Abstract
We studied predation risk in relation to nest location and subcolony size in Southern Rockhopper Penguins (Eudyptes chrysocome chrysocome) during the chick-rearing period. Striated Caracaras (Phalcoboenus australis), the main predator, preferentially attacked from tussock grasses which are found in the periphery of all subcolonies (peripheral tussocks) and often scattered within them (central tussocks). The greatest numbers of predation and attempted predation events were observed on nests in the periphery of the subcolony next to peripheral tussocks, and on those nests next to central tussocks. Central tussocks offer Striated Caracaras an additional “edge” area from which to prey, much in the same way as do the peripheral tussocks. Predation rate per individual was not correlated with subcolony size possibly due to the presence of central tussocks which, by creating an extra edge area, change the subcolony shape. There is a suggestion (P = 0.06) of increased probability of nest success with subcolony size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the proposed advantages of coloniality, reduced probability of predation has been widely studied. Animals living in colonies may lower rates of nest predation in several ways: (1) by early detection of predators (given that group vigilance increases with the number of individuals present), (2) by deterring predators through group mobbing and defense, and/or (3) by dilution of predation risk (“dilution effect”) either through synchronized reproduction, or by clustering nests to create the “selfish herd effect” (see reviews in Wittenberger and Hunt 1985; Brown and Brown 2001). Hamilton’s selfish herd model predicts that, if a predator always takes the prey item closest to it, prey will seek to minimize the distance between themselves and their neighbors, and maximize the number of neighbors (Hamilton 1971).
Several studies have shown lower predation at higher densities of prey or in larger colonies (Spear 1993; Anderson and Hodum 1993; Hernández-Matías et al. 2003). Conversely, other studies have shown an opposite trend (Brunton 1999; Stokes and Boersma 2000). The “selfish herd” concept has been extended to predict the center as the optimal location for a nest in a colony. Because peripheral nests have neighbors only on one side, individuals breeding at the edge of a colony should suffer higher losses due to predation than individuals breeding near the center (Tenaza 1971). In support of this concept, several authors have reported higher predation rates on colony edges than on the center (Taylor 1962; Tenaza 1971; Spear 1993; Emslie et al. 1995; Yorio and Quintana 1997; Descamps et al. 2005), though others have shown the reverse (Bellinato and Bogliani 1995; Brunton 1997).
In penguins, impacts and activities of predators have been studied mainly in the Gentoo (Pygoscelis papua) and Adélie Penguins (Tenaza 1971; Davis 1982; Ainley et al. 1983; Young 1994; Emslie et al. 1995), King Penguin (Aptenodytes patagonicus) (Hunter 1991; Le Bohec et al. 2003; Descamps et al. 2005) and Chinstrap Penguin (Pygoscelis antarctica) (Barbosa et al. 1997), but not in Rockhopper Penguins (Eudyptes chrysocome chrysocome).
Here, we present data on the occurrence and impact of predator–scavengers associated with Rockhopper Penguins during the first part of the chick rearing period on Staten Island, Argentina, to determine the effects of nest position and subcolony size on predation risk. First, we describe and quantify the activities of various predator and scavenger species associated with Rockhopper Penguin subcolonies; and evaluate temporal variations in these activities. Second, based on the selfish herd hypothesis, we tested the following predictions: (1) Predation and predation attempts would be higher on the edge than on the center of the subcolonies, (2) Larger subcolonies would experience less predation per individual than smaller ones. Lastly, we examine the simultaneous effect of subcolony size, nest location and year on the probability of nest success.
Materials and methods
Study area
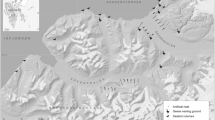
Staten Island (Isla de los Estados), east of the Tierra del Fuego archipelago, has two of the three known breeding colonies of Southern Rockhopper penguins (Eudyptes chrysocome chrysocome) for Argentina, representing 27.3% of the breeding population. Our study was conducted at Bahía Franklin, Staten Island (54°50′S, 64°40.5′W; Fig. 1), where the largest colony of Southern Rockhopper Penguins on the island is located (167,000 breeding pairs in 102 subcolonies) (Schiavini 2000). Within colonies, Rockhopper penguins form distinct nest aggregations or subcolonies that are easily identifiable on the ground or from aerial photos by differences in soil and vegetation modified by the bird’s activities. Nests are distributed mainly on areas of tussock grass (Poa flabellata) which are found surrounding the subcolonies (peripheral tussocks) and often scattered within the subcolony perimeter (central tussocks) as well.
