Abstract
Colobanthus quitensis (Kunth) Bartl. is widely distributed from Mexico to the Antarctic. C. quitensis is a freezing resistant species that accumulates sucrose in response to cold. We tested the hypothesis that low temperature modifies the kinetic properties of C. quitensis sucrose phosphate synthase (SPS) to increase its activity and ability to synthesize sucrose during cold acclimation. Cold acclimation caused a fourfold increment in sucrose concentration and a 100% increase in SPS activity, without changes in the level of SPS protein. Cold acclimation did not affect the optimal temperature and pH for SPS activity. However, it caused a tenfold increase in the inhibition constant (K i) for inorganic phosphate (Pi) calculated as a function of fructose-6-phosphate (Fruc-6-P). SPS from cold acclimated plants also exhibited a higher reduction of its Michaelis constant (K m) for glucose-6-phosphate (Gluc-6-P) with respect to non-acclimated plants. We suggest that the increase in C. quitensis SPS K i for Pi and the increase in activation by Gluc-6-P in response to cold keep SPS activated, leading to high sucrose accumulation. This may be an important adaptation that allows efficient accumulation of sucrose during the harsh Antarctic summer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Antarctic is characterized by low temperature with strong winds. Photosynthetic active radiation (PAR) may vary between 50 and 2,000 μmol photons m−2 s−1, while the average summer temperature in the Maritime Antarctic is about 3°C. These conditions make it a hostile territory for vascular plants (Alberdi et al. 2002). Considering these harsh conditions, it is remarkable that two vascular plants, Deschampsia antarctica and Colobanthus quitensis have been able to colonize the Maritime Antarctic, (Xiong et al. 1999; Bravo et al. 2001; Lewis-Smith 2003). Both plants grow in summer, remaining under the snow during winter.
Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) is a dicot that inhabits from Southern Mexico 17°N down to 68°42′S in the Maritime Antarctic (Lewis-Smith and Poncet 1987). This latitudinal amplitude and habitat isolation have allowed the generation of ecotypes (Gianoli et al. 2004). The Antarctic ecotype is cold resistant (Bravo et al. 2001), with numerous biochemical properties that could be advantageous for colonizing some parts of the Antarctic. For example, the photosynthetic rate of this plant at 0°C is about 30% of its optimum (Xiong et al. 1999). Furthermore, it has the capability to increase heat dissipation when exposed to a combination of high light and low temperature (Pérez et al. 2004). C. quitensis also accumulates compatible solutes, mainly sucrose, in response to cold treatment (Bravo et al. 2001).
Plants accumulate sucrose in response to several environmental stress factors, including water deficit (Zrenner and Stitt 1991) and low temperature (Guy et al. 1992; Uemura and Steponkus 2003). This compound may play an essential role in osmoregulation and cryoprotection in many plant tissues (Santarius 1992). Sucrose is synthesized from triose phosphates (TP) from the Calvin Cycle and maltose from starch. These compounds are transported to the cytoplasm by a triose phosphates/phosphate translocator (TPT) and a maltose translocator (Niittyla et al. 2004). Sucrose is then synthesized in the cytoplasm by sucrose phosphate synthase (SPS, EC 2.4.1.14) and sucrose phosphate phosphatase (SPP, EC 3.13.24) from fructose-6-phosphate (Fruc-6-P) and UDP-Glucose (UDP-Gluc). The kinetic properties of leaf SPS vary in different species. Some leaf SPS are allosterically activated by glucose-6-phosphate (Gluc-6-P) and inhibited by inorganic phosphate (Pi). These compounds appear to compete for the same binding site and have antagonistic effects (Amir and Preiss 1982; Sinha et al. 1997). The regulatory site, however, has not been characterized (Huber and Huber 1996).
Low temperature can modify the kinetic properties of SPS. In potato tubers, cold acclimation increases the affinity for the substrates and decreases the affinity for Pi, probably because new isoforms are expressed (Reimholz et al. 1997). It has been suggested that these mechanisms increase sucrose synthesis, even though SPS decreases its activity at low temperature (Deiting et al. 1998). Little is known on the mechanisms of SPS regulation by low temperature in C. quitensis. Therefore, the purpose of this work was to characterize SPS from C. quitensis and determine the effect of cold acclimation on SPS kinetic properties. We hypothesized that cold acclimation increases SPS activity in C. quitensis due to an increase in the amount of enzyme or a modification of SPS kinetic properties.
