Abstract
Samples taken at two stations in the northern and southern parts of the Scotia Arc, at depths of 277 and 307 m, respectively, were analysed for metazoan meiofauna with special attention to the nematodes. Identification to species level was performed for two closely related subdominant nematode genera (Desmodora and Desmodorella) in samples from the two Scotia Arc stations and in other available samples from adjacent areas (Magellan Region, Drake Passage, Weddell Sea). Seven Desmodora species and three Desmodorella species were found, of which, respectively five and two species are new to science. The Scotia Arc stations show relatively high densities and average diversity on meiofauna and nematode level compared to adjacent areas. The distribution patterns of the various Desmodora and Desmodorella species suggest the Scotia Arc as a shallow bridge and a possible exchange route for meiofauna between the Antarctic and South America, especially since these species seem to be constrained by water depth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Separated from other continents by at least 1,000 km (South America), and surrounded by some of the deepest and coldest seas of the world, Antarctica is without doubt the most isolated continent on this planet. In Paleozoic times, however, Antarctica was part of the Gondwana supercontinent, the climate of which was considerably warmer than that of today’s southern continents. Rifting between the Eastern (comprising Antarctica, Australia and India) and Western Gondwana (comprising South America and South Africa) started in the Early Jurassic (208–178 Ma) (Crame 1999) and eventually led to the complete disconnection of South America and the Antarctic continent by means of a middle- to deep-water passage somewhere in the Oligocene epoch (23–32 Ma) (Lawver and Gahagen 2003; Thomson 2004; Barker and Burrell 1982). Further opening of the Drake Passage eventually led to a complete Antarctic Circumpolar Current (ACC) around 22–17 Ma (Barker 2001), driven by the west wind drift and further isolating Southern Ocean biota (Crame 1999).
The cold, deep waters around Antarctica, the strong ACC and the steep gradients in temperature, phytoplankton abundance, distribution of zooplankton and climatic conditions at the Antarctic Convergence contribute to the isolation of Antarctica and are therefore considered as important biological barriers (Knox 1994). These factors, along with the long period of isolation and the occurrence of succeeding glacial and interglacial periods, drove evolution, leading to the present-day diversity and biogeography (Clarke and Crame 1992; Brey et al. 1996) in which the Southern Ocean benthic fauna is characterised by endemism on different taxonomic levels (Knox 1994; Clarke and Johnston 2003). However, continuing exchange through remaining migration routes may lead to characteristic faunistic links between Antarctica and the surrounding continents. Of particular interest is the Scotia Arc, which represents the remains of the last land bridge that connected Antarctica with another continent and now comprises different archipelagos creating a unique shallow chain between Antarctica and South America.
Meiobenthos (32–1,000μm) is the most abundant eukaryotic size class in marine sediments and the small, relatively easily collectable samples of seabed sediments yield thousands of individuals of many different species. Nematodes form the most abundant taxon within the metazoan meiobenthos, usually comprising more than 90% and displaying a tremendous diversity with over 4,000 free-living marine species described worldwide (Heip et al. 1982; Lambshead 1993) and a vast number of undescribed species. Yet in spite of their high abundances and species diversity, and their importance in marine ecosystems (Heip et al. 1982), nematodes have received relatively little attention in Antarctic research and consequently little is known about their biodiversity and biogeography. Only a few studies have dealt with ecological and biogeographical information on Antarctic nematodes at species level (Vermeeren et al. 2004; De Mesel et al. 2006; Fonseca et al. 2006).
By identifying all species within two closely related subdominant genera (Desmodora and Desmodorella) in samples collected from the Scotia Arc and adjacent areas (Magellan Region, Drake Passage, Weddell Sea), we present information about nematode biodiversity and biogeography at the species level. The aims of this study are to: (1) investigate both local and regional biodiversity at the genus level and (2) study the distribution of species within the two subdominant genera along and at both sides of the Scotia Arc. In addition, the effect of sample-size on diversity measures is evaluated.
Materials and methods
Sampling and sampling area
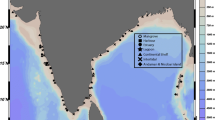
During the Latin AMerican POlarstern Study (LAMPOS, from 3 April 2002 to 5 May 2002) campaign along the Scotia Arc on board of the German research vessel Polarstern (Arntz and Brey 2003), meiofauna samples were taken using a multicorer (MUC), equipped with 12 core tubes with an internal diameter of 57 mm, equivalent to a 25.5 cm2 cross-sectional area. Two stations on opposite sides of the Scotia Arc were sampled: station PS61-177 (277 m depth), near South Georgia on the northern part of the Scotia Arc (NSA) and station PS61-242 (307 m depth), in the vicinity of Signy Island in the southern part of the Scotia Arc region (SSA) (Table 1, Fig. 1). The distance between the stations is ca. 960 km. Stations PS61-177 and PS61-242 are hereafter referred to as NSA 177 and SSA 242, respectively.