Map of southern Argentina (upper left) showing the location of Staten Island (Isla de los Estados; box and enlargement upper right) where the two Rockhopper Penguin colonies (boxes) are located. Detail of Bahía Franklin (bottom) on west end of Staten Island and location of the study subcolonies (SC 1–9) and camp site (black square)
Study species
Rockhopper Penguins arrive at Staten Island in late September, lay two eggs in late October and hatch chicks in late November. The chick rearing period includes the brooding or guard stage which extends from the end of November to mid December, and the crèche stage which extends from there until the end of January/beginning of February. During the brooding stage chicks are guarded at the nest mostly by the male. As chicks get older they are left unguarded and form crèches (Raya Rey 2006; Raya Rey et al. 2007).
Previous observations suggest that the Striated Caracara (Phalcoboenus australis) is an important predator at Rockhopper Penguin colonies on Staten Island (J. Meiburg, personal communication; M. Liljesthröm, personal observation). The IUCN lists the Striated Caracara as Near-Threatened due to its small numbers and restricted range (Birdlife International 2005). Its distribution includes isolated shores and islands off southern South America (Narosky and Yzurieta 1987).
Other predator/scavengers at Staten Island include Andean Condors (Vultur gryphus), Crested Caracaras (Polyborus plancus), American Kestrels (Falco sparverius), Peregrine Falcons (Falcos peregrinus anatum), Variable Hawks (Buteo polysoma), Turkey Vultures (Cathartes aura), Black-chested Buzzard-eagles (Geranoaetus malanoleucus), Kelp Gulls (Larus dominicanus) and Chilean Skuas (Catharacta chilensis) (J. Meiburg, personal communication).
Data collection
We observed eight subcolonies ranging from 69 to 1,520 nests and nine subcolonies ranging from 72 to 1,682 nests during 7–30 December 2003 and 8–25 December 2004, respectively (Table 1). We estimated the size of each subcolony as the mean total number of occupied nests (either by adults and chicks/eggs or by adults only), determined from 3 to 6 repeated counts by four different observers during the first observation day. Study subcolonies were chosen because of their relatively easy access from the camp site, their near circular shape, their different sizes and the clear field of view of all nests. Subcolony SC8 was the most isolated from the other subcolonies (Fig. 1), the most exposed to the wind, the closest to the water, and bordered a 10–15 m cliff on which Kelp Gulls nest. Subcolonies SC1, SC3, SC5, and SC7 and subcolonies SC2, SC6 and SC9 were in close proximity to each other (Fig. 1) in an area generally occupied by groups of adult/juvenile Striated Caracaras, Turkey Vultures and Crested Caracaras. Subcolony SC4 was about 400 m away from these last subcolonies and, instead, was part of the feeding territory of a breeding pair of Striated Caracaras.
We observed the subcolonies for 3 h periods alternating periodically between 08:00–20:00 each day (time periods 08:00–11:00, 11:00–14:00, 14:00–17:00, and 17:00–20:00). Observations were made with 8 × 10 binoculars from observation points located on elevated areas behind tussock grasses ∼6–7 m from the subcolony. We completed a total of 461 and 386 h of observation during the study periods of 2003 and 2004, respectively (Table 1). Approximately equal numbers of observations were made in each of the four time periods and similar numbers of observation periods were obtained for all subcolonies each year. During each observation period we recorded species and activities of predators and scavengers. Following the methods of Emslie et al. (1995), we classified activities as searches, predation, attempted predation and scavenging. Searches were recorded either when birds flew low (<∼15 m above the subcolony) and circled slowly over the subcolony (“search from air”), approached the subcolony near the edges on the ground (“search from ground”) or when they stood on tussocks in the center or in the periphery of the subcolony (“search from central or peripheral tussock”, respectively). Predation occurred when a bird successfully took a chick from the subcolony and attempted predation when a bird took and lost or attempted to take a chick. We also noted whether predation events and attempts were from the air, from the ground, or from a central/peripheral tussock. Scavenging was recorded when a predator fed on food remains (abandoned eggs, dead chicks, left overs from chick feedings) from the subcolony. For predation and attempted predation events, we also recorded nest location within the subcolony (see below in “Data Analysis”).