Materials and methods
Plant material and growth conditions
Colobanthus quitensis (Kunth) Bartl. was collected in King George Island, Maritime Antarctic (62°22′S; 59°43′W). Plants were reproduced vegetatively in plastic pots, using a soil: peat mixture (3:1) and maintained at 13 to 15°C in a growth chamber (Forma Scientific. Inc., Marietta, OH, USA) with a photon flux density of 100–120 μmol m−2 s−1 at the top of the canopy and a 16/8 h light/dark period. Plants were fertilized with Phostrogen (Solaris, Buckinghamshire, UK) using 0.2 g l−1 once every 2 weeks.
Cold acclimation treatment
Plants kept for at least 2 months in 16/8 day length, 15°C and 100 ± 10 μmol m−2 s−1 (non-acclimated) were transferred at 4°C (cold-acclimated) for 7 or 21 days. Samples of mature leaves were taken at 0, 7 and 21 days. SPS activity, kinetic constants, and relative amounts of SPS protein were measured.
Sugar extraction
Samples of mature leaves were homogenized and extracted with ethanol (86%) for 24 h, and then centrifuged at 12,000 g for 10 min. The supernatant was depigmented in a mixture of 1:3 (v/v) with chloroform. The aqueous fraction was freeze-dried overnight. The dry residue was resuspended in 500 μl of methanol. Xylose was used as an internal standard to calculate carbohydrate losses during the extraction procedure.
Sucrose content was determined by high performance thin layer chromatography (HPTLC) according to Lee et al. 1979, using a Silicagel plate 60 F 254 (Merck, Darmstadt, Germany) pretreated with a 0.1 M methanolic potassium phosphate solution. HPTLC plates were developed five times in acetonitrile: water (85:15 v/v). For quantitative analyses a standard calibration curve was performed using sucrose (Merck, Darmstadt, Germany) from 0.1 to 0.3 mg/ml. The position of sucrose was visualized by the diphenylamine/aniline reagent at 105°C. For quantitation, the plate was scanned at 520 nm.
Enzyme extraction
Samples of fresh tissue were frozen in liquid nitrogen and stored at −80°C until used. The samples were ground to a fine powder using mortar and pestle with liquid nitrogen and suspended in extraction buffer (0.15 g of fresh tissue per 900 μl buffer), consisting of 40 mM Hepes (pH 7.5), 15 mM MgCl2, 10 mM DTT, 2% w/v PEG, 0.1 % Triton X-100 and 2 mM benzamidine. The homogenized tissue was subsequently centrifuged at 12,000 g at 4°C and the supernatant was desalted and depleted of endogenous substrates in a G-25 Sephadex column that had been previously equilibrated with assay buffer consisting of 40 mM Hepes (pH 7.5), 15 mM MgCl2 and 1 mM DTT.
Enzyme assay
SPS activity was measured in a reaction mixture, which contained 40 mM Hepes (pH 7.0), 15 mM MgCl2, 1 mM DTT, 20 mM UDP-Gluc, 15 mM Fruc-6-P. (pyruvate kinase [7.2 U ml−1], lactate dehydrogenase [11.3 U ml−1] ), using 0.8 mM phosphoenol pyruvate and 0.3 mM NADH as substrates. Uridine 5′-diphosphate, one of the products formed in the reaction between UDP-Gluc and Fruc-6-P, was then used as a substrate for the conversion of phosphoenol pyruvate to pyruvate by pyruvate kinase. Finally, pyruvate was reduced to lactate by lactate dehydrogenase using NADH as a reductant. SPS activity was measured at 30°C following the oxidation of NADH at 340 nm (Hauch and Magel 1998). The values are expressed as velocity of NADH oxidation (μmol of oxidized NADH per minute) at 30°C and pH 7.5 in conditions of initial velocity. Blanks without Fruc-6-P were used to evaluate formation of uridine 5′-diphosphate independently of SPS activity. These values were subtracted from the total activity. The contribution of these blanks to the total velocity of NADH oxidation was always less than 10%.
For kinetic studies, the concentrations of substrates and effectors (Pi and Gluc-6-P) were varied as specified in the plots. Kinetics parameters were determined from three independent enzymatic assays by means of nonlinear regression Lineweaver-Burk plots (GraphPad Prism 4.0, San Diego, CA, USA) and confirmed by Eisenthal Cornish-Bowden direct linear plots (not shown).