In addition to the two samples processed in this study, the presence of species belonging to the genera Desmodora and Desmodorella was verified in several other samples from Subantarctic and Antarctic regions in the Atlantic sector of the Southern Ocean, near Rothera on the Pacific side of the Antarctic Peninsula, and in the Ross Sea. Samples for these areas were taken with a boxcorer or multicorer and sometimes subsampling took place (this information is integrated in Table 2). These samples were already analysed to genus level in previous unpublished and published studies (Chen 1999; H.J. Lee, unpublished; Manachini 1997; Vanhove 1997; Vermeeren 2002; Luyten 1999; Vanhove et al. 2004; Table 2).
Meiofauna and nematode analysis
At the two Scotia Arc stations, three pseudo-replicate (different cores from the same MUC deployment) sediment samples were sliced (0–1 cm, 1–3 cm, 3–5 cm, 5–10 cm) and fixed in buffered 4% formalin solution. Afterwards, the samples were passed through a 1,000-μm mesh and then sieved on a 32-μm mesh to retrieve the meiofauna size class, which was then resuspended and centrifuged with LUDOX HS 40% as described by Heip et al. (1985) and Vincx (1996). After staining with Rose Bengal, all metazoan meiobenthic organisms were classified at higher taxon level and counted under a stereoscopic microscope using the work of Higgins and Thiel (1988). The samples taken at the additional stations were sliced at variable sediment depths and treated similarly to the NSA 177 and SSA 242 samples prior to analysis. However, sometimes the minimum mesh width was 38 μm instead of 32 μm. The number of nematodes identified to genus level and the used mesh width were mentioned per station in Table 2.
Six sets of 50 nematodes were picked out randomly from the top 0–1 cm slice of one pseudoreplicate for both Scotia Arc stations. They were transferred first to an alcohol–glycerine solution and then to glycerine and mounted on Cobb slides (Cobb 1917). The total number of nematodes identified was always lower than 300 due to, e.g., damaged specimens, juveniles. Nematodes were identified to genus level using the pictorial key to nematode genera of Platt and Warwick (1998), relevant taxonomic literature (Bussau 1993; Jensen 1978; Platt and Warwick 1998; Vermeeren et al. 2004; Verschelde et al. 1998) and reference drawings of the Department of Marine Biology of Ghent University. Identification to species level of the genera Desmodora and Desmodorella was done by comparing detailed morphological drawings (made with a camera lucida on a Leica DMLS microscope) and measurements of adult specimens with relevant literature concerning these genera in the nematode library of Ghent University and the NeMys database (Deprez et al. 2005).
As a measure of diversity we used Hill’s indices (Hill 1973) and applied them to genus level. Hill’s indices or diversity numbers are variably sensitive to sample size and are commonly used to probe different aspects of the community, i.e. with increasing order they become more sensitive to dominant taxa and vice versa. Another advantage is that they are mathematically related to commonly used diversity indices such as Shannon–Wiener diversity index and Simpson’s index (Heip et al. 1998; Soetaert and Heip 1990).
-
N0 = number of genera,
-
N1=exp (H′) with H′ = Shannon Wiener index = \( - {\sum_{i = 1}^n {p_{i}\ln p_{i}}}\) and p i = relative abundance of the ith genus,
-
\(N_{2} = \frac{1}{D}\) with \(D = {\sum_{i = 1}^n {p_{i}}^2}\) = Simpson’s index,
-
\(N_{\rm inf} = \frac{1}{{p_{1}}}\) with p1 = the proportional abundance of the most common genus.
Results
Meiofauna
Although a high diversity of major taxa was observed at both stations, the total number of taxa was higher at station NSA 177 (21 taxa) than at station SSA 242 (15 taxa). Other regularly occuring taxa, in addition to the Nematoda (ca. 90%), were harpactoid copepods and nauplii (3–5%), Kinorhyncha (ca. 1%), Polychaeta (0.8%, station NSA 177) and Ostracoda (0.6%, station SSA 242). Total meiofauna density was higher at station NSA 177 (8,804 ind./10 cm2) compared to station SSA 242 (3,409 ind./10 cm2).
At station NSA 177, nearly 40% of all meiofauna was situated in the upper centimetre of the sediment and 78.2% in the upper 3 cm. At station SSA 242, the density decrease with increasing depth is even clearer; 55.3% and more than 90% of the meiofauna resided in the first centimetre and the upper 3 cm of the sediment, respectively. Between 5 and 10 cm depth, the total meiofauna fraction is reduced to less than 1% (Fig. 2).