At each subcolony we plotted on a map nests along a radial transect and monitored them every 3 days in 2003 and every other day in 2004 until the crèche period (in 2004, for subcolonies <140 nests, SC1 and SC6, we monitored all nests in the subcolony and a posteriori we randomly picked a transect from the map). Nest monitoring was completed by one observer with 8 × 10 binoculars from outside each subcolony to minimize disturbances. Since the study was restricted to the first weeks of the chick-rearing period, the only breeding variable we recorded was the number of chicks surviving at each nest during this study-period. The cause of chick death was listed as predation based on observed predator attacks or when chicks were missing from the nest; if chicks were found dead next to the nest it was assumed that they had died by other causes because during observed predation events chicks were always taken away from the nest by the predator.
Data analysis
Predators and scavengers at Rockhopper Penguin subcolonies
We used observed activity events to calculate activity rates as the number of activity events recorded divided by the number of hours of observation. Rate categories included total activities (searches, predation, attempts and scavenging), searches and predation and attempted predation.
To evaluate temporal variations in predator’s activities, these rates were determined for each species and analyzed by year, subcolony, time of day and time of season (divided into 2-day intervals that included between 28 and 53 h of observations each and during which each subcolony had been observed at least twice). We only analyzed “total activity rates” because predation and attempts were too low to analyze separately. To examine diurnal patterns, we compared total activity rates among the four 3-h observations periods for each species (Kruskal–Wallis tests). To assess variation in total activity rates within our study period, we divided it into “early” and “late” periods based on the day the first crèche was observed and used a Wilcoxon rank sum-test to compare the distribution of total activity rates between these two time periods.
To examine the proportion of observed predation and attempted predation events and predators method of approach on different nest locations and to test whether the pattern varied between years we used a Mantel–Haenszel chi-square analysis stratified by year. Based on previous observations of Striated Caracaras preying on Rockhopper Penguin chicks from tussock grasses (M. Liljesthröm, personal observation), we classified nest location as geometric edge, functional edge or central (Fig. 2). Those nests in the most external ring of the subcolony and not completely surrounded by other nests were considered as “geometric edge”; nests at least one nest away from the periphery of the subcolony and next to a tussock were considered as “functional edge”; and “central” nests were those at least one nest away from the edge of the subcolony and not next to a tussock. Subcolonies <200 nests (SC1, SC6 and SC8) had only geometric edge and central locations because no central tussock were present. Nests on the geometric edge can be accessed from a peripheral tussock, from the ground or from the air while nests on the functional edge can be accessed from a central tussock or from the air, and central nests can be accessed only from the air. Although nests with a functional edge or central location could potentially be accessed from the ground, this seems unlikely since little free ground space occurs between nests, making it difficult for a predator to land within the subcolony.
Predation, nest location and subcolony size
To test whether predation and predation attempts are higher on the edge than on the center of subcolonies (prediction1), we used a chi-square test to compare the proportion of observed predation and attempted predation events on “edge” versus “center” nest locations. For this analysis, geometric edge and functional edge were pooled into “edge”. Because Striated Caracaras accounted for most predation and attempt predation events and preyed more frequently from peripheral and central tussocks than from the air or ground (see “Results” below), whether prey are on the geometric edge or in the functional edge represents a similar risk. Moreover, for the Striated Caracara, tussocks simply offer an “attack platform”, regardless of their position in the subcolony. Thus, central tussocks essentially act as additional “edge” space.
We used observed predation events at each subcolony to calculate the following measures: subcolony predation rate as the total number of predation events by all predators per hour (this may indicate whether a given subcolony is more or less attractive and/or conspicuous to predators) and individual predation rate as the subcolony predation rate divided by subcolony size (this is related to the probability of chick predation). To test whether subcolony predation rate and individual predation rate were related to subcolony size (prediction 2) we used partial correlation to adjust for the effect of search rate by all predators. Including search rate in the model corrects for the effect of differences of predation pressure among subcolonies (e.g., a predator might be attracted to a subcolony because it sees other predators searching there and not because of subcolony size or other factors).