Protein extraction and protein quantification
After treatments 0.2 g of C. quitensis leaves were macerated and homogenized with 600 μl of extraction buffer at 4°C. The extraction buffer was 0.06 M Tris–HCl, pH 6.8 containing 1% SDS, 2,5% of β-mercaptoethanol and 5% glycerol (Laemli 1970). The extracts were kept for 2 min at 80°C and centrifuged for 10 min in a micro centrifuge at 14,000 g. Protein was measured in the supernatants by the colorimetric method of Bradford (1976), using a BSA calibration curve (Bio-Rad, Hercules, CA, USA). The extracted protein was kept at −20°C until the western analysis was carried out.
Western analysis
Extracted proteins were separated on a SDS-PAGE gel as described by Laemli (1970). The stacking gel was made at 5% of polyacrylamide (w/v) and the resolving gel was made at 10% of polyacrylamide (w/v). Each gel was loaded with 15 μg of total proteins. The electrophoresis was run at 100 v in the stacking gel and 200 v in the resolving gel using a Mini-Protean II chamber (Bio-Rad, Hercules, CA, USA). Prestained protein standards of known molecular weights were run in the same gel. The proteins were transferred to a polyvinylidenefluoride (PVDF) membrane (Immobilon-P, Millipore, Bedford, MA) by using a mini trans blot cell (Bio-Rad, Hercules, CA, USA) for 1 h. The membranes were blocked with 10% non-fat powdered milk solution made in 20 mM Tris pH 7.5 containing 500 mM NaCl (TBS buffer). The membrane was then incubated over night with a rabbit polyclonal primary antibody against potato tuber SPS (1:300). The membrane was washed three times for 15 min with TTBS (TBS buffer containing 0.1% Tween 20). The secondary antibody was an alkaline phosphatase-goat-anti-rabbit IgG (Sigma, St Louis MO, USA) diluted 1:1000 in 5% non-fat milk solution in TBS buffer. The membrane was developed with 5bromo-4chloro-3indolyl phosphate (BCIP) and nitroblue tetrazolium (NBT) in 100 mM Tris-HCl, pH 9, containing 100 mM NaCl and 5 mM MgCl2. Band intensities in the westerns blots were densitometrically quantified with the Sigma Gel Program 1.05 (SPSS, Inc. Chicago IL, USA).
The polyclonal anti-SPS antibody was donated by Dr. Donald Ort from the University of Illinois, Urbana, IL, USA. A further description of this antibody can be found in Deiting et al. (1998).
Results
Effect of cold acclimation on sucrose accumulation and SPS amount and specific activity
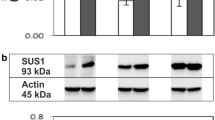
After 21 days of cold acclimation, sucrose concentration increased fourfold with respect to non-acclimated controls (Fig. 1a). In the same period, SPS specific activity increased only two times (Fig. 1b). An additional band of higher molecular mass was recognized by the antibody in cold acclimated plants. Total SPS protein, however, remained nearly constant with just a slight increase of 18% at 7 days of cold acclimation (based on densitometric measurements of inset in Fig. 1b).
Effect of cold acclimation on sucrose accumulation (a) and specific activity of SPS (b) from leaves of Colobanthus quitensis. Bars represent the average of three independent determinations. The inset in b shows western blots with 30 μg of protein loaded in each lane. An SPS antibody against potato SPS was used. See Materials and methods
Effect of cold acclimation on SPS kinetic parameters
The assays to determine kinetic constants were performed at 30°C and pH 7.0 because these were the optimal conditions for SPS activity in extracts from control plants and cold acclimated ones (Fig. 2). Cold acclimated plants exhibited higher SPS activity over a wide range of pH, from 5.5 to 8.5 (Fig. 2a) and of temperature, from 10 to 35°C (Fig. 2b). Interestingly, a twofold higher SPS activity was observed at 0°C in cold acclimated plants, with respect to non-acclimated plants (Fig. 2b).
