Abstract
Feeding habits of ringed (Phoca hispida), bearded (Erignathus barbatus), spotted (Phoca largha) and ribbon (Phoca fasciata) seals and walrus (Odobenus rosmarus) were studied using stomach contents and stable carbon and nitrogen isotopes. Bearded seals fed benthically, primarily crustaceans and mollusks. Both zooplankton and fish were significant prey for ringed seals, while fish was principal spotted seal prey. Few gastric contents were available from ribbon seals. δ15N was positively correlated with age in ribbon seals and δ13C was positively correlated with age in ringed and ribbon seals. δ15N was highest in spotted seals, in agreement with their fish-dominated diet. δ15N was not different between Alaskan-harvested ringed and bearded seals, while δ15N was lowest in ribbon seals and walrus. Carbon-13 was most enriched in bearded seals and walrus reflecting benthic ecosystem use. Canadian ringed seals were depleted in 13C compared to Alaskan pinnipeds, likely because of Beaufort Sea versus Chukchi and Bering seas influence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distribution, movement and feeding ecology of ringed (Phoca hispida), bearded (Erignathus barbatus), spotted (Phoca largha) and ribbon (Phoca fasciata) seals are strongly associated with Arctic sea ice (Braham et al. 1984). They are commonly referred to as ice or pagophilic seals, but are adapted to different niches within the sea ice environment, and only some overlap occurs among species (Burns 1970; Simpkins et al. 2003). These seals are important prey to higher trophic level organisms, including Arctic fox (Alopex lagopus), polar bear (Ursus maritimus), humans and some walrus (Odobenus rosmarus) (Smith 1976; Lowry and Fay 1984; Hammill and Smith 1991; Derocher et al. 2002). Ice seals have significant nutritional and cultural importance to the Native coastal population of Alaska and other Arctic areas. Trophic relationships are of key importance to the understanding, management and conservation of free-ranging marine mammals. Nutritional status of marine mammals is a factor that can limit reproductive output and thus population growth.
In the past, analysis of stomach contents has been used extensively to determine feeding ecology of pagophilic seals (Frost and Lowry 1980; Lowry et al. 1980a, b; Bradstreet and Finley 1983; Finley and Evans 1983; Smith 1987). Recently, it was suggested that analysis of stomach contents or scats of marine mammals is strongly biased and overestimates prey with chitinous structures (e.g., crustaceans, cephalopod beaks) and fish otoliths that resist digestion or are retained in the stomach, and underestimates soft prey such as snails and mussels that are digested within hours (Murie and Lavigne 1986; Gales and Cheal 1992; Bowen 2000; Sheffield et al. 2001). Secondary ingestion of prey, such as digestive tract contents of ingested fish, could also lead to biased prey assessments (Santos et al. 2001). It is therefore difficult to accurately assess the importance of prey species, prey preference or dietary shifts as stomach contents only present a single point in time and empty stomachs yield no prey-based information. The use of other methods has been suggested by Sheffield et al. (2001) to identify diet, and a combination of classic methods with chemical feeding ecology has proven powerful in dietary reconstructions (Hobson et al. 1997; Burns et al. 1998).
Stable isotopes of carbon and nitrogen have become increasingly important in trophic ecology studies. They occur naturally and nitrogen isotope ratios become enriched in consumer tissues as trophic level increases due to selective incorporation of the heavier isotope (15N) in tissues (DeNiro and Epstein 1981). Thus, nitrogen isotope analysis is helpful in establishing trophic level and predator-prey relationships (Kelly 2000). However, turnover rates and tissue- and species-specific enrichment factors are poorly understood and make evaluation of stable isotope ratios difficult (Gannes et al. 1997; Adams and Sterner 2000; McCutchan et al. 2003). Further, biological variables, e.g., age, body condition, gestation, lactation, water stress, body protein catabolism and urea recycling can have substantial impact on stable isotope ratios and their interpretation (Hobson et al. 1993; Hobson et al. 1997; Fernandez-Mosquera et al. 2001).
Stable carbon isotopes may enrich in consumer tissues to a minor degree and are therefore less useful in the determination of trophic position or predator-prey relationships (Tieszen et al. 1983; France 1995a). However, species differences in δ13C can provide insights on spatial habitat use and carbon sources (France 1995b, Smith et al. 1996; Schell et al. 1998; Burton and Koch 1999).
Feeding ecology of ice seals in the Alaskan Arctic has been determined almost solely via stomach contents analysis, and few data are available on stable isotopes in ice seals. However, stable isotope analysis provides a cost effective and potentially minimally invasive (via biopsy sampling) alternative to monitor trophic level and possibly nutritional status of free-ranging ice seals. In addition, stable isotopes have become important tools for ecotoxicological studies to assess contaminant transfer and magnification (Fisk et al. 2001; Hobson et al. 2002; Dehn et al. 2006a). Interpretation of baseline stable carbon and nitrogen isotope ratios and their natural variability can be more conclusive when prey composition and seal age are known. Simultaneous examination of stomach contents and stable isotope ratios promises the most success with dietary reconstructions.
The objectives of this study are threefold and aim to (1) evaluate and compare feeding ecology of Arctic phocids harvested in Alaska and Canada using stomach contents and stable isotope analysis, (2) provide baseline data for stable isotopes in muscle of apparently healthy pinnipeds and typically ingested prey and (3) discuss isotope ratios in muscle and prey composition in stomachs with regard to age.
Materials and methods
Sample collection
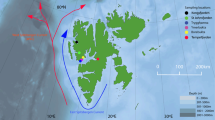
All marine mammal samples were obtained during Native subsistence harvests and thus most seals were acquired in nearshore areas. Basic morphometrics, e.g., body length, blubber thickness and sex were recorded. Seals were grossly examined for lesions and parasites. Lumbar muscle samples and stomachs were collected from ringed and bearded seals in Barrow, Alaska mainly during the summer period from 1996 to 2003. Ringed seal samples also were collected in Holman, NWT, Canada during summer, 2001. Tissues of spotted seals were collected in Little Diomede and Shishmaref, Alaska primarily during summer and fall in 2000–2001 and 2003. Ribbon seal samples were acquired in Little Diomede, Alaska, during summer 2003. Walrus muscle was obtained opportunistically in Barrow and Little Diomede during summer 1998 and 2003 and serves mainly as a comparison to bearded seal muscle. Figure 1 shows communities where samples were collected, and Table 1 summarizes sample sizes. Muscle tissue was sub-sampled under clean conditions with titanium or ceramic blades on a Teflon covered surface, following the sampling protocol for contaminants by Becker et al. (1999) and stored at −20°C in acid-washed vials or whirlpacks™ until analysis. Several potential prey species were collected or donated by subsistence hunters in Barrow and the Alaskan Bering Strait region. Marine mammal samples were collected and analyzed under Permit Nos. 782–1399, 358–1585 and 932-1489-03.
Stomach contents analysis
Stomachs of ringed and bearded seals were collected by tying off cardiac and pyloric sphincters to avoid spillage, placed into a bag and frozen at −20°C until analysis in Fairbanks. Stomachs obtained from spotted and ribbon seals near Little Diomede and Shishmaref were archived and analyzed by the Alaska Department of Fish and Game (ADF& G) in Fairbanks.