Nest success
We used nest monitoring data to estimate nest success as number of nests that had at least one chick at the last monitoring divided by the total number of nests on the transect. We estimated this index for each subcolony and for the different nest locations within each subcolony (in the latter case we calculated them with respect to the total number of nests on that nest location in the transect). For this analysis geometric edge and functional edge were also pooled into a single category as “edge”. To simultaneously test the effect of subcolony size, year, and nest location on the probability of nest success we used a logistic regression model with nesting outcome (successful or not) as a binary dependant variable. Explanatory variables included subcolony size, nest location (edge or center) and year. Subcolony was treated as the sampling unit in the “repeated” statement of the GEE analysis in the PROC GENMOD procedure in SAS. This allowed for the identification of the relative contribution of each explanatory variable while controlling for the non-independence of nests in the same subcolony.
Statistics
We used a significance level of P < 0.05 for all statistical tests. All analyses were completed using SAS 9.1 (SAS 2006) and JMPIN 3.2.6 software.
Results
Predator and scavenger activities
The predator/scavenger community associated with Rockhopper Penguin subcolonies at Staten Island included Striated Caracaras, Kelp Gulls, Dolphin Gulls (Larus scoresbii), Turkey Vultures, Southern Giant Petrels (Macronectes giganteus), Chilean Skuas and Crested Caracaras. In both years almost 50% of the total activity events at all subcolonies combined (2003: n = 2923 total activity events, 2004: n = 2025) were by Striated Caracaras (2003: 51.8%, 2004: 46.6%). Kelp Gulls, Dolphin Gulls and Turkey Vultures were the second most common species with 10–20% of the total activity events (2003: 17.2, 10.8 and 13.6%; 2004: 17.5, 14.8 and 12.4%, respectively). Chilean Skuas, Giant Petrels and Crested Caracaras were rarely seen and accounted for less than 5% of the total activity events (2003: 1.2, 4.6 and 0.7%; 2004: 0.9, 4.6 and 3.1%, respectively).
In both years, considering all subcolonies together, searching was the predominant predator–scavenger activity recorded. For Kelp Gulls, Dolphin Gulls and Turkey Vultures, as well as for the less common species, searching from the air was the predominant activity, accounting for over 85% of their total activity events. For Striated Caracaras, searches from the air and from peripheral tussocks were the most predominant activity accounting for 30–50% of their total activities, followed by searches from central tussocks which comprised ∼10% of the total activity events. Predation and attempted predation events were rare and were only observed for Kelp Gulls (four attempts and one predation) and Striated Caracaras (32 attempts and 34 predations, Table 1). In both years, the main predator was the Striated Caracara, which accounted for 93% (n = 71) of all observed predations and attempts. Total activity rate by all species combined and search rate by all species combined were both positively correlated with subcolony size (total activity rate: r s = 0.71, P = 0.001, search rate: r s = 0.71, P = 0.04, both: n = 17 subcolonies).
Temporal variation in activity rates
We analyzed temporal variation only for Striated Caracaras, Kelp Gulls, Dolphin Gulls and Turkey Vultures because the other three species (Southern Giant Petrels, Chilean Skuas and Crested Caracaras) were rarely seen and accounted for less than 5% of the total activity events. There was no significant diurnal pattern in total activity rates for any of the species in neither year (Kruskal–Wallis test, for all species P > 0.05, df = 3). The distribution of total activity rates by Striated Caracaras and Turkey Vultures early in the study period was similar as that for later in the study period, in both years (Wilcoxon test, both years P > 0.05, n = 8 2-day intervals; Fig. 3). However, total activity rates by Kelp Gulls (in 2004) and Dolphin Gulls (in both years) were higher during the last week of the study, once penguin crèches had formed, than during the first weeks (Wilcoxon test, 2003: P = 0.22 for Kelp Gulls, P = 0.04 for Dolphin Gulls; 2004: P = 0.05 for Kelp Gulls, P = 0.03 for Dolphin Gulls, all n = 8 2-day intervals; Fig. 3). Early in the chick-rearing period activity by gulls was low. Later in the sampling period, as crèches formed and both penguin parents were foraging, the open ground space between nests increased and gulls continually searched the subcolonies from the air, on occasion landing and walking inside the subcolony attempting to take dead chicks or abandoned eggs. Even though scavenging rates were too low for statistical analysis, scavenging events by gulls were not observed until after 24 December in 2003 (n = 4) and 18 December in 2004 (n = 15).
