach sizes of these penguins. There is no indication from this relationship that humpback whales have a negative effect on the foraging success of Adelie penguins around Anvers Island.
Krill can grow to reach 25 mm after 1+ year (Quetin and Ross 2003). With limited recruitment events due to climate forcing, a single dominant krill cohort will spend several years at a size great than 30 mm. If humpback whales and penguins have preference for krill of this size-class, and without the introduction of a new and smaller age class into the krill population, the possibility exists that they could help deplete local prey abundance and compete for this resource. We find no significant relationship; however, between annual whale abundance and the persistence of a single dominant krill cohort in the area. Thus, it appears that the episodic recruitment of krill has, at least in the past, maintained enough of the size-preferred prey for penguins and whales to both forage efficiently.
Sea ice around the WAP is decreasing in a number of ways. Aside from a decrease in the overall extent of sea ice during the last decade, sea ice is also forming later and retreating earlier (Smith et al. 2003). Fraser and Hofmann (2003) suggest that while progress has been made toward understanding krill–sea ice interactions, the mechanisms by which variability in sea ice cover effects krill demography, and cascades to apex predators remains poorly understood. Climate variability is important to the biological components of marine ecosystems because many species have evolved complex life-history strategies to cope with or exploit environmental variability (Johnston et al. 2005). However, unidirectional climate warming may induce changes in ecosystem structure and function by disrupting these evolved life-history strategies of key species (Rhodes and Odum 1996). The long life-span (>6 years) of Antarctic krill has evolved to minimize mismatches in the highly variable Antarctic marine environment, but it has been suggested that this strategy is also a potential “weak link” in the processes maintaining ecosystem function (Fraser and Hofmann 2003). Siegel et al. (1998) offer that multiple age classes buffer the krill population from short term and rapid changes in abundance to maintain ecosystem structure and function, however recent trends indicate that the number of strong cohorts in the population may be decreasing, thus making the ecosystem more vulnerable to climate-induced perturbations if multiple poor recruiting classes or senescent events occur in succession (Fraser and Hofmann 2003). Therefore, quantifying the links between krill predators, prey, and the physical environment is a fundamental step toward predicting and understanding the effects of climate change and variability on predators such as penguins and baleen whales in Antarctica.
Humpback whales may play a role in limiting local availability of prey for Adelie penguins around Anvers Island; however, no empirical data exist on the consumption rates and threshold prey required for humpback whales. Focused research on the foraging behavior and consumption rates of humpback whales in this area would greatly increase our knowledge of how these krill predators and climate changes both may affect penguin populations around the Antarctic Peninsula.
References
Ainley DG, Sydeman WJ, Parrish RH, Lenarz WH (1993) Oceanic factors influencing distribution of young rockfish (Sebastes) in central California: a predator’s perspective. Calif Coop Ocean Fish Invest Rep 34:133–139
Ainley DG, Ballard G, Dugger KM (2006) Competition among penguins and cetaceans reveal trophic cascades in the Western Ross Sea, Antarctica. Ecology 87(8):2080–2093
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Boyd IL, Arnould JPY, Barton T, Croxall JP (1994) Foraging behavior of Antarctic fur seals during periods of contrasting prey abundance. J Anim Ecol 63:703–713
Chappell MA, Shoemaker VH, Janes DH, Maloney SK, Bucher TL (1993) Energetics of foraging in breeding Adelie penguins. Ecology 74:2450–2461
Croxall JP, Reid K, Prince PA (1999) Diet, provisioning and productivity of marine predators to differences in availability of Antarctic krill. Mar Ecol Progress Ser 177:115–131
Fraser WM, Hofmann EE (2003) A predator’s perspective on causal links between climate change, physical forcing and ecosystem response. Mar Ecol Progress Ser 265:1–15
Fraser WM, Trivelpiece WZ, Ainley DG, Trivelpiece SG (1992) Increases in Antarctic penguin populations: reduced competition with whales or a loss of sea ice due to global warming? Polar Biol 11:525–531
Friedlaender AS (2006) Spatial ecology of Humpback and Minke Whales off the Western Antarctic Peninsula. Doctoral Dissertation, Duke University, Durham, pp 199
Friedlaender AS, Halpin PN, Qian SS, Lawson GL, Wiebe PH, Thiele D, Read AJ (2006) Whale distribution in relation to prey abundance and oceanographic processes in the Western Antarctic Peninsula shelf waters. Mar Ecol Progress Ser 317:297–314