Nematode diversity
Both stations are characterised by a relatively high generic nematode diversity. Hill’s indices (especially N 2 and N inf; Fig. 3) showed a higher generic nematode diversity at station NSA 177. At this station, nematodes belonged to 44 different genera and 16 families, while at station SSA 242, 43 genera were found, belonging to 15 different families. The two stations have 27 genera and 13 families in common and show a similar dominance pattern (Table 3). Nine of the most abundant genera (> 1%) are common for both stations. The most abundant of these nine genera are Microlaimus and Daptonema, followed by Monhystera, Desmodorella and Desmodora of which the latter two belong to the family Desmodoridae. For station NSA 177 and station SSA 242, respectively 90.8% and 73.5% of the individuals that could not be identified to genus-level, almost exclusively juveniles, belonged to the family Desmodoridae (most likely to the genera Desmodora and Desmodorella), resulting in a very high Desmodoridae abundance. This observation (Table 3) provides a logical basis for choosing the genera Desmodora and the closely related Desmodorella for a more detailed biodiversity and biogeography study.
Taxonomical considerations for Desmodora and Desmodorella species
The genera Desmodora and Desmodorella have only recently been raised to the genus level. Before, the two genera were considered as subgenera within the genus Desmodora (Verschelde et al. 1998). The adult Desmodora specimens encountered at stations NSA 177 and SSA 242 are identified as Desmodora campbelli Allgén, 1932 due to the characteristic morphology of the head, setae, cuticle and the structure of the spicules and the precloacal supplements of the males (Fig. 4f, g). Although D. campbelli bears the genus name Desmodora, its characteristics are not fully consistent with the genus description according to Verschelde et al. (1998) and Pastor de Ward (1988): D. campbelli carries subcephalic setae like all Desmodora species, excluding it from Pseudochromadora which does not posses such setae, but it is also equipped with lateral alae on the cuticle, a typical characteristic for the genus Pseudochromadora. Because of these conflicting characteristics, Verschelde et al. (1998) regarded this species as incertae sedis. For convenience, we continue to refer to it as D. campbelli.
Morphological drawings of the cephalic region and the tail (with spicules when male specimens were available) of Desmodora species: (a) Desmodora sp. A (♂), (b) Desmodora sp. B (♂), (c) Desmodora sp. C (♂), (d) Desmodora sp. D (♀), (e) Desmodora sp. E (♂), (f) Desmodora campbelli (♂) (note the precloacal supplements), (g) Desmodora campbelli (♀) (drawing of the vulva region instead of the tail; note the lateral alae)
Five new Desmodora species (sp. A, sp. B, sp. C, sp. D, sp. E) (Fig. 4a–e) could be distinguished. Desmodora sp. A and Desmodora. sp. B are morphologically very similar and differentiated from the other Desmodora species, mainly by their body shape and small size. Desmodora sp. C shows an affinity with Desmodora sp. D and Desmodora sp. E but does not appear to have the numerous denticles in the buccal cavity and the distinct longitudinal rows of short, stout somatic setae which characterise these species. There is a striking similarity between Desmodora sp. D and Desmodora sp. E which could only be distinguished from each other by the long somatic setae in the tail region (present with Desmodora sp. D) and the number of amphid turns.
The adult Desmodorella specimens from stations NSA 177 and SSA 242 are identified as Desmodorella aff. balteata (After Desmodorella balteata Verschelde, Gourbault and Vincx 1998) (Fig. 5c). They are distinguished from Desmodorella tenuispiculum (Allgén 1928) by the dimensions of the amphid and tail morphology and show striking resemblance with D. balteata. Yet, small variations in morphological characteristics (stoutness of precloacal setae, amphid dimensions) are observed. These are, however, not obtrusive enough to classify the specimens as new species. Interesting is the presence of cuticular rings which did not originate from the animal itself, resembling the trapping rings of nematophagous fungi (Barron 1977) and the presence of small Suctoria attached to the cuticle as was observed by Verschelde et al. (1998). However, these rings are not encountered not only with Desmodorella and are therefore unlikely to be species-specific.
In addition to Desmodorella aff. balteata, two new Desmodorella species (sp. A, sp. B) (Fig. 5a, b) occurred in the samples. Desmodorella sp. A has an amphid coiling only 1.5 times (vs. 2.6 turns with D. balteata) and the males have extremely long spicules, clearly distinguishing them from D. balteata. The body morphology, head structures and conspicuously long spicules of this species resemble the characteristics of Desmodorella filispiculum Lorenzen 1976 (description based on specimens from Southern Chile). However, Desmodorella sp. A has less regular and smaller somatic setae, a shorter and narrower, conical-shaped tail and the males are equipped with a row of short precloacal setae. Desmodorella sp. B was characterised by a long and slender body shape, a spiral amphid (1.5 turn) and a relatively large tail.