Total activity rate (searches, predation, attempts and scavenging events/h) of the four most common predator/scavenger species with time of season, divided into 12 (7–30 December in 2003) and 8 (8–25 December in 2004) 2-day intervals. The 2-days intervals are grouped in “early” and “late” in the season based on the day the first crèche was observed in the study subcolonies (see text)
Predator’s method of approach
Highest predation and attempted predation events were observed on the functional and geometric edge of the subcolony (Fig. 4). There was a significant association between predator’s method of approach and nest location and this pattern did not differ significantly between years (Mantel–Haenszel χ2 = 120.62, P < 0.0001; Fig. 4). Predation and attempted predation events from peripheral and central tussocks were associated with nests on the geometric and functional edges, respectively; events from the air were associated with central nests; and those from the ground (which were very rare) were associated with nests on the geometric edge. Kelp Gulls were only observed approaching from the air (predation and attempted predation events from the air for 2003 and 2004 combined, n = 5). On the other hand, for Striated Caracaras, predation and attempted predation events varied significantly with the method of approach (both years combined χ2 = 42.73, P < 0.0001, df = 3). They were observed using all four methods of approach, but they used peripheral and central tussocks more frequently than the other two methods (predation and attempted predation events from air, n = 5; from central tussocks, n = 33; from peripheral tussocks, n = 26; from ground, n = 2). Normally, they would land on a tussock and remain as long as it took the adult penguins to habituate to their presence or become distracted. Occasionally, penguins from nests near that tussock would chase the attacking bird away.
Percent of predation and attempted predation events (% P & AP; n = 25 in 2003, n = 46 in 2004) on Rockhopper Penguin chicks by Striated Caracaras and Kelp Gulls, collectively, during 2003 and 2004. Events were recorded from the air, from central or peripheral tussocks or from the ground on nests on the geometric edge, functional edge or center of the subcolonies. Numbers above bars are sample sizes
Predation, nest location and subcolony size
In both years, total predation and attempted predation events were significantly higher on the edge than in the center of subcolonies (2003: χ2 = 6.76, P = 0.01, df = 1, n = 25; 2004: χ2 = 31.3913, P < 0.001, df = 1, n = 46).
Subcolony predation rate was not correlated with subcolony size (partial correlation, r = 0.14, P = 0.6, n = 17; Fig. 5). Predation events were rare, and in several small as well as large subcolonies no predation events were observed. We observed a negative though non-significant correlation of individual predation rate with subcolony size (partial correlation, r = −0.17, P = 0.5, n = 17; Fig. 5). However, for those subcolonies which suffered predation rates >∼0.05 events/h individual predation rate was lower for larger subcolonies (Fig. 5).
Nest success
Year was a significant predictor of the probability of nest success, while subcolony size was marginally significant (Table 2), suggesting that there is a tendency for nest success to increase with subcolony size. Alternatively, there was no significant effect of nest location on the probability of nest success.
Discussion
A potential problem of our study is that observation periods were limited to daylight hours, thus possibly underestimating predation rates. There are records of nocturnal predation by Striated Caracaras on Rockhopper Penguin chicks on Beauchene Island (Strange 1996 in Meiburg 2006) and, though rarely seen at our subcolonies, Southern Giant Petrels are known to prey on King Penguins chicks during the night (LeBohec et al. 2003). Also, our study could not include the entire chick-rearing period and was limited to only the first few weeks after hatching. However, the data do provide an estimate of total predation that is occurring throughout the breeding period.
Predators and scavengers at Rockhopper Penguin subcolonies
Striated Caracaras at Bahía Franklin were the main predators on Rockhopper Penguin chicks. This result agrees with previous observations which indicated that these subcolonies are usually occupied by either a single adult breeding pair of Striated Caracaras or by a “gang” of juveniles, immatures and/or adult nonbreeders; these groups sometimes also included Turkey Vultures and juvenile Crested Caracaras (J. Meiburg, personal communication). On a survey conducted in November 2001 at Bahía Franklin, 20 adult Striated Caracara pairs (five of them with nests) and five immature foraging groups were located and their territories mapped (Meiburg 2006). All of the territories were within the Rockhopper colony at Bahía Franklin.