Stomach contents were weighed to the nearest gram for ringed seals and with a chatillon scale (0.1 pound increments) for bearded seals and sequentially washed through three sieves with mesh sizes 3.96, 1.4 and 0.5 mm. Spotted and ribbon seal stomach contents were sequentially washed through sieves with mesh sizes 1.0 and 0.5 mm. Standard reference keys (Rathbun 1929; Akimushkin 1965; Keen and Coan 1974; Butler 1980; Frost and Lowry 1980; Frost 1981; Härkönen 1986; Kathman et al. 1986; Foster 1991; Jensen 1995; Harvey et al. 2000) were used for the identification of fish otoliths and invertebrate prey to the lowest possible taxonomic level. Identifiable prey were sorted, counted and weighed to the nearest milligram. Due to digestive biases on diagnostic tissues of varying endurance (e.g., overestimation of chitinous prey versus under-representation of soft prey, such as echiurid worms and polychaetes) a ranking of prey by weight or numerical frequency of prey in the stomach was not determined and only the frequency of occurrence of prey species i (FO i method) was noted for all seals. FO i is defined as the percentage of stomachs that contained one or more individuals of the prey species i:
where p i is the number of stomachs with the prey species i and p t is the number of stomachs with digesta (Hjelset et al. 1999). Nematodes in the stomach and cestodes migrating from the duodenum to the stomach after death were found in all seals, in particular bearded seals on a regular basis and were considered normal (Lauckner 1985). They were not analyzed as a food item and hence not included in p t .
Stable isotopes
Lumbar muscle of pinnipeds and total body homogenates of potential prey were freeze-dried and ground into a fine powder. For each sample, 0.2–0.4 mg of tissue was weighed into a 4.75 × 4 mm tin capsule and folded into a cube. Samples were analyzed for both stable carbon and nitrogen ratios at the University of Alaska Fairbanks (UAF) using a Finnigan MAT DeltaPlusXL Isotope Ratio Mass Spectrometer (IRMS) directly coupled to a Costech Elemental Analyzer (ESC 4010). Samples were flash combusted at 1,020°C, followed by on-line chromatographic separation of sample N2 and CO2 with He as carrier gas. Samples analyzed for 15N/14N and 13C/12C were standardized against atmospheric N2 and PeeDee Belemnite limestone, respectively. Enrichment of a particular isotope was reported using the following notation and equation:
where the differential notation (δR) represents the relative difference between isotopic ratios of the sample and standard gases (i.e., 13C/12C, 15N/14N). A laboratory-working standard (Peptone No. P-7750) was analyzed every 10 samples during analysis, and tin capsule blanks were run every 20 samples. Calibrations were made with the use of stable isotope reference materials provided by the National Institute of Standards and Technology (NIST). External instrument reproducibility for both carbon and nitrogen isotope analysis was ±0.2‰.
Aging
Jaws and claws were collected from seals after 1997 and stored at −20°C until analysis. Ringed seals harvested in 1996 and 1997 were aged using the keratin layers of claws, which represent a minimum age estimate for the animals (Benjaminsen 1973). Two canines or canine and postcanine (if only one canine was available) were extracted from upper or lower jaw (depending on availability), submerged in a hot water bath for 30 min to avoid damage to the structure of the cementum, and stored in paper envelopes (Matson 1981). All teeth were shipped to Matson’s Laboratory, Milltown, MT, USA for slide preparation. Teeth were prepared in 14 μm sections, placed on glass slides and stained with Giemsa histological stain suitable for cementum analysis. Age was estimated by counting annual growth layers in the cementum of teeth by two independent readers at UAF. Preparation and evaluation of teeth were done doubly blind by randomly assigning an identification number to each tooth with two teeth analyzed per animal. Animal identification and matching teeth were revealed only after all ages were estimated in duplicate. One growth layer per year of age was assumed for all seals (Benjaminsen 1973; Stewart et al. 1996). Maximum variation in age estimates was ±1 year for seals younger than 15 years and ±5 years in animals older than 15.
Statistical analysis
The response variables in the data set (age, δ15N and δ13C) were ranked prior to analysis to reduce the risk of violations of normality and homogeneity of variance assumptions. Variables were analyzed for sex and location differences using the Mann–Whitney test within a seal species. If no significant differences were detected for each variable, sexes and localities were pooled. A residual analysis was conducted to determine possible violations of assumptions. Spearman rank correlation was calculated within a species to determine correlations between age and stable isotope ratios. LOESS non-parametric smoothing was used on non-ranked raw data to estimate suitable functions between two variables and compare regression surfaces between species. The Kruskal–Wallis test followed by Tukey’s multiple comparison procedure was used to compare variable means between seal species. For statistical analysis of stomach contents, indicator variables were established for each seal species based on presence and absence of prey items (0 = absent, 1 = present). These indicator variables were analyzed using logistic regression with interaction term (forward selection) with age and sex (indicator variable) as independent variables for each species. The odds-ratio (OR) is a term used in logistic regression and assesses the strength of an association. An OR of 1 indicates no association of variables, while an OR greater than 1 suggests that the “event” (i.e., presence of prey) is more likely to occur in one group than the other, e.g., male versus female. All statistical analyses were performed using SAS (Version 8) with α = 0.05. Sigma-Plot (Version 7.0) was used for graphic presentation of data. Results are reported as mean ± standard deviation (SD) unless otherwise noted.
Results
Stomach contents analysis
Table 2 presents frequency of occurrence (FO i ) of prey in stomachs of bearded, ringed and spotted seals harvested in Alaska. FO i of prey was calculated for all stomachs containing digesta. For ringed seals, 50 stomachs were analyzed and 11 were empty or contained only bile, blood or parasites. Of 37 bearded seal stomachs, one was empty and contained only parasites. For spotted seals 43 stomachs were analyzed and 5 contained no food. All 25 stomachs of ringed seals harvested in Holman, Canada were empty. Of 37 stomachs collected from ribbon seals only two contained prey. The small sample size did not allow for further statistical analysis. Arctic cod (Boreogadus saida) otoliths were identified from both ribbon seal stomachs containing digesta (a 3-year-old male and a 2-year-old female). Additionally, the male contained one twospine crangon (Crangon communis) and the female had fragments of yellowleg pandalid (Pandalus tridens) in the stomach.
The frequency of fish was 62% in stomachs of ringed seals containing prey (Table 2). Of all fish identified, gadids like Arctic cod and saffron cod (Eleginus gracilis) were identified most often, followed by Pacific sand lance (Ammodytes hexapterus). Zooplankton in stomachs of ringed seals occurred at a frequency of 64%. Both euphausids and mysids were consumed in similar proportions, as were amphipods and pandalid shrimp. All other prey were present in less than 10% of stomachs with contents.