Friedlaender AS, Lawson GL, Halpin PN (2008) Evidence of resource partitioning and niche separation between humpback and minke whales in Antarctica. Mar Mammal Sci (in review)
Hilborn R, Mangel M (1997) The ecological detective: confronting models with data. Princeton University Press, New Jersey
Johnston DW, Friedlaender AS, Torres LG, Lavigne DM (2005) Variation in sea ice cover on the east coast of Canada from 1969 to 2002: climate variability and implications for harp and hooded seals. Clim Res 20:200–222
Kasamatsu F, Ensor P, Joyce GG, Kimura N (2000a) Distribution of minke whales in the Bellingshausen and Amundsen Seas (60°W–120°W), with special reference to environmental/physiographic variables. Fish Oceanogr 9:214–223
Kasamatsu F, Matsuoka K, Hakamada T (2000b) Interspecific relationships in density among the whale community in the Antarctic. Polar Biol 23:466–473
Kawamura A (1994) A review of baleen whale feeding in the Southern Ocean. Rep Int Whaling Comm 44:261–271
King JC, Turner J (1997) Antarctic meteorology and climatology. Cambridge University Press, Cambridge
Loeb V, Seigel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900
Mackintosh NA (1965) The stocks of whales. Fishing News (Books) Ltd, London
Murase H, Matsuoka K, Ichii T, Nishiwaki S (2002) Relationship between the distribution of euphausiids and baleen whales in the Antarctic (35°E–145°W). Polar Biology 25:135–145
Nicol S, Pauly T, Bindoff NL, Wright S, Thiele D, Hosie GW, Strutton PG, Woehler E (2000) Ocean circulation off east Antarctica affects ecosystem structure and sea-ice extent. Nature 406:504–507
Piatt JF, Methven DA (1992) Threshold foraging behavior of baleen whales. Mar Ecol Progress Ser 84:205–210
Quetin LB, Ross RM (2003) Episodic recruitment in Antarctic krill Euphausia superba in the Palmer LTER study region. Mar Ecol Progress Ser 259:185–200
Redfern JV, Ferguson MC, Becker EA, Hyrenbach KD, Good C, Barlow J, Kaschner K, Baumgartner MF, Forney KA, Balance LT, Fauchald P, Halpin P, Hamazaki T, Pershing AJ, Qian SS, Read A, Reilly SB, Torres L, Werner F (2006) Techniques for Cetacean-habitat modeling: a review. Mar Ecol Progress Ser 310:271–295
Reid K, Brierley AS, Nevitt GA (2000) An initial examination of relationships between the distribution of whales and Antarctic Krill Euphausia superba at South Georgia. J Cetacean Res Manage 2:143–149
Reid K, Croxall JC (2001) Environmental responses of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proc R Soc Lond 268:377–384
Rhodes OE Jr, Odum EP (1996) Spatiotemporal approaches in ecology and genetics: the road less traveled. In: Rhodes OE Jr, Chesser RK, Smith MH (eds) Population dynamics in ecological space and time. University of Chicago Press, Chicago, pp 1–8
Siegel V, Loeb V (1995) Recruitment of Antarctic krill Euphausia superba and possible causes for its variability. Mar Ecol Progress Ser 123:45–56
Siegel V, Loeb V, Groger J (1998) Krill (Euphausia superba) density, proportional recruitment and absolute recruitment and biomass in the Elephant Island region (Antarctic Peninsula) during the period 1997–1997. Polar Biol 19:393–398
Smith RC, Stammerjohn SE, Baker KS (1996) Surface air temperature variations in the western Antarctic Peninsula region. Antarct Res Ser 70:105–121
Smith RC, Fraser WR, Stammerjohn SE (2003) Climate variability and ecological response of the marine ecosystem in the Western Antarctic Peninsula (WAP) region. In: Greenland D, Goodin D, Smith R (eds) Climate variability and ecosystem response at long-term ecological research sites. Oxford Press, Oxford, pp 158–173
Thiele DC, Chester ET, Moore SE, Sirovic A, Hildebrand JA, Friedlaender AS (2004) Seasonal variability in whale encounters in the Western Antarctic Peninsula. Deep Sea Res II 51:2311–2325
Tynan CT (1998) Ecological importance of the Southern Boundary of the Antarctic circumpolar current. Nature 392:708–710
Wilson RP (1984) An improved stomach pump for penguins and other seabirds. J Field Ornithol 55:109–112
Acknowledgments
Data for this study were collected as part of the Palmer Long-Term Ecological Research project. This work would not have been possible without the data and intellectual contributions of Drs. Robin Ross and Langdon Quentin. We are grateful to the many visual observers and field personnel who helped throughout the course of this project. We offer special thanks to the officers and crew of the Polar Duke and LM Gould and the support staff of the US Antarctic Program. This manuscript was enhanced by the comments and guidance from Drs. Eileen Hofmann, Richard Barber, Andy Read, and Deb Thiele, as well as two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friedlaender, A.S., Fraser, W.R., Patterson, D. et al. The effects of prey demography on humpback whale (Megaptera novaeangliae) abundance around Anvers Island, Antarctica. Polar Biol 31, 1217–1224 (2008). https://doi.org/10.1007/s00300-008-0460-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-008-0460-x