At Rockhopper penguin colonies on New Island, Falkland Islands, skua predation is a major cause of egg mortality (St. Clair and St. Clair 1996) and they were also observed taking penguin chicks during the guard period (St. Clair and St. Clair 1996) and during the crèche period (Pettingill 1960). At Staten Island, Chilean Skuas have been seen on occasion feeding on adult/chick penguin remains in the periphery of the subcolonies or close to their nests (A. Raya Rey, personal communication). However, during this study, skuas were never seen taking penguin chicks or scavenging, and accounted for less than 5% of the total activity events in all subcolonies for both years. Further study is needed on their potential impact on Rockhopper penguins during the incubation period and during the rest of the chick-rearing period.
Searches were the most common activity for all species. This result agrees with other studies of South Polar Skuas (Catharacta maccormicki) at Adélie Penguin colonies (Young 1994) and Brown Skuas (Catharacta lonnbergi) at Adélie and Gentoo Penguin colonies (Emslie et al. 1995). Emslie et al. (1995) suggested that frequent searches by predators might help them locate vulnerable prey or assess prey for later attacks.
Predation, nest location and subcolony size
The effect each species of predator has on a colony depends on the size of the predator in relation to the defending species or risk, and the method of approach by the predator (Brunton 1997). In Least Terns (Sterna antillarum), small colonies appeared to be more vulnerable to predators that approached from the periphery, whereas large colonies were more vulnerable to predators approaching from the air directly into the center of the colony (Brunton 1999). Brunton (1997) concluded that the selfish herd hypothesis may be limited in application to only when predators are restricted to approach from the edge due to the type of predator, the effectiveness of the antipredator behavior by the prey species, or the physical characteristics of the nesting site.
Our results support the prediction of higher predation and predation attempts on the edge than on the center of subcolonies (prediction 1). In accordance to these results, several studies have reported higher nest predation on the edge of the colonies (Young 1994; Emslie et al. 1995; Yorio and Quintana 1997; Descamps et al. 2005) and in all these studies predators approached almost exclusively from the ground. In Rockhopper penguin subcolonies at Staten Island predation and attempted predation events from the ground were rare. Instead, Striated Carcaras, the main predator, preferentially attacked from tussocks which are found in the periphery of all subcolonies and are often scattered within them. These central tussocks offer Striated Caracaras an additional “edge” area from which to prey on penguin chicks, much in the same way as do the peripheral tussocks.
Larger subcolonies offer a greater number and variety of prey, and might attract more predators than smaller ones, resulting in higher predation rates. Hunter (1991) calculated predation rates on King Penguins by collecting carcasses and found that they increased with colony size. Emslie et al. (1995) calculated predation rates on Adélie Penguins from direct observations and found the highest rates at larger colonies. However, a surprising result of our study is the absence of a correlation between subcolony predation rate and subcolony size. The non-significance of our results may be due to low sample sizes or to differences in the number and kind of predators associated with each subcolony. Fore example, Striated Caracaras at Staten Island often hold territories that include entire penguin subcolonies (M. Liljesthröm, personal observation). We found that those subcolonies that were not part of a Striated Caracara territory could have up to five caracaras simultaneously within them, whereas a similar sized subcolony that was part of a territory would have no more than two caracaras (the breeding pair) within it at any one time, resulting in a lower predation rate at that subcolony. Further study is needed to determine if territory is a major factor in predation rates at specific subcolonies.
Breeding in larger colonies can still be adaptive for reducing predation risk as long as predation rates are proportionally lower in larger colonies. Davis (1982) found that the number of Adélie chicks lost to predation was greatest at the largest crèche, although the proportion was smallest. Hernández-Matías and Ruiz (2003) found that larger subcolonies of Common Terns had a higher absolute number of depredated nests, but a lower percentage of nest predation than smaller subcolonies and observed that the probability of predation was negatively related to subcolony size. When predation occurs mainly on the edge at a subcolony with a circular shape, the proportion of vulnerable nests is higher at smaller subcolonies because smaller areas expose a proportionally greater number of individuals to the edge than in larger subcolonies. Thus, according to the selfish herd hypothesis, individual predation rate should be higher at smaller subcolonies. However, contrary to our second prediction, we found that individual predation rate was not correlated with subcolony size. A possible explanation may be the presence of central tussocks which create an extra edge area and, in a way, fragment the subcolony by changing its shape. Jackson et al. (2005) pointed out that one factor not considered explicitly in the selfish herd hypothesis is shape: for equal group sizes, different shapes will expose different numbers of individuals to the edge. Even though almost all Rockhopper penguin subcolonies at Bahía Franklin are nearly circular in shape along the outer edges, the number and size of central tussocks within them varies. Thus, similar sized subcolonies will expose different number of nests to the edge depending on the presence of central tussocks. Further studies accounting for the effect of subcolony shape on predation risk are needed.