Prey diversity in bearded seal stomachs was higher than in ringed and spotted seals with more than 20 different species consumed representing more than 10 animal phyla (Table 2). Prey was ingested intact in most cases though only feet of ingested clams and snails were present and only abdomens of predominately gravid female spider crabs (Hyas coarctatus) were identified from stomach contents. Crustaceans were found in 97% of stomachs. Of the prey species consumed, sculptured shrimp (Sclerocrangon boreas) was present most often. The frequency of fish in bearded seal stomachs was 81%, with eelpout (Lycodes spp.) making up the majority of teleost prey. Other prey identified in more than 50% of the stomachs consisted of northern shrimp (Pandalus spp.), amphipods, spider crabs, octopus, Greenland cockle (Serripes groenlandicus) and echiurid worms.
Fishes were identified in all spotted seal stomachs containing prey (Table 2). Most frequently found was Pacific herring (Clupea pallasii), followed by gadid fish and rainbow smelt (Osmerus mordax). Capelin (Mallotus villosus), sand lance and flatfish were found in more than 10% of the stomachs, while other teleosts (e.g., sculpin) were present in less than 10%. Invertebrate prey was mainly comprised of crustaceans with amphipods making up the largest proportion. Mollusks occurred in less than 10% of the stomachs.
Generally, there was no statistical difference in the frequency of prey types consumed by males and females for either bearded or spotted seals. However, bearded seal males were 6 times more likely to contain smelt (OR = 6.06, P = 0.04) and 7.5 times more likely to contain sea cucumber than females (OR = 7.52, P = 0.01). In ringed seals, male stomachs were about 16 times more likely to contain zooplankton than females (OR = 15.87, P = 0.0004). This relationship was also significant when mysids and euphausids were analyzed separately (P = 0.007 and 0.002 for euphausids and mysids, respectively). Similarly, stomachs of female ringed seals were more likely to contain fish than those of males (OR = 5.42, P = 0.04). Sex was not significant when teleost species were analyzed separately.
Older bearded seals were less likely to consume whelks (Buccinum spp.) (OR = 0.87, P = 0.049). No other age effects were noted in bearded seal diets. In spotted seals, presence of capelin and flatfish increased with increasing age (P = 0.003, OR = 1.90 and P = 0.005, OR = 1.41, for capelin and flatfish, respectively). In ringed seals only the consumption of gadid fish was related to age (P = 0.01, OR = 1.22), while zooplankton ingestion was age independent.
Stable isotopes
Age and isotope composition of spotted seals harvested near Shishmaref and Little Diomede were not statistically different (P = 0.09, 0.25 and 0.68 for age, δ15N and δ13C, respectively). Therefore these data were pooled to increase sample size and power. Ringed seals harvested in Holman were significantly more depleted in 13C than ringed seals from Barrow (P < 0.0001). Stable nitrogen isotope ratios and age were higher in Canadian ringed seals (P = 0.01 for both δ15N and age) than in animals sampled in Barrow and therefore seals from Alaska and Canada were analyzed separately. Generally, there were no sex differences within species in age composition, stable carbon or stable nitrogen isotope ratios. However, female ringed seals harvested in Barrow had higher stable nitrogen isotope ratios than males harvested in Barrow (P = 0.02) and they were analyzed separately for this variable. Male and female ringed seals from Holman as well as bearded, ribbon and spotted seal sexes were pooled.
Spearman rank test showed no significant correlation between variables (age, δ15N and δ13C) in spotted seals and ringed seals harvested in Holman. Age was positively correlated with δ15N in ribbon seals (P = 0.004), and was positively correlated with δ13C in ringed seals from Barrow (P = 0.0006) and ribbon seals (P = 0.009). Age was negatively correlated with δ15N in bearded seals (P = 0.03).
Mean ratios of stable carbon and nitrogen isotopes in seals, walrus and some prey species (analyzed in this study and compiled from literature) are given in Table 3. Kruskal–Wallis testing showed significant differences in stable carbon and nitrogen isotope ratios between seal species (P < 0.0001 for δ15N and δ13C). Figure 2 illustrates δ15N versus δ13C in pinnipeds analyzed. Stable nitrogen isotope ratios were significantly higher in spotted seals and ringed seals from Holman than walrus, bearded and ribbon seals and ringed seals harvested in Barrow. δ15N was not statistically different in bearded seals and ringed seals from Barrow and was lowest in walrus and ribbon seals. Carbon-13 was significantly more enriched in bearded seals and walrus than the other species, while ringed seals from Holman were most depleted in 13C. No difference in δ13C was detected between ringed seals from Barrow, spotted seals and ribbon seals.
Discussion
Ringed seals
Stomach contents analysis
Analysis of ringed seal stomachs showed Arctic cod prevalence increased with age and could be related to hunting experience or habitat when foraging. This finding agrees with observations by Lowry et al. (1980a) that ringed seal pups consume less gadids than adults, and Bradstreet and Finley (1983) noted a decline in the presence of crustaceans in stomachs with age in ringed seals. However, age was not a significant variable for consumption of zooplankton, crustaceans or invertebrate prey in general in this study. This could be related to the use of the FO i method, as it tends to overestimate the importance of less commonly or unintentionally ingested prey (Hjelset et al. 1999). It is possible that seals preying on schooling fish, such as Arctic cod, will also ingest krill and amphipods as fish schools feed on zooplankton patches (Lowry and Frost 1981; Hop et al. 1997). Hence a decline in the importance of crustaceans with age cannot be ruled out, as the relationship of numerical frequency of krill with age was not determined. It is possible that ingestion of zooplankton is necessary nutritionally for these seals. Very little is known about nutritional quality of most marine forage or nutritional requirements of seals. Geraci (1975) reported high levels of thiaminase, an enzyme that breaks down thiamine (Vitamin B1), in herring, smelt and capelin. As a result, captive and wild seal populations sustained exclusively on these fish can suffer from thiamine deficiency. Inclusion of krill in the diet, even in adult animals, could serve to fulfill a dietary requirement.
Analysis of stomach contents in this study showed significant differences in prey composition between male and female ringed seals harvested in Barrow. This difference in prey composition between sexes was also detected by means of stable isotope analysis. δ15N was higher in females than males and stomach contents analysis showed that females were more likely to eat fish, while males consumed more zooplankton. Lowry et al. (1980a) reported that female ringed seals from the Bering Sea ate more fish and less shrimp than did males, but differences were minimal and similar differences in prey selection could not be found in other Arctic regions. Possible explanations for the differences in foods ingested by male and female ringed seals could include spatial segregation of sexes and associated differential use of resources. Fedoseev (2000) described segregation of ice-associated seals by age and sex outside the breeding period. Differences in foraging strategy and prey selection between sexes have been indicated for northern elephant seals (Mirounga angustirostris) and northern fur seals (Callorhinus ursinus) (Hobson et al. 1997; Le Boeuf et al. 2000). Das et al. (2003) showed that female harbor porpoise (Phocoena phocoena) were enriched in 15N over males and suggested higher consumption or feeding on larger prey by pregnant or lactating animals.