Biogeography of Desmodora and Desmodorella
From the 64 Antarctic and Magellanic stations analysed, 25 stations were characterised by the presence of the genera Desmodora and/or Desmodorella (Table 4). In total, ten different species were identified: seven Desmodora species and three Desmodorella species. Fifteen out of the 25 stations contained only one species of Desmodora or Desmodorella while the maximum number of species per station was three. The stations NSA 177 and SSA 242 were each characterised by two species: Desmodora campbelli (type material from Campbell island, ca. 700 km South of New Zealand, Pacific) and Desmodorella aff. balteata (similar to D. balteata which was described based on specimens from hydrothermal vents in the East Pacific Rise, Guyamas, at 2,000 m depth). These two species were each present in 12 of the 25 stations while the other species were restricted to 1 or 2 stations.
At station NSA 177, 11 adult specimens (5 males, 6 females) belonged to the species D. campbelli and 8 adult Desmodorella aff. balteata specimens (5 males, 3 females) were found. At station SSA 242, 5 adult specimens (3 males, 2 females) belonged to the species D. campbelli and 2 adult females were classified as Desmodorella aff. balteata.
Desmodora campbelli is relatively widely spread over the shallowest stations (100–405 m) investigated and was completely absent in the deeper samples, including the South Sandwich Trench, Drake Passage and Ross Sea. Desmodorella aff. balteata was present in all areas (even the Ross Sea) except for the South Sandwich Trench and the Magellan Region. The maximum depth observed for Desmodorella aff. balteata was 1,028 m in the Drake Passage. Desmodora sp. A, sp. B and sp. C were only observed in the South Sandwich Trench samples at depths between 747 and 6,315 m, while only Desmodora sp. D was found in the Weddell Sea as well as in the South Sandwich Trench. Desmodora sp. E, Desmodorella sp. A and Desmodorella sp. B are characteristic for the Weddell Sea. Finally, Desmodora minuta Wieser 1954 was found in the Magellan region. Distribution patterns are given in Table 4 and Fig. 6.
Discussion
Scotia Arc meiofauna
Antarctic benthic fauna is characterised by a high diversity which has been formed under the influence of the combined effects of speciation and extinction, abiotic environmental conditions and biotic interactions (Arntz et al. 1994, 1997; Brey et al. 1994, 1996; Clark and Crame 1992). The metazoan meiofauna is a prominent member of the Antarctic benthic fauna as indicated by Heip et al. (1982). However, this group remains less studied in polar regions compared to macrofauna. Moreover, little is known about their biodiversity in the Antarctic.
For stations NSA 177 and SSA 242, respectively 21 and 15 (total 22) higher meiofauna taxa were found, showing a high but not exceptional diversity compared to what was found in previous studies in the Atlantic sector of the Southern Ocean (Herman and Dahms 1992; Vanhove 1997; Vanhove et al. 1995, 1998, 1999, 2000, 2004). In the two stations studied, meiofauna densities were higher (8,804 ind./ 10 cm2 for NSA 177 and 3,409 ind./ 10 cm2 for SSA 242) than in other oceans at comparable depths (Soltwedel 2000). The previous Antarctic studies mentioned above demonstrated that meiofauna can be very abundant in the Southern Ocean. This might be a consequence of the very high Antarctic primary production during the short but intensive summer bloom (Bathman et al. 1991; von Bodungen et al. 1986; von Bröckel 1985; Figueiras et al. 1998; Korb and Whitehouse 2004; Vanhove et al. 1995). The results show that densities decrease fast with increasing sediment depth (down to 1% between 5 and 10 cm depth), illustrating the importance of the topmost sediment layer. The spatial distribution of meiobenthos within the sediment shows a classic vertical decreasing pattern, and corresponds with a similar trend in food availability.
Scotia Arc nematodes
The nematode community of the two stations studied is dominated by the genera Microlaimus and Daptonema, followed by Monhystera, Desmodorella and Desmodora. The first three genera are similarly dominant in other oceans, across comparable depth ranges, whilst the latter two are much less abundant in oceans all over the world (Jensen 1988; Vanreusel et al. 1992; Soetaert and Heip 1995; Vanaverbeke et al. 1997; Vanhove et al. 2004) and in the Magellan Region (0–3.8%) and the Weddell sea (0–4.12%). Perhaps the high Desmodoridae abundance can be explained by the very fine sediment structure (a higher percentage of silt-clay than stations at Kapp Norvegia, Halley Bay and Magellan Strait which clearly contained higher percentages of sand) providing preferential habitats for large burrowers (Tita et al. 1999; Wieser 1959; Coull 1988) such as Desmodora and Desmodorella. Thus, although the genera of the family Desmodoridae are widely distributed, their densities show a distinct variation on a global and local Antarctic scale.