SPS K m for Fruc-6-P did not significantly change during acclimation at 4°C, being 1.4 mM in all assayed conditions (Fig. 3a). The K m for UDP-Gluc was 4.6 mM in the non-acclimated control and 2.7 and 3.0 mM after 7 and 21 days of cold acclimation, respectively (Fig. 3b). SPS V max for Fruc-6-P and UDP-Gluc doubled from 0.02 μmol/min in non-acclimated control to 0.046 μmol/min after 21 days of cold acclimation (Fig. 3).
Effect of Gluc-6-P and Pi on SPS kinetic parameters
Pi decreased SPS activity in all conditions studied, while Gluc-6-P increased it (Fig. 4). The increase in SPS activity by Gluc-6-P was higher in cold acclimated plants than in non-acclimated controls (Fig. 4a). Conversely, the inhibition of SPS activity by Pi was higher in non-acclimated controls than in cold acclimated plants (Fig. 4b). These results allowed us to determine the concentrations to be used in the studies of activation by Gluc-6-P and inhibition by Pi of SPS. Gluc-6-P behaved as an activator of C. quitensis SPS, as in other species. In order to evaluate the influence of cold acclimation on the activation produced by Gluc-6-P, 5 mM of this compound was added to the protein extract on non-acclimated plants. This addition induced a threefold decrease in the K m for Fruc-6-P and sixfold in the K m for UDP-Gluc, with respect to the untreated extract from non-acclimated plants. In cold acclimated plants (21 days at 4°C), the addition of 5 mM Gluc-6-P induced a fivefold decrease in the K m for Fruc-6-P and 14-fold for UDP-Gluc with respect to untreated extracts (Table 1).
Effect of Gluc-6-P (a) and Pi (b) on the initial velocity of leaf SPS of Colobanthus quitensis from (filled circle) non-acclimated control, (open circle) cold acclimated for 7 days, and (open triangle) cold acclimated for 21 days. These assays were performed under limiting concentrations of substrates (4 mM UDP-Gluc and 2mM Fruc-6-P). a Gluc-6-P concentrations were 0, 1, 2, 3, 4, 5, 6 mM, b Pi concentrations were 0, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 15, 20, 30 mM
The inhibitory effect of Pi on SPS was studied at different substrate concentrations and increasing Pi concentration. Double reciprocal graphs as a function of Fruc-6-P exhibited typical parallel lines as Pi increased in extracts from cold acclimated and non-acclimated plants, indicating an uncompetitive type of inhibition for Fruc-6-P (Fig. 5 a, c, e). However, the same graph as a function of UDP-Gluc exhibited increasing slope lines as Pi concentration increased in all acclimation treatments, indicating competitive inhibition of this compound for UDP-Gluc (Fig. 5 b, d, f).
Double-reciprocal plots of initial velocity of SPS versus Fruc-6-P concentration (a, c, e) or UDP-Gluc concentration (b, d, f) in the presence of (open triangle) 5 mM, (filled diamond) 3 mM (open circle) 1 mM and the absence (filled circle) of Pi in controls plants (a, b), in presence of (open triangle) 15 mM, (filled diamond) 10 mM (open circle) 4 mM and the absence (filled circle) of Pi in cold acclimated plants for 7 days (c, d), in presence of (open triangle) 20 mM, (filled diamond) 15 mM (open circle) 6 mM and the absence (filled circle) of Pi in cold acclimated for 21 days (e, f). In order to calculate Ki the intercepts and slopes of the double-reciprocal plots were replotted as a function of the Fruc-6-P (insets in a, c, e) or as a function of UDP-Gluc (inset b, d, f)
When the influence of cold acclimation on Kii (inhibition constant calculated from the intercepts) and Kis (inhibition constants calculated from the slopes) for Pi was determined, we found that Kii as a function of Fruc-6-P increased tenfold with respect to controls in plants exposed to 21 days of cold acclimation (from 3 to 30 mM, insets of Fig. 5 a, c, e). Kis as a function of UDP-Gluc increased from 5 to 12 mM (insets of Fig. 5 b, d, f) in 21 day cold acclimated plants with respect to the non-acclimated controls. This decrease in the affinity for Pi induced by cold acclimation is consistent with the cold induced increase in SPS activity.