Several circumpolar studies have noted that ringed seals prey only on a few key taxa, and Arctic cod and a variety of crustaceans were found as important food items (Lowry et al. 1980a; Bradstreet and Finley 1983; Smith 1987). Results of this study also show that krill and fishes (in particular Gadidae) make up the majority of ringed seal diet and are consumed in similar frequencies. Lowry et al. (1978) noted that amphipods had a high frequency (69%) in ringed seal stomachs taken near Barrow, but comprised only 4.6% of the total combined volume. Similarly, frequency of amphipods in this study was 39%, but biomass consumed was negligible. Amphipods have been reported as a major food item for ringed seals from Svalbard and the Canadian Arctic (Bradstreet and Finley 1983; Smith 1987; Weslawski et al. 1994). Based on stable nitrogen isotope ratios in ringed seals from Barrow and Holman, and assuming an enrichment factor of 2.4‰ for δ15N in seal muscle (Hobson et al. 1996), it is highly improbable that amphipods make up a large proportion of their diet in either region (Table 3). However, large variation in prey availability by region may confound a large-scale geographical comparison.
Ringed seals
Stable isotopes
Stable nitrogen isotopes varied over the range of one trophic level in ringed seals from both Barrow (15.6–18.0‰) and Holman (14.6–18.0‰). This likely reflects the consumption of different trophic level prey, e.g., either krill or Arctic cod. Stable nitrogen isotope ratios in assumed prey of ringed seals range from 9.9 ± 0.8 to 15.5 ± 1.0‰ for zooplankton and Arctic cod, respectively (Table 3), reflecting the upper, but not lower ranges of δ15N found in ringed seals (2.4‰ enrichment factor after Hobson et al. 1996). This suggests minor significance of a zooplankton-exclusive diet, but points to a krill and gadid mix for these seals.
While the gastric prevalence of Arctic cod was positively correlated with age, δ15N showed no age dependence. Several young animals (< 1 year) and one fetus displayed elevated 15N values. Similar nitrogen-15 enrichment has been observed in northern fur seal pups and suckling harbor porpoises, and it was suggested that milk has isotope signatures comparable to other maternal tissues (Hobson et al. 1997; Das et al. 2003). Trophic enrichment could therefore occur in nursing pups or in fetuses during gestation compared to their mothers. Roth and Hobson (2000) theorized that high rate of protein synthesis and catabolism in tissues of juveniles causes excretion of predominantly light nitrogen while the heavier isotope is incorporated and magnified in tissues.
A positive correlation of carbon isotope ratios with age was shown for ringed seals from Barrow, but not for ringed seals from the Canadian Arctic (Fig. 3). In both locations a similar relationship was noticeable after a LOESS non-parametric smoothing. However, the positive relationship observed in seals harvested in Barrow was most likely driven by some young-of-the-year (YOY) and one fetus [highlighted as black triangles (▴) in Fig. 3] that are depleted in 13C compared to the majority of seals. Body fat is usually depleted in 13C due to selective fractionation (DeNiro and Epstein 1977) and the ratios for the fetus and YOY suggest mobilization and transfer of carbon from maternal lipid stores to fetal development and milk production (Jenkins et al. 2001; Polischuk et al. 2001; Dehn et al. 2006b).
δ13C in muscle versus age based on cementum analysis of teeth of ringed seals harvested in Barrow, AK, USA, 1996–2003 and Holman, Canada, 2001. Four YOY and one fetus harvested in Barrow are highlighted as black triangles. A LOESS nonparametric smoothing (dashed lines) was employed to estimate and compare regression surfaces for ringed seals from both locations
Carbon isotope signatures in ringed seals from Holman were significantly depleted compared to the other seals. Schell et al. (1998) reported more depleted carbon-13 values in the Beaufort Sea versus continental shelf waters of the Bering and Chukchi seas and a similar pattern can be detected in baleen and muscle of bowhead whales (Balaena mysticetus) migrating between these two regions (Schell et al. 1989; Hoekstra et al. 2002). Figure 3 illustrates that δ13C in ringed seals from the Canadian Arctic can clearly be distinguished from ringed seals harvested in Barrow. Since many phocid seals migrate (Lowry et al. 1998) and ringed seals of the Canadian Arctic are known to move toward the Chukchi and Bering seas (Smith 1987; Harwood and Smith 2003), it is possible that some of the ringed seals harvested in the Barrow area had migrated or dispersed from the Beaufort Sea and hence show the characteristic low carbon-13 signature. Geographically, Point Barrow is the point of separation for Beaufort and Chukchi seas (Fig. 1) and seals from either area and with either carbon signature are common and taken by subsistence hunters.
Bearded seals
Stomach contents analysis
Stomachs of bearded seals examined in this study contained a wide variety of benthic and epibenthic prey and these seals can be characterized as generalists. Some aspects of bearded seal diet were similar to those previously reported, but others were markedly different. Antonelis et al. (1994) noted high frequencies of fish in bearded seals sampled in the Bering Sea in spring; the most common teleost species consumed was capelin. Finley and Evans (1983) also found large occurrences of fish in bearded seal stomachs from the Canadian Arctic with gadids being most common. In contrast, Lowry et al. (1980b) did not consider fish important prey for bearded seals based on volumetric measurements, even though frequency of fish ranged from 78 to 82%. However, volume of fish would be severely underestimated when only otoliths or bones are present. Frequency of fish found in stomachs of seals from the Barrow area in this study was similar to occurrences reported by Lowry et al. (1980b), but the most common species consumed was eelpout followed by gadids.
Large regional differences in consumption of clams are seen in bearded seal diets. While frequencies of Greenland cockle in this study are high and are in agreement with prevalence of cockle reported by Lowry et al. (1980b) in the Bering Sea, others only documented infrequent occurrences of less than 10% in the Canadian Arctic and Svalbard (Finley and Evans 1983; Hjelset et al. 1999) to absence in diet in the Okhotsk Sea (Pikharev 1941).
Frequency of octopus and echiurid worms in stomachs also varied among locations. Finley and Evans (1983) noted a high frequency of occurrence of octopus in bearded seal stomachs from the Canadian Arctic, but amounts consumed were minimal, while echiurid worms were not found. They concluded that neither octopus nor marine worms were important prey. Antonelis et al. (1994) and Hjelset et al. (1999) came to the same conclusion for bearded seals harvested in the Bering Sea and Svalbard, respectively. Lowry et al. (1980b) found no cephalopods, and echiurids were of minor importance. In contrast, this study showed high frequencies of octopus and echiurids in the diet of bearded seals harvested near Barrow and is in accordance with studies in the Sea of Okhotsk (Pikharev 1941).
The high frequency of occurrence of crustacean prey is in agreement with previous studies, though some regional differences are notable in species composition (Pikharev 1941; Lowry et al. 1980b; Finley and Evans 1983; Antonelis et al. 1994; Hjelset et al. 1999). Sea cucumber was reported as a minor food item in bearded seal stomachs analyzed from the Canadian Arctic and Okhotsk Sea (Pikharev 1941; Finley and Evans 1983), but was fairly common in seals in this study. These variations in bearded seal diet are likely area specific and reflect the local distribution of available prey in the Arctic. However, there are some deviations in prey composition reported from the Bering and Chukchi seas (Lowry et al. 1980b; Antonelis et al. 1994) compared to seals collected in this study from the Chukchi Sea. This could reflect changes in prey distribution or abundance over the past 10–20 years, although even small seasonal or regional differences in prey availability between studies make a temporal comparison difficult.