Nest success
Our results suggest a tendency for increased nest success with subcolony size. Several studies have found higher breeding success in large colonies. In Chinstrap Penguins, nests in large subcolonies, whatever their position, were more successful than nests in small ones (Barbosa et al. 1997). In Adélie Penguins, lower breeding success in small subcolonies has been explained by the increased proportion of peripheral nests which suffer higher rates of nest predation (Tenaza 1971). Similarly, Emslie et al. (1995) reported low breeding success by penguins in small colonies and attributed this, in part, to predation losses. In our study, smaller subcolonies had lower nest success than larger ones, though predation rates were low or absent at several of them. Differences in breeding success within a subcolony are not always attributable to predation; factors such as the quality of birds nesting at the edge versus the center of the colony might be involved (Coulson 1968). For instance, in Adélie Penguins the lower breeding success of peripheral nests has been explained by the presence of older and more experienced birds in the center of the colony and a larger proportion of young and inexperienced birds on the periphery (Ainley et al. 1983). The affects of age-related factors on nest success and the impact of predation during the entire breeding period remain to be explored.
We also found that year was a significant predictor on the probability of nest success. Differences in breeding success could be due to differences in hatching date. Some authors have reported negative effects of late breeding on penguin breeding success (Taylor 1962; Viñuela et al. 1996); however, contrary to these results, Barbosa et al. (1997) did not find any relationship between mean hatching date and mean chick survival neither for large nor for small subcolonies. Raya Rey et al. (2007) investigated provisioning rates and chick survival of Southern Rockhopper penguins at Staten Island during the breeding seasons of 2002–2003 and 2003–2004. Their results show no difference in mean hatch date between years and they found evidence that differences in chick survival between years could be affected by differences in provisioning rates by parents, especially during the guard stage of the breeding season (Raya Rey et al. 2007). Thus, even though we were not able to determine hatching dates because our study began after hatching, differences in nest success associated with different hatching dates between years are unlikely.
References
Ainley AG, LeResche RE, Sladen WJL (1983) Breeding biology of the Adélie Penguin. University of California Press, Berkeley
Anderson D J, Hodum PJ (1993) Predator behavior favors clumped nesting in an oceanic seabird. Ecology 74:2462–2464
Barbosa A, Moreno J, Potti J, Merino S (1997) Breeding group size, nest position and breeding success in the chinstrap penguin. Polar Biol 18:410–414
Bellinato F, Bogliani G (1995) Colonial breeding imposes increased predation: experimental studies with herons. Ethol Ecol Evol 7:347–353
Birdlife International (online)(2005) Species fact sheet: Phalcoboenus australis. http://www.birdlife.org(8 November 2005)
Brown CR, Brown MB (2001) Avian coloniality, progress and problems. Curr Ornithol 16:1–82
Brunton DH (1997) Impacts of predators: center nests are less successful than edge nests in a large colony of Least Terns. Condor 99:372–380
Brunton D (1999) “Optimal” colony size for Lest Terns: an intercolony study of opposing selective pressures by predators. Condor 101:607–615
Coulson JC (1968) Differences in the Quality of Birds nesting in the Center and on the Edges of a Colony. Nature 217:478–479
Davis L S (1982) Creching behaviour of Adélie penguin chicks (Pygoscelis adeliae). NZJ Zool 9:279–286
Descamps S, Gauthier-Claerc M, Le Bohec C, Gender JP, Le Maho Y (2005) Impact of predation on King Penguin Aptenodytes patagonicus in Crozet Archipelago. Polar Biol 28:303–310
Emslie SE, Karnovsky N, Trivelpiece W (1995) Avian predation at penguin colonies on King George Island, Antarctica. Wilson Bull 107:317–327