Discussion
Exposure of C. quitensis to low temperature resulted in a 300% increase in sucrose accumulation in leaves after 21 days of cold acclimation. A similar change has been described for Deschampsia antartica, the other vascular plant that has colonized the Maritime Antarctic (Zuñiga-Feest et al. 2005). The activity of SPS, the key enzyme in sucrose biosynthesis appears to be positively modulated, leading to sucrose accumulation under cold acclimation conditions. Although, the specific activity of SPS after 21 days of cold acclimation doubled, there was not a concomitant increase in SPS synthesis, exhibiting just a transient increase of 18% at 7 days of cold acclimation. Thus, the increase in SPS activity is mainly due to changes in the regulatory properties of the enzyme.
Gluc-6-P and Pi are modulators of SPS activity. Gluc-6-P is a positive allosteric regulator and Pi is a negative allosteric regulator (Amir and Preiss 1982; Sinha et al. 1997). We propose that the increase in SPS activity caused by cold acclimation is mainly due to an increase in the affinity of SPS for Gluc-6-P, as shown by the greater decrease in the K m (using the same concentrations of Gluc-6-P) in cold acclimated plants than in non acclimated ones (Table 1). This increase in activity results in a higher sucrose accumulation. An increase in the concentration of Gluc-6-P has been observed in cold acclimated Festuca arundinacea (Pérez et al. 2001). If a similar change would occur in C. quitensis, the activation of SPS would be favoured even more.
Pi content increases tenfold during cold acclimation in Arabidopsis thaliana (Hurry et al. 2000). This would cause a decrease in SPS activity because Pi is an inhibitor of the enzyme. However, in C. quitensis, SPS Ki for Pi as a function of both substrates increased significantly during cold acclimation (Fig. 5). This loss of affinity for Pi would favour a higher SPS activity under cold acclimation conditions and, therefore, higher sucrose accumulation even if an increase in Pi similar to that described for Arabidopsis would occur in cold acclimated plants of C. quitensis.
Several hypotheses could explain the changes in the inhibition constants for Pi and in the K m induced by the activator Gluc-6-P observed after cold acclimation: (1) a conformational change in the enzyme could modify the affinity of allosteric binding sites, as a response to cold acclimation. (2) It is also possible that a posttranslational modification could be triggering these changes in the enzyme, as in other systems. For example, phosphorylation plays a key role in the regulation of enzymes related to osmotic stress (Toroser and Huber 1997). Finally, (3) it is possible that the new band observed in cold acclimated plants (inset of Fig. 1b) is a new isoform of SPS, which possesses this new allosteric affinity, as it has been reported for potatoes (Reimholz et al. 1997). Nonetheless, this remains to be demonstrated for C. quitensis during cold acclimation.
Our experiments allow us to conclude that upon cold acclimation of C. quitensis intracellular sucrose doubles, most likely due to an increase in the Vmax of SPS. This increase in V max would be produced by activation of SPS caused by an increase in the affinity for Gluc-6-P and reduction in the affinity for Pi. Considering the physiological concentrations of these effectors in other plants (Stitt et al. 1984; Theodorou and Plaxton 1993), it is likely that the detected changes in the kinetic parameters are physiologically relevant in the acclimation of C. quitensis to low temperature and, therefore, for its survival in the Antarctic.