Bearded seal versus walrus
Stable isotopes
Stable nitrogen isotope ratios in bearded seal muscle range widely (15.2–18.8‰), and reflect the diverse feeding habits of these seals. Similarly, isotopic signatures of potential prey consumed by bearded seals vary considerably and some scavenging species, e.g., sculptured shrimp, can occupy equally high trophic levels as teleosts (Table 3). This makes it difficult to discern the importance of any particular prey species to bearded seals by means of stable isotope analysis and demonstrates the greatest limitation of this technique when potential prey species are numerous and cover a range of trophic levels.
The low nitrogen isotope ratios in walrus muscle indicate the reliance on lower trophic level prey. The significance of clams to walrus diet has been emphasized by a variety of reports (Lowry et al. 1980b; Fay 1982; Fay et al. 1984) and is further supported by the δ15N findings of this study. Fish are generally not present in walrus stomachs and frequency of octopus is negligible (Fay et al. 1984). While seal-eating walrus have been described, they do not represent the norm of the population (Lowry and Fay 1984). Based on nitrogen isotope ratios obtained it is not likely that walrus included in this study had consumed other pinnipeds in the recent past.
Values of δ13C in bearded seals show a large distribution, while carbon isotope ratios of walrus have a smaller range (−18.7 to −15.8 and –17.3 to –16.8‰ in bearded seals and walrus, respectively). The more enriched carbon isotope signatures in bearded seals and walrus compared to ringed, ribbon and spotted seals (Fig. 2) are likely due to benthic feeding habits (France 1995b), as confirmed by analyses of stomach contents. Enrichment of 13C has also been described for benthic feeding gray whales (Eschrichtius robustus) (Dehn et al. 2006b). The larger δ13C range in bearded seals could be related to migration routes between Beaufort and Chukchi Seas as discussed for ringed seals, but could also be associated with opportunistic feeding on both benthos and plankton. In contrast, walruses are specialists and rely almost exclusively on benthic prey. However, sample size for walrus muscle was small and could account for the smaller variation in their isotope ratios. Based on these results it is unlikely that walrus and bearded seal have a large overlap in prey utilization, but it is possible that competition between these two pinnipeds is the driving force for a dietary change in bearded seals as suggested by Lowry et al. (1980b).
Ribbon seals
Stable isotopes
Very little is known about ribbon seal biology due to their remote distribution in the pack ice (Braham et al. 1984; Simpkins et al. 2003). Ribbon seals sampled for this study were harvested during their annual molt. During this time they generally do not forage (Shustov 1965; Frost and Lowry 1980) and only two ribbon seal stomachs contained food. Prey in both stomachs was comprised of diagnostic hard parts, i.e., Arctic cod otoliths, indicating advanced digestion (Sheffield et al. 2001; Christiansen et al. 2005) and consequently a break in feeding activity for more than 10 h. Analysis of stable carbon and nitrogen isotopes can provide some additional insights in the assessment of ribbon seal diet. δ15N was positively correlated with age, suggesting that trophic level increases with increasing age of ribbon seals (Fig. 4). A change from low to high trophic level prey is in accordance with available studies suggesting that ribbon seal pups and juveniles feed mainly on small crustaceans and that the importance of fish and nektobenthos, e.g., cephalopods and pollock (Theragra chalcogramma) increases with age (Shustov 1965; Frost and Lowry 1980; Fedoseev 2000). Adult ribbon and spotted seals have similar nitrogen isotope ratios. Stable nitrogen isotopes in typical prey of adult ribbon seals range from 13.6 ± 1.2‰ in cephalopods to 14.2 ± 2.0 and 15.5 ± 1.0‰ for pollock and Arctic cod, respectively (Table 3). δ15N in small crustaceans ranges from 9.9 ± 0.8 to 11.7 ± 0.7‰, for zooplankton (euphausids and copepods) and hyperiid amphipods (Parathemisto libellula), respectively (Table 3). Assuming a seal feeding exclusively on small crustaceans and further assuming an enrichment factor of 2.4‰ (Hobson et al. 1996), a muscle δ15N value of approximately 13‰ can be expected while a seal preying on gadids would approximate 18‰. These extremes reflect the ranges of δ15N found in ribbon seals. The high values of δ15N in YOY (Fig. 4) are in agreement with enrichment of nitrogen-15 in pups and juveniles due to an increased demand of nitrogen for protein synthesis during growth or mobilization of maternal nitrogen to the offspring as described above (Hobson et al. 1997; Roth and Hobson 2000).
δ15N in muscle versus age based on cementum analysis of teeth of ribbon seals harvested near Little Diomede, 2003. A sigmoid function was fitted to the data set and a LOESS nonparametric smoothing (dashed line) was employed to estimate the regression surface. Gastric contents were present in two seals, highlighted as open circles
δ13C is positively correlated with age in ribbon seals such that YOY are depleted in 13C compared to adult seals and as discussed for ringed seals this likely indicates maternal carbon transfer and mobilization. Mean δ13C in ribbon seals is similar to pelagic feeding ringed and spotted seals and is significantly lower than in benthic feeding bearded seals. This is in agreement with pelagic feeding behavior of ribbon seals during the ice-free months (Burns 1970).
Spotted seals
Stomach contents analysis
Only in the past 20 years have spotted seals been differentiated from their close relative, the harbor seal (Phoca vitulina) (Burns et al. 1984). Feeding habits of spotted and harbor seals are similar and schooling fish, such as pollock, herring, smelt and salmonids dominate their diets (Bukhtiyarov et al. 1984; Iverson et al. 1997; Orr et al. 2004). The high frequency of fish in spotted seal diet reported herein is in accordance with studies conducted in the Alaskan and Russian Arctic and the Sea of Okhotsk (Bukhtiyarov et al. 1984; Sobolevskii 1996). The most common species present in spotted seal stomachs analyzed in this study was herring followed by gadids. Bukhtiyarov et al. (1984) noted that herring and smelt are minor foods for spotted seals in spring, but increase in prevalence during summer and fall. In late spring and early summer, spotted seals in the Sea of Okhotsk consumed mostly walleye pollock, followed by herring (Kato 1982). Pacific herring are abundant in coastal waters during spawning in summer and migrate offshore to their wintering grounds after spawning (Lassuy 1989). Hence spotted seals likely are responding to seasonal availability of forage fish. However, salmonids have been described as an important component of the diet in summer during spawning (Sobolevskii 1996; Lowry et al. 2000) but were not identified from stomachs of spotted seals in this study.
Kato (1982) and Bukhtiyarov et al. (1984) reported high frequencies of crustacean prey in younger spotted seals, while fish and cephalopods made up the majority of the adult diet. However, no age-related differences were found in the consumption of invertebrate prey in this study. This could be associated with the abundance of spawning herring that would make this species seasonally accessible and easy prey for spotted seal pups. However, age was a significant factor in the prevalence of flatfish and capelin in older seals. Bukhtiyarov et al. (1984) reported that older seals are more likely to feed on benthic organisms. Benthic prey may only be available to adults due to restrictions in diving performance of juveniles, as has been suggested for harbor seal pups (Jørgensen et al. 2001). This could explain the presence of flatfish or other benthic prey in adult spotted seals and relative absence in pups.