Hamilton WD (1971) Geometry for the selfish herd. J Theor Biol 31:295–311
Hernández-Matías A, Jover L, Ruiz X (2003) Predation on Common Tern Eggs in Relation to Sub-colon Size, Nest Aggregation and Breeding Synchrony. Waterbirds 23:280–289
Hernández-Matías A, Ruiz X (2003) Predation on common tern eggs by the yellow-legged gull at the Ebro Delta. Sci Mar (Barc) 67:95–101
Hunter S (1991) The impact of avian predator-scavengers on King Penguin Aptenodytes patagonicus chicks at Marion Island. Ibis 133:343–350
Jackson AL, Bearhop S, Thompson DR (2005) Shape can influence the rate of colony fragmentation in ground nesting seabirds. Oikos 111:473–478
Le Bohec C, Gauthier-Clerc M, Gender J-P, Chatelain N, Le Maho Y (2003) Nocturnal predation of king penguins by giant petrels on the Crozet Islands. Polar Biol 26:587–590
Meiburg JA (2006) The biogeography of Striated Caracaras Phalcoboenus australis. Unpublished Master of Arts thesis, The University of Texas at Austin
Narosky T, Yzurieta D (1987) Guía para la identificación de las aves de Argentina y Uruguay. Asociación Ornitológica del Plata. Buenos Aires
Pettingill Jr OS (1960) Crèche behavior and individual recognition in a colony of Rockhopper Penguins. Wilson Bull 72:213–221
Raya Rey A (2006) Ecología trofica de Eudyptes chrysocome chrysocome en el mar Austral. Unpublished PhD thesis, University of Buenos Aires
Raya Rey A, Trathan P, Schiavini A (2007) Inter-annual variation in provisioning behaviour of Southern Rockhopper Penguins Eudyptes chrysocome chrysocome at Staten Island, Argentina. Ibis (online early articles)
SAS version 9.1 (2006) SAS Institute Inc., Cary, NC
Schiavini A (2000) Staten Island, Tierra del Fuego: the largest breeding ground for Southern Rockhopper Penguins? Waterbirds 23:286–291
Spear LB (1993) Dynamics and effect of western gulls feeding in a colony of guillemots and Brandt’s cormorants. J Anim Ecol 62:399–414
St. Clair CC, St. Clair RC (1996) Causes and consequences of egg loss in rockhopper penguins, Eudyptes chrysocome. Oikos 77:459–466
Stokes DL, Boersma PD (2000) Nesting density and the reproductive success in a colonial seabird, the Magellanic Penguin. Ecology 81:2878–2891
Taylor RH (1962) The adelie penguin Pygoscelis adeliae at Cape Royds. Ibis 104:176–204
Tenaza R (1971) Behavior and nesting success relative to nest location in Adélie Penguins (Pygoscelis adeliae). Condor 73:81–92
Viñuela J, Moreno J, Carrascal LM, Sanz JJ, Ferrer M, Amat JA, Belliure J, Cuervo JJ (1996) The effect of hatching date on parental care, chick growth and chick mortality in the chinstrap penguin Pygoscelis antarctica. J Zool Lond 240:51–58
Wittenberger JF, HuntGL (1985) The adaptive significance of coloniality in birds. In: Farner DS, King JR, Parkes KC (eds) Avian Biology, vol. 8. Academic, New York, pp 1–78
Yorio P, Quintana F (1997) Predation by Kelp Gulls Larus dominicanus at a mixed-species colony of Royal Terns Sterna maxima and Cayenne Terns Sterna eurygnatha in Patagonia. Ibis 139:536–541
Young E (1994) Skua and penguin. Cambridge University Press, England
Acknowledgments
This research was completed as part of the senior author’s Masters thesis at UNCW. We thank M. Galizio and M. McCartney for their help and comments while serving on the thesis committee. We also thank Soledad Albanese, Carlos Cabrera, Christine Calleri, Carolina Gargiulo, Fernanda Malacrida, and Marcelo Turus for assistance in the field. We also thank Jonathan Meiburg and Andrea Raya Rey for sharing their observations on Striated Caracaras and Rockhopper Penguins at Staten Island. Funding for this research was provided in part by grants from the National Geographic Society (7040-01 and 7572-03) and NSF (OPP-0125098) to S. Emslie. We also appreciate the support of the Argentinean Navy and the “Ushuaia” tour vessel for transportation to and from Staten Island.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liljesthröm, M., Emslie, S.D., Frierson, D. et al. Avian predation at a Southern Rockhopper Penguin colony on Staten Island, Argentina. Polar Biol 31, 465–474 (2008). https://doi.org/10.1007/s00300-007-0372-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-007-0372-1