References
Alberdi M, Bravo LA, Gutiérrez A, Gidekel M, Corcuera LJ (2002) Ecophysiology of Antarctic vascular plants. Physiol Plant 115:479–486
Amir J, Preiss J (1982) Kinetic characterization of spinach leaf sucrose phosphate. Plant Physiol 69:1027–1030
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bravo LA, Ulloa N, Zuñiga GE, Casanova A, Corcuera LJ, Alberdi M (2001) Cold resistance in Antarctic angiosperms. Physiol Plant 111:55–65
Deiting U, Zrenner R, Stitt M (1998) Similar temperature requirement for sugar accumulation and for the induction of new forms of sucrose phosphate synthase and amylase in cold-stored potato tubers. Plant Cell Environ 21:127–138
Gianoli E, Inostroza P, Zúñiga-Feest A, Reyes-Díaz M, Cavieres LA, Bravo LA, Corcuera LJ (2004) Ecotypic differentiation in morphology and cold resistance in populations of Colobanthus quitensis (Cariophyllaceae) from the Andes of Central Chile and Maritime Antarctica. Arctic Antarctic and Alpine Research 36:484–489
Guy CL, Huber JL, Huber SC (1992) Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiol 100:502–503
Hauch S, Magel E (1998) Extractable activities and protein content of sucrose-phosphate synthase, sucrose synthase and neutral invertase in trunk tissues of Robinia pseudoacacia are related to cambial wood production and heartwood formation. Planta 207:266–274
Huber SC, Huber JL (1996) Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol 17:431–444
Hurry V, Strand A, Furbank R, Stitt M (2000) The role of inorganic phosphate in the development of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutants of Arabidopsis thaliana. Plant J 24:383–396
Laemli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee KY, Nurok D, Zlatkis A (1979) Determination of glucose, fructose and sucrose in molasses by high-performance thin-layer chromatography. J Chromatogr 174:187–193
Lewis-Smith RI, Poncet S (1987) Deschampsia antarctica and Colobanthus quitensis in the Terra Firma Islands. Br Antarct Surv Bull 74:31–35
Lewis-Smith RI (2003) The enigma of Colobanthus quitensis and Deschampsia antarctica in Antyarctica. In: Huiskes AHL, Gieskes WWC, Rozema J, Schorno RML, van der vies SM, Wolff WJ (eds) Antarctic biology in a global context. Backhuys Publishers, Leiden, pp 234–239
Niittyla T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303:87–89
Perez P, Morcuende R, Martin Del Molino I, Sanchez De La Puente L, Martinez-Carrasco R (2001) Contrasting responses of photosynthesis and carbon metabolism to low temperatures in tall fescue and clovers. Physiol Plant 112:478–486
Pérez-Torres E, Dinamarca J, Bravo LA, Corcuera LJ (2004) Responses of Colobanthus quitensis (Kunth) Bartl. to high light and low temperature. Polar Biol 27:183–189
Reimholz R, Geiger M, Deiting U, Krause KP, Sonnewald U, Stitt M (1997) Potato plants contain multiple forms of sucrose phosphate synthase, that show differences in their tissue distribution, their response during development, and their response to low temperature. Plant Cell Environ 20:291–305
Santarius KA (1992) Freezing of isolated thylakoid membranes in complex media VIII Differential cryoprotection by sucrose, proline and glycerol. Physiol Plant 84:87–93
Sinha AK, Pathre UV, Sane PV (1997) Purification and characterization of sucrose-phosphate synthase from Prosopis juliflora. Phytochemistry 46(3):441–447
Stitt M, Kürzel B, Heldt HW (1984) Control of photosynthetic sucrose synthesis by fructose 2,6-bisphosphate II partitioning between sucrose and starch. Plant Physiol 75:554–560
Theodorou ME, Plaxto WC (1993) Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol 101:339–344
Toroser D, Huber SC (1997) Protein phosphorylation as a mechanism for osmotic-stress activation of sucrose-phosphate synthase in spinach leaves. Plant Physiol 114:947–55
Uemura M, Steponkus PL (2003) Modification of the intracellular sugar content alters the incidence of freeze-induced membrane lesions of protoplasts isolated from Arabidopsis thaliana leaves. Plant Cell Environ 26:1083–1096
Xiong FS, Ruhland CT, Day TA (1999) Photosynthetic temperature response of the Antarctic vascular plants Colobanthus quitensis and Deschampsia antarctica. Physiol Plant 106:276–286
Zrenner R, Stitt M (1991) Comparison of the effect of rapidly and gradually developing water-stress on carbohydrate metabolism in spinach leaves. Plant Cell Environ 14:939–946
Zúñiga-Feest A, Ort D, Gutiérrez A, Gidekel M, Bravo LA, Corcuera LJ (2005) Light regulation of sucrose-phosphate synthase activity in the freezing-tolerant grass Deschampsia antarctica. Photosynth Res 83:75–86
Acknowledgements
Permits to collect plant material in protected areas in the Maritime Antarctic were provided by Instituto Antártico Chileno. The authors thank the financial support of DIUC 205.111.042-1S. Luisa Bascuñán thanks MECESUP UCO 0214 for a graduate fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bascuñán-Godoy, L., Uribe, E., Zúñiga-Feest, A. et al. Low temperature regulates sucrose-phosphate synthase activity in Colobanthus quitensis (Kunth) Bartl. by decreasing its sensitivity to Pi and increased activation by glucose-6-phosphate. Polar Biol 29, 1011–1017 (2006). https://doi.org/10.1007/s00300-006-0144-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-006-0144-3