Spotted seals
Stable isotopes
Nitrogen isotope ratios of spotted seals demonstrate feeding on a higher trophic level than other pinnipeds in this study and results are comparable to stable isotope ratios reported for harbor seal muscle (Hobson et al. 1997). A higher trophic level (based on δ15N) for spotted seals compared to other ice-associated seals is in accordance with stomach contents findings as fish occurs at a high frequency in their diet. Though there is some indication that nitrogen isotope ratios increase in seals younger than 5 years, the relationship of δ15N with age was not significant. Kato (1982) and Bukhtiyarov et al. (1984) reported a change from low to high trophic level prey and suggested that spotted seal pups feed mainly on crustaceans and that the importance of fish increases with age. However, analysis of stomach contents in this study did not show any age effects for invertebrate prey. Considering that stable isotopes in muscle tissue show a signature that reflects feeding habits over about one month (Tieszen et al. 1983) it is possible that younger seals were feeding on a lower trophic level earlier in the year and switched their diet to take advantage of the abundant spawning herring in early summer. Hence the importance of invertebrates to immature seals was not detectable by means of stomach contents and stable isotope analysis.
Carbon isotope ratios of spotted seal muscle were not statistically different from δ13C in ringed and ribbon seals harvested in Alaska. This suggests that these species rely on the planktonic food web rather than the benthic ecosystem as seen for bearded seals and walrus. However, stable carbon isotope ratios were not correlated with age in spotted seals as described for Alaskan ringed and ribbon seals. Lowry et al. (1998) showed long-distance migration of spotted seals equipped with satellite transmitters in the Chukchi Sea during the open-water season. Seals migrating between the Beaufort and Chukchi seas could therefore have different carbon isotope ratios. This possible movement and cyclical foraging of spotted seals in offshore areas and coastal haul-out sites as demonstrated by Lowry et al. (1998) or feeding in freshwater versus saltwater habitats could also result in highly variable carbon signatures (Smith et al. 1996; Hobson et al. 1997).
Conclusion
In conclusion, dietary habits of pinnipeds analyzed in this study are markedly different. Bearded seals and walruses relied heavily on the benthic food chain, and ringed, ribbon and spotted seals foraged mainly pelagically. Stable nitrogen and carbon isotope analysis are in agreement with most dietary compositions based on stomach contents. However, the interpretation of stable isotope ratios in pinnipeds (in particular bearded seals) consuming prey with different nitrogen contents and from a wide range of trophic levels can be challenging and sometimes inconclusive. This study documented that age is an important factor when reconstructing pinniped diets and accounts for much of the variability found in stable carbon and nitrogen isotope ratios. We recommend the use of traditional methods, e.g., stomach contents or scat analysis, in combination with chemical feeding ecology to assess dietary habits most accurately when direct observation of feeding behavior is not possible.
References
Adams TS, Sterner RW (2000) The effect of dietary nitrogen content on trophic level 15N enrichment. Limnol Oceanogr 45:601–607
Akimushkin II (1965) Cephalopods of the seas of the U.S.S.R. Israel Program for Scientific Translations, Jerusalem
Antonelis GA, Melin SR, Bukhtiyarov YA (1994) Early spring feeding habits of bearded seals (Erignathus barbatus) in the Central Bering Sea, 1981. Arctic 47:74–79
Becker PR, Porter BJ, Mackey EA, Schantz MM, Demiralp R, Wise SA (1999) National Marine Mammal Tissue Bank and Quality Assurance Program: protocols, inventory, and analytical results. U.S. Department of Commerce, Gaithersburg
Benjaminsen T (1973) Age determination and the growth and age distribution from cementum growth layers of bearded seals at Svalbard. Fiskeridirektoratets skrifter. Ser Havunders 16:159–170
Bowen WD (2000) Reconstruction of pinniped diets: accounting for complete digestion of otoliths and cephalopod beaks. Can J Fish Aquat Sci 57:898–905
Bradstreet MSW, Finley KJ (1983) Diet of ringed seals (Phoca hispida) in the Canadian High Arctic. LGL Limited, Toronto
Braham HW, Burns JJ, Fedoseev GA, Krogman BD (1984) Habitat partitioning by ice-associated pinnipeds: distribution and density of seals and walruses in the Bering Sea, April, 1976. In: Soviet–American cooperative. Research on marine mammals, Pinnipeds, vol 1. U.S. Department of Commerce, NOAA, NMFS
Bukhtiyarov YA, Frost KJ, Lowry LF (1984) New information on foods of the spotted seal, Phoca largha, in the Bering Sea in spring. In: Soviet–American cooperative. Research on marine mammals, Pinnipeds, vol 1. U.S. Department of Commerce, NOAA, NMFS
Burns JJ (1970) Remarks on the distribution and natural history of pagophilic pinnipeds in the Bering and Chukchi Seas. J Mamm 51:445–454
Burns JJ, Francis HF, Fedoseev GA (1984) Craniological analysis of harbor and spotted seals of the North Pacific Region. In: Soviet–American cooperative. Research on marine mammals, Pinnipeds, vol 1. U. S. Department of Commerce, NOAA, NMFS
Burns JM, Trumble SJ, Castellini MA, Testa JW (1998) The diet of Weddell seals in McMurdo Sound, Antarctica as determined from scat collections and stable isotope analysis. Polar Biol 19:272–282
Burton RK, Koch PL (1999) Isotopic tracking of foraging and long-distance migration in northeastern Pacific pinnipeds. Oecologia 119:578–585
Butler TH (1980) Shrimps of the Pacific coast of Canada. Can Bull Fish Aquat Sci 202:280
Christiansen JS, Gamst Moen A-G, Hansen T, Nilssen KT (2005) Digestion of capelin, Mallotus villosus (Müller), herring, Clupea harengus L., and polar cod, Boreogadus saida (Lepechin), otoliths in a simulated seal stomach. ICES J Mar Sci 62:86–92
Das K, Lepoint G, Leroy Y, Bouquegneau JM (2003) Marine mammals from the southern North Sea: feeding ecology data from δ13C and δ15N measurements. Mar Ecol Prog Ser 263:287–298
Dehn L-A, Follmann EH, Thomas DL, Sheffield GG, Rosa C, Duffy LK, O’Hara TM (2006a) Trophic relationships in an Arctic food web and implications for trace metal transfer. Sci Total Environ 362:103–123