Hill’s indices (N 0, N 1, N 2, N inf) (Hill 1973), were calculated for the nematodes for both stations and represented in a rarefaction curve (Fig. 3). They are generally lower than for the Weddell Sea stations (Kapp Norvegia and Halley Bay; Vanhove et al. 1999), and higher than for the Rothera stations (Luyten 1999). The sample size dependency of many diversity indices is a well-known problem for nematode diversity studies and asks for specific methodological considerations. The question of whether sample size is large enough to estimate real diversity has an ambiguous answer because both community type variability and the large variety of available diversity indices must be considered, a problem clearly pointed out by Heip et al. (1998) and Soetaert and Heip (1990). For station NSA 242, doubling the amount of identified nematodes from ca. 100 to 200 resulted in an increase of 48% of the number of genera (N 0). A similar trend is found for station NSA 177, where an increase of 93% of identified nematodes resulted in an increase of 22% in genera numbers. After identification of ca. 100 nematodes, the rarefaction curve of N 0 is flattening, making the identification of more nematodes relatively unimportant for estimating genus diversity. Except for N 0 (both stations) and N 1 (station SSA 242), an imaginary asymptote is reached within the ca. 200 nematodes identified. When analysing more than 200 specimens, new genera will be found but their low abundances in most cases will hardly influence higher order Hill’s indices. In stations NSA 177 and SSA 242 there are respectively 22 and 25 single-individual genera or singletons, covering more than half of the total number of genera in these stations (respectively, 44 and 43).
The Scotia Arc as possible migration route for interstitial meiofauna
The origin of Antarctic benthic biota has fascinated scientists for a long time and several hypotheses have been put forward. It may (a) represent a relict autochthonous fauna, or consist of (b) eurybathic species derived from adjacent deep-water basins, (c) abyssal species and sub-Antarctic species of predominantly northern origin, (d) species of Magellanic origin which have migrated to Antarctica via the Scotia Arc and vice versa (Knox 1994). Knox (1994) summarised some major distribution patterns observed for Antarctic fauna (Circumpolar distribution, Circum-Antarctic and Circum-sub-Antarctic). Of specific interest for this study is the role of the Scotia Arc as a migration path to and from the Antarctic.
The genus Desmodora occurred in all areas except for the Antarctic Peninsula (Drake Passage and Rothera), while Desmodorella was not recorded in the South Sandwich Trench area. In general Desmodora and Desmodorella specimens occurred mainly at shallow depths, except Desmodora sp. A, sp. B and sp. C which are confined to the deeper stations at the South Sandwich Trench.
Desmodora campbelli was found in the Atlantic and Pacific sector (Campbell Island) suggesting a circumpolar distribution. However, this species only occurred at shallower depths and was absent in deeper samples (South Sandwich Trench), presumably excluding its dispersion through the deep sea. It was also absent around the Peninsula, possibly due to low food availability or sediment characteristics: samples from the Rothera area were characterised by a relatively low silt–clay fraction. However, the absence of D. campbelli in a few samples does not necessarily mean that it is completely absent in the general sampling area. The Scotia Arc may very well have served as a migration path from or to the Antarctic for this species. Its dispersion could be influenced by hydrodynamics (Palmer 1990) since dispersal of nematodes is assumed to be primarily by passive transport in the bedload and water column. Epigrowth-feeders such as Desmodora and Desmodorella prefer the surficial sediment and are most susceptible to erosion and transport (Commito and Tita 2002 and references therein). Although the nematodes are assumed to be permanent sediment inhabitants, their occurrence in the water column is not exceptional in high-energy areas (Ullberg and Ólafsson 2003). Considering that Antarctic waters are characterised by a complex current system (ACC, East Wind Drift, Weddell Sea gyre, eddies, etc.) and movement of vast water masses (Antarctic Bottom Water, Circumpolar Deep Water, Antarctic Surface Water, Sub Antarctic Surface Water, etc.), the dispersion of benthic meiofauna cannot be excluded since turbulent water masses are encountered at considerable depth and have enough energy to transport small animals (Angel and Fasham 1983; Clarke et al. 2005). Another explanation for the distribution of D. campbelli could be that it inhabited parts of South America and Antarctica before the two continents drifted apart, and remained unaltered ever since, partly due to a slow evolution rate which is typical in very cold conditions. This view is consistent with the assumption that the Antarctic fauna is very old. Preliminary molecular results based on ten partial COI-gene sequences (primers JB2 and JB5) of D. campbelli from NSA 177 and SSA 242 show little genetic divergence between populations from these stations, which again could point to either a very slow “evolver” or an extremely high gene flow. Considering the large distance (ca. 960 km) between both stations, the latter may seem very improbable, but the complex hydrodynamic situation around the Scotia Arc leaves scope for discussion.