Dehn L-A, Follmann EH, Rosa C, Duffy LK, Thomas DL, Bratton GR, Taylor RJ, O’Hara TM (2006b) Stable isotope and trace element status of subsistence-hunted bowhead and beluga whales in Alaska and gray whales in Chukotka. Mar Pollut Bull 52:301–319
DeNiro MJ, Epstein S (1977) Mechanisms of carbon isotope fractionation associated with lipid synthesis. Science 197:261–263
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Derocher AE, Wiig O, Andersen M (2002) Diet composition of polar bears in Svalbard and the western Barents Sea. Polar Biol 25:448–452
Fay FH (1982) Ecology and biology of the Pacific Walrus, Odobenus rosmarus divergens Illiger. North American Fauna 74, United States Department of the Interior, Washington, DC
Fay FH, Bukhtiyarov YA, Stoker SW, Shults LM (1984) Foods of the Pacific walrus in winter and spring in the Bering Sea. In: Soviet–American cooperative. Research on marine mammals, Pinnipeds, vol 1. U. S. Department of Commerce, NOAA, NMFS
Fedoseev GA (2000) Population biology of ice-associated forms of seals and their role in the northern Pacific ecosystems. Center for Russian Environmental Policy, Moscow
Fernandez-Mosquera D, Vila-Taboada M, Grandal-d’Anglade A (2001) Stable isotopes data (δ13C, δ15N) from the cave bear (Ursus spelaeus): a new approach to its palaeoenvironment and dormancy. Proc R Soc Lond B 268:1159–1164
Finley KJ, Evans CR (1983) Summer diet of the bearded seal (Erignathus barbatus) in the Canadian high Arctic. Arctic 36:82–89
Fisk AT, Hobson KA, Norstrom RJ (2001) Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the northwater polynya marine food web. Environ Sci Technol 35:732–738
Foster NR (1991) Intertidal bivalves—a guide to the common marine bivalves of Alaska. University of Alaska Press, Fairbanks
France RL (1995a) Carbon isotopic variability in the composite pelagic foodweb of four oligotrophic lakes: feeding diversity or metabolic fractionations? J Plankton Res 17:1993–1997
France RL (1995b) Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar Ecol Prog Ser 124:307–312
Frost KJ (1981) Descriptive key to the otoliths of gadid fishes of the Bering, Chukchi, and Beaufort Seas. Arctic 34:55–59
Frost KJ, Lowry LF (1980) Feeding of ribbon seals (Phoca fasciata) in the Bering Sea in spring. Can J Zool 58:1601–1607
Gales NJ, Cheal AJ (1992) Estimating diet composition of the Australian sea-lion (Neophoca cinerea) from scat analysis: an unreliable technique. Wildl Res 19:447–456
Gannes LZ, O’Brien DM, Martinez Del Rio C (1997) Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78:1271–1276
Geraci JR (1975) Pinniped nutrition. Rapp P-v Reun Cons Int Explor Mer 169:312–323
Härkönen T (1986) Guide to otoliths of the bony fishes of the Northeast Atlantic. Danbiv ApS, Hellerup, Denmark
Harvey JT, Loughlin TR, Perez MA, Oxman DS (2000) Relationship between fish size and otolith length for 63 species of fishes from the Eastern North Pacific Ocean. NOAA Technical Report NMFS 150. U.S. Department of Commerce, Seattle, Washington
Hammill MO, Smith TG (1991) The role of predation in the ecology of the ringed seal in Barrow Strait, Northwest Territories, Canada. Mar Mamm Sci 7:123–135
Harwood LA, Smith TG (2003) Movements and diving of ringed seals in the Beaufort and Chukchi seas, 1999–2003. In: 15th Biennial conference on the biology of marine mammals, Greensboro, North Carolina
Hjelset AM, Andersen M, Gjertz I, Lydersen C, Gulliksen B (1999) Feeding habits of bearded seals (Erignathus barbatus) from the Svalbard area, Norway. Polar Biol 21:186–193
Hobson KA, Welch HE (1992) Determination of trophic relationships within a High Arctic marine food web using d13C and d15N analysis. Mar Ecol Prog Ser 84:9–18
Hobson KA, Alisauskas RT, Clark RG (1993) Stable-nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress: implications for isotopic analyses of diet. Condor 95:388–394
Hobson KA, Schell DM, Renouf D, Noseworthy E (1996) Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive seals: implications for dietary reconstructions involving marine mammals. Can J Fish Aquat Sci 53:528–533
Hobson KA, Sease JL, Merrick RL, Piatt JF (1997) Investigating trophic relationships of pinnipeds in Alaska and Washington using stable isotope ratios of nitrogen and carbon. Mar Mamm Sci 13:114–132
Hobson KA, Fisk AT, Karnovsky N, Holst M, Gagnon J-M, Fortier M (2002) A stable isotope (δ13C, δ15N) model for the North Water foodweb: implications for evaluating trophodynamics and the flow of energy and contaminants. Deep-Sea Res II 49:5131–5150
Hoekstra PF, Dehn L-A, George JC, Solomon KR, Muir DCG, O’Hara TM (2002) Trophic ecology of bowhead whales (Balaena mysticetus) compared with that of other arctic marine biota as interpreted from carbon-, nitrogen-, and sulfur-isotope signatures. Can J Zool 80:223–231
Hop H, Welch HE, Crawford RE (1997) Population structure and feeding ecology of Arctic cod schools in the Canadian high Arctic. Am Fish Soc Symp 19:68–80
Iverson SJ, Frost KJ, Lowry LF (1997) Fatty acid signatures reveal fine scale structure of foraging distribution of harbor seals and their prey in Prince William Sound, Alaska. Mar Ecol Prog Ser 151:255–271
Jenkins SG, Partridge ST, Stephenson TR, Farley SD, Robbins C (2001) Nitrogen and carbon isotope fractionation between mothers, neonates, and nursing offspring. Oecologia 129:336–341
Jensen GC (1995) Pacific coast crabs and shrimps. Sea Challengers, Monterey
Jørgensen C, Lydersen C, Brix O, Kovacs KM (2001) Diving development in nursing harbour seal pups. J Exp Biol 204:3993–4004
Kathman RD, Austin WC, Saltman JC, Fulton JD (1986) Identification manual to the mysidacea and euphausiacea of the Northeast Pacific. Can Spec Publ Fish Aquat Sci 93:411
Kato H (1982) Food habits of largha seal pups in the pack ice area. Sci Rep Whales Res Inst 43:123–136
Keen AM, Coan E (1974) Marine molluscan genera of Western North America. An illustrated key. Stanford University Press, Stanford
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Lassuy DR (1989) Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (Pacific Northwest). Pacific herring. US Fish Wildl Serv Biol Rep 82:1–18
Lauckner G (1985) Diseases of mammalia: pinnipedia. In: Diseases of marine mammals. Westholsteinische Verlagsdruckerei Boyens & Co., Heide
Lawson JW, Hobson KA (2000) Diet of harp seals (Pagophilus groenlandicus) in nearshore northeast Newfoundland: inferences from stable-carbon (δ13C) and nitrogen (δ15N) isotope analyses. Mar Mamm Sci 16:578–591
Le Boeuf BJ, Crocker DE, Costa DP, Blackwell SB, Webb PM, Houser DS (2000) Foraging ecology of northern elephant seals. Ecol Monogr 70:353–382
Lowry LF, Frost KJ, Burns JJ (1978) Food of ringed seals and bowhead whales near Point Barrow, Alaska. Can Field-Nat 92:67–70
Lowry LF, Frost KJ (1981) Distribution, growth, and foods of Arctic cod (Boreogadus saida) in the Bering, Chukchi, and Beaufort seas. Can Field-Nat 95:186–191
Lowry LF, Fay FH (1984) Seal eating by walruses in the Bering and Chukchi Seas. Polar Biol 3:11–18
Lowry LF, Frost KJ, Burns JJ (1980a) Variability in the diet of ringed seals, Phoca hispida, in Alaska. Can J Fish Aquat Sci 37:2254–2261
Lowry LF, Frost KJ, Burns JJ (1980b) Feeding of bearded seals in the Bering and Chukchi Seas and trophic interaction with Pacific walrus. Arctic 33:330–342
Lowry LF, Frost KJ, Douglas RD, DeMaster DP, Suydam RS (1998) Movements and behavior of satellite-tagged spotted seals (Phoca largha) in the Bering and Chukchi Seas. Polar Biol 19:221–230
Lowry LF, Burkanov VN, Frost KJ, Simpkins MA, Davis R, DeMaster DP, Suydam R, Springer A (2000) Habitat use and habitat selection by spotted seals (Phoca largha) in the Bering Sea. Can J Zool 78:1959–1971
Matson GM (1981) Workbook for cementum analysis. Matson’s Laboratory, Milltown, p 30
McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. OIKOS 102:378–390
Muir DCG, Segstro MD, Hobson KA, Ford CA, Stewart REA, Olpinski S (1995) Can seal eating explain elevated levels of PCBs and organochlorine pesticides in walrus blubber from Eastern Hudson Bay (Canada)? Environ Pollut 90:335–348
Murie DJ, Lavigne DM (1986) Interpretation of otoliths in stomach content analyses of phocid seals: quantifying fish consumption. Can J Zool 64:1152–1157
Ostrom PH, Lien J, Macko SA (1993) Evaluation of the diet of Sowerby’s beaked whale, Mesoplodon bidens, based on isotopic comparisons among northwestern Atlantic cetaceans. Can J Zool 71:858–861
Orr AJ, Banks AS, Mellman S, Huber HR, DeLong RL, Brown RF (2004) Examination of the foraging habits of Pacific harbor seal (Phoca vitulina richardsi) to describe their use of the Umpqua River, Oregon, and their predation on salmonids. Fish Bull 102:108–117
Pikharev GA (1941) Some data on the feeding of the far eastern bearded seal. Izv TINRO 20:101–120
Polischuk SC, Hobson KA, Ramsay MA (2001) Use of stable-carbon and -nitrogen isotopes to assess weaning and fasting in female polar bears and their cubs. Can J Zool 79:499–511
Rathbun MJ (1929) Canadian Atlantic Fauna. Arthropoda—Decapoda. The Biological Board of Canada. The Atlantic Biological Station. St. Andrews, N. B., Canada
Roth JD, Hobson KA (2000) Stable carbon and nitrogen isotopic fractionation between diet and tissue of captive red fox: implications for dietary reconstructions. Can J Zool 78:848–852
Santos MB, Clarke MR, Pierce GJ (2001) Assessing the importance of cephalopods in the diets of marine mammals and other top predators: problems and solutions. Fish Res 52:121–139
Schell DM, Saupe SM, Haubenstock N (1989) Natural isotope abundance in bowhead whale (Balaena mysticetus) baleen: markers of aging and habitat usage. In: Stable isotopes in ecological research. Springer, Berlin Heidelberg New York
Schell DM, Barnett BA, Vinette KA (1998) Carbon and nitrogen isotope ratios in zooplankton of the Bering, Chukchi and Beaufort seas. Mar Ecol Prog Ser 162:11–23
Sheffield G, Fay FH, Feder H, Kelly BP (2001) Laboratory digestion of prey and interpretation of walrus stomach contents. Mar Mamm Sci 17:310–330
Shustov AP (1965) The food of ribbon seals in the Bering Sea. Izv TINRO 59:178–183
Simpkins MA, Hiruki-Raring LM, Sheffield G, Grebmeier JM, Bengtson JL (2003) Habitat selection by ice-associated pinnipeds near St. Lawrence Island, Alaska in March 2001. Polar Biol 26:577–586
Smith RJ, Hobson KA, Koopman HN, Lavigne DM (1996) Distinguishing between populations of fresh-and saltwater harbour seals (Phoca vitulina) using stable-isotope ratios and fatty acid profiles. Can J Fish Aquat Sci 53:272–279
Smith TG (1976) Predation of ringed seal pups (Phoca hispida) by the arctic fox (Alopex lagopus). Can J Zool 54:1610–1616
Smith TG (1987) The ringed seal, Phoca hispida, of the Canadian Western Arctic. Can Bull Fish Aquat Sci 216:1–81
Sobolevskii EI (1996) The distribution and seasonal dynamics of feeding of the spotted seal Phoca largha in the Bering Sea. Rus J Mar Biol 22:199–204
Stewart REA, Stewart BE, Stirling I, Street E (1996) Counts of growth layer groups in cementum and dentine in ringed seals (Phoca hispida). Mar Mamm Sci 12:383–401
Tieszen LL, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57:32–37
Weslawski JM, Ryg M, Smith TG, Oritsland NA (1994) Diet of ringed seals (Phoca hispida) in a fjord of West Svalbard. Arctic 47:109–114
Acknowledgments
This study would not have been possible without the samples provided by Alaskan and Canadian subsistence hunters in the communities of Barrow, Holman, Little Diomede and Shishmaref, and we thank them all for their support. We greatly appreciate the assistance of C. D. N. Brower, H. Brower Jr., T. Olemaun, B. Akootchook, T. Hepa, L. Hopson, V. Woshner, R. Elsner, T. Zenteno-Savin, S. Visalli, D. Burnett, G. York and many others in the field and T. Bentzen, T. Howe, N. Haubenstock and P. Hoekstra for support with analysis. We also thank L. Harwood for providing tissues and jaws of ringed seals harvested in Holman, R. Highsmith and B. Bluhm for collection of amphipod samples from the Bering Strait, and J. Bengtson and D. DeMaster for training in cementum aging and stomach contents analysis. The Frozen Tissue Collection of the University of Alaska Museum provided some of the spotted seal muscle samples. The comments of two anonymous reviewers improved the manuscript.
This study was primarily funded by the Cooperative Institute for Arctic Research. Additional support was provided by the Experimental Program for Stimulation of Competitive Research; the North Slope Borough Department of Wildlife Management; the Institute of Arctic Biology and the Department of Biology and Wildlife, UAF; the US Geological Survey; the Barrow Arctic Science Consortium; and the National Science Foundation OPP Grant 9910319.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dehn, LA., Sheffield, G.G., Follmann, E.H. et al. Feeding ecology of phocid seals and some walrus in the Alaskan and Canadian Arctic as determined by stomach contents and stable isotope analysis. Polar Biol 30, 167–181 (2007). https://doi.org/10.1007/s00300-006-0171-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-006-0171-0