Desmodorella aff. balteata occurs at all areas studied except for the Magellan region and South Sandwich Trench. Based on these records, which include the Ross Sea, we assume that its distribution is circum-Antarctic. Although this species is mainly observed at shallower depths, the type specimens of D. balteata were found at a hydrothermal vent at a depth of 2,000 m (Verschelde et al. 1998).
While both D. campbelli and Desmodorella. aff. balteata show a wider distribution, most of the Desmodora and Desmodorella species seem to be confined in their distribution because of geographical or environmental barriers. However, evidence drawn from the distributions of D. campbelli and other species should be carefully assessed since, in addition to the real turn-over, environmental variables (e.g. sediment structure, food availability) may limit small-scale distribution of species. Moreover, we should keep in mind the possible effect of undersampling on species distribution patterns, especially when considering absence data. The maximum number of Desmodora and/or Desmodorella species occurring at one station was three out of a total of ten observed, leading to a high turn-over of species within these genera between stations and suggesting that regional diversity may increase significantly compared to local diversity
The South Sandwich Trench is characterised by a very distinctive Desmodora assemblage and the absence of the genus Desmodorella. Three of the four Desmodora species found here, were apparently confined to this area; the fourth (Desmodora sp. D) also occurred at Kapp Norvegia. Environmental conditions (depth, sediment properties, food availability) peculiar for this area could explain the distinct and characteristic deep-sea nematode fauna observed.
Desmodora minuta is restricted to the Magellan Strait, an area characterised by a very different ecosystem. The different environmental conditions linked to this ecosystem may prevent its dispersion southward. Observation by Clasing (1980) off the coast of Puerto Mont, Chile suggests that this species is distributed around the southern part of South America.
Vermeeren et al. (2004) did a similar study of the distribution of the genus Dichromadora without including the Scotia Arc. Therefore, both Scotia arc stations were also checked on the presence of Dichromadora species. Vermeeren et al. (2004) came to the conclusion that nearly all Antarctic Dichromadora species were new to science. All the Dichromadora species encountered in the Scotia Arc samples (Dichromadro sp. B, Dichromadro aff. weddellis, Dichromadro polaris) are identified as species described by Vermeeren et al. (2004). We identified specimens as Dichromadro aff. weddellis because of a slight difference in tail length and spicule thickness compared to the type specimens, traits not distinct enough to regard them as a new species. An overview of the Dichromadora species distributions is given in Table 5. Assemblages of Dichromadora in NSA 177 and SSA 242 are similar and resemble those found in the SE Weddell Sea. Dichromadora sp. B and D. polaris are widely distributed over all areas at different depths (277–2,000 m) suggesting migration unaffected by bathymetry along deeper and shallower routes.
Conclusions
The Scotia Arc stations show high densities and average diversity on meiofauna and nematode level, compared to adjacent areas. In addition, the identification of ca. 200 nematodes provides a relatively accurate estimate of diversity at the generic level using Hill’s diversity indices.
In this study the species level turn-over between different stations did not reflect regional diversity, due to the restricted distributions of some species. Bathymetrical and sedimentary constraints were observed, transcending biogeographical confinements, hence shaping these species’ distributions.
The distribution patterns of Desmodora campbelli and Desmodorella aff. balteata which are present across the Scotia Arc, suggest either (1) that this shallow island chain serves as a possible migration path between South America and the Antarctic, or (2) that under the cold Antarctic conditions the evolution of these species is extremely slow.
Nematode species data for the Antarctic region are very scarce, despite the valuable information they can yield on biodiversity and biogeography. The development of molecular techniques combined with intensive morphological study of deep-sea nematodes may provide a solution for the lack of taxonomic knowledge for the Antarctic, and especially the deep sea.
References
Angel MV, Fasham MJR (1983) Eddies and biological processes. In: Robinson AR (ed.) Eddies in marine science. 1983 series: Topics in atmospheric and oceanographic sciences. Springer, Berlin Heidelberg New York
Arntz WE, Brey T (2003) The expedition ANTARKTIS XIX/5 (LAMPOS) of RV “Polarstern” in 2002. Berichte zur Polar- und Meeresforschung 462
Arntz WE, Brey T, Gallardo VA (1994) Antarctic zoobenthos. Ocean Mar Biol: Ann Rev 32:241–304
Arntz WE, Gutt J, Klages M (1997) Antarctic marine biodiversity: an overview. In: Batagli B, Valencia J, Walton DHW (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 3–14
Barker PF (2001) Scotia Sea regional tectonic evolution: implications for mantle flow and palaeocirculation. Earth Sci Rev 55(1, 2):1–39
Barker PF, Burrell J (1982) The influence upon Southern Ocean circulation, sedimentation, and climate of the opening of the Drake Passage. In: Craddock C (ed) Antarctic geoscience, University of Wisconsin Press, Madison, pp 377–385
Barron GL (1977) The nematode-destroying fungi. Topics in mycobiology: 1. Canadian Biological Publications, Guelph
Bathman U, Fischer G, Muller PJ, Gerdes D (1991) Short-term variations in particulate matter sedimentation off Kapp Norvegia, Weddell Sea, Antarctica: relation to water mass advection, ice cover, plankton biomass and feeding activity. Polar Biol 11:185–195
von Bodungen B, Smetacek VS, Tilzer MM, Zeitzschel B (1986) Primary production and sedimentation during spring in the Antarctic Peninsula region. Deep Sea Res Pt I 33:177–194
Brey T, Klages M, Dahm C, Gorny M, Gutt J, Hain S, Stiller M, Arntz WE (1994) Antarctic benthic diversity. Nature 368:297
Brey T, Dahm C, Gorny M, Klages M, Stiller M, Arntz WE (1996) Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarctic Sci 8(1):3–6
von Bröckel K (1985) Primary production data from the south-eastern Weddell Sea. Polar Biol 4:75–80
Bussau C (1993) Taxonomische und ökologische Untersuchungen an Nematoden des Peru-Beckens. PhD thesis, University Kiel, Kiel
Chen G (1999) Ecology and systematics of the meiofauna and nematode communities in the Strait of Magellan and the Beagle Channel (Chile). PhD thesis, Ghent University, Belgium
Clarke A, Crame JA (1992) The Southern Ocean Benthic fauna and climate change—a historical perspective. Philos T Roy Soc B 1285:299–309
Clarke A, Johnston NM (2003) Antarctic marine benthic diversity. Ocean Mar Biol: Ann Rev 41:47–114
Clarke A, Barnes DKA, Hodgson DA (2005) How isolated is Antarctica? Trends Ecol Evol 20(1):1–3
Clasing E (1980) Postembryonic Development in Species of Desmodoridae, Epsilonematidae and Draconematidae. Zool Anz 204(5, 6):337–344
Cobb NA (1917) Notes on nemas. Contrib Sci Nematol 5:117–128
Commito JA, Tita G (2002) Differential dispersal rates in an intertidal meiofauna assemblage. J Exp Mar Biol Ecol 268:237–256
Coull BC (1988) Ecology of the marine meiofauna. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution Press, London, pp 18–38
Crame JA (1999) An evolutionary perspective on marine faunal connections between southernmost South America and Antarctica. Sci Mar 63(Suppl 1):1–14
De Mesel I, Lee HJ, Vanhove S, Vincx M, Vanreusel A (2006) Species diversity and distribution within the deep-sea nematode genus Acantholaimus on the continental shelf and slope in Antarctica. Polar Biol (in press)
Deprez T, et al (2005) NeMys. World Wide Web electronic publication. http://www.nemys.ugent.be, version (10/2005)
Figueiras FG, Estrada M, López O, Arbones B (1998) Photosynthetic parameters and primary production in the Bransfield Strait: relationships with mesoscale hydrographic structures. J Mar Syst 17:129–141
Fonseca G, Vanreusel A, Decraemer W (2006) Taxonomy and biogeography of Molgolaimus Ditlevsen, 1921 (Nematoda: Chromadoria) with reference to the origins of deep-sea nematodes. Ant Sci (in press)
Heip C, Vincx M, Smol N, Vranken G (1982) The systematics and ecology of free-living marine nematodes. Helminthol Abs Ser B, Plant Nematol 51(1):1–31
Heip C, Vincx M, Vranken G (1985) The ecology of marine nematodes. Ocean Mar Biol: Ann Rev 23:399–489
Heip C, Herman P, Soetaert K (1998) Indices of diversity and evenness. Océanis 24(4):61–87
Herman RL, Dahms HU (1992) Meiofauna communities along a depth transect off Halley Bay (Weddell Sea Antarctica). Polar Biol 12:313–320
Higgins RP, Thiel H (1988) Introduction to the study of meiofauna. Smithsonian Institution Press, London
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Jensen P (1978) Revision of Microlaimidae, erection of Molgolaimidae fam.n., and remarks on the systematic position of Paramicrolaimus (Nematoda, Desmodorida). Zool Scr 7:159–173
Jensen P (1988) Nematode assemblages in the deep-sea benthos of the Norwegian Sea. Deep-Sea Res Pt I 35(7):1173–1184
Knox GA (1994) The biology of the Southern Ocean. Studies in polar research. Cambridge University Press, Cambridge
Korb RE, Whitehouse M (2004) Contrasting primary production regimes around South Georgia, Southern Ocean: large blooms versus high nutrient, low chlorophyll waters. Deep-sea Res Pt I 51:721–738
Lambshead PJD (1993) Recent developments in marine benthic biodiversity research. Océanis 19(6):5–24
Lawver LA, Gahagan LM (2003) Evolution of Cenozoic seaways in the circum-Antarctic region. Palaeogeogr Palaeoclimatol Palaeoecol 198(1–2):11–37
Luyten C (1999) Meiofauna van Antarctica: structurele en trofische aspecten. Licentiate thesis, Ghent University
Manachini B (1997) Biodiversity of Nematoda assemblages in the Antarctic sea bed. MSc thesis, Ghent University
Palmer MA (1990) Understanding the movement dynamics of a stream-dwelling meiofauna community using marine analogs. Stygologia 5(2):67–74
Pastor de Ward CT (1988) Nematodes Marinos de la Ría Deseado (Desmodoroidea): Desmodoridae, Draconematidae), Santa Cruz, Argentina. VII. Physis (Buenos Aires), Seccion. A. 46:61–72
Platt HM, Warwick RM (1998) Freeliving marine nematodes. Part II Chromadorids. Synopses of the British Fauna (New Series) 53. The Linnean Society and The Estuarine and Coastal Sciences Association, London
Soetaert K, Heip C (1990) Sample-size dependence of diversity indices and the determination of sufficient sample size in a high-diversity deep-sea environment. Mar Ecol Prog Ser 59:305–307
Soetaert K, Heip C (1995) Nematode assemblages of deep-sea and shelf break sites in the North Atlantic and Mediterranean Sea. Mar Ecol Prog Ser 125:171–183
Soltwedel T (2000) Metazoan meiobenthos along continental margins: a review. Prog Oceanogr 46:59–84
Thomson MRA (2004) Geological and palaeoenvironmental history of the Scotia Sea region as a basis for biological interpretation. Deep-Sea Res 51(Pt II):1467–1487
Tita G, Vincx M, Desrosiers G (1999) Size spectra, body with and morphotypes of intertidal nematodes: an ecological interpretation. J Mar Biol Assoc UK 79:1007–1015
Ullberg J, Ólafsson E (2003) Free-living marine nematodes actively choose habitat when descending from the water column. Mar Ecol Prog Ser, 260:141–149
Vanaverbeke J, Soetaert K, Heip C, Vanreusel A (1997) The metazoan meiobenthos along the continental slope of the Goban Spur (NE Atlantic). J Sea Res 38:93–107
Vanhove S (1997) Antarctic sublittoral meiofauna: focus on the ecology of free-living marine nematodes. PhD thesis, Ghent University, Belgium
Vanhove S, Wittoeck J, Desmet G, Van den Berghe B, Herman RL, Bak RPM, Nieuwland G, Vosjan JH, Boldrin A, Rabitti S, Vincx M (1995) Deep-sea meiofauna communities in Antarctica: structural analysis and relation with the environment. Mar Ecol Prog Ser 127:65–76
Vanhove S, Lee HJ, Beghyn M, Van Gansbeke D, Brockington S, Vincx M (1998) The metazoan meiofauna in its biogeochemical environment: the case of an Antarctic coastal sediment. J Mar Biol Assoc UK 78:411–434
Vanhove S, Arntz W, Vincx M (1999) Comparative study of the nematode communities on the southeastern Weddell Sea shelf and slope (Antarctica). Mar Ecol Prog Ser 181:237–256
Vanhove S, Beghyn M, Van Gansbeke D, Bullough LW, Vincx M (2000) A seasonally varying biotope at Signy Island, Antarctic: implications for meiofauna structure. Mar Ecol Prog Ser 202:13–25
Vanhove S, Vermeeren H, Vanreusel A (2004) Meiofauna towards the deep South Sandwich Trench (750–6300 m). Deep-Sea Res Pt II 51:1665–1687
Vanreusel A, Vincx M, Van Gansbeke D, Gijselinck W (1992) Structural analysis of the meiobenthos communities of the shelf break area in two stations of the gulf of Biscay (N.E. Atlantic). Belg J Zool 122(2):185–202
Vermeeren H (2002) Biogeografie van Antarctische diepzeenematoden: species turn-over in dominante genera van de familie Chromadoridae. Licentiate thesis, Ghent University, Belgium
Vermeeren H, Vanreusel A, Vanhove S (2004) Species distribution within the free-living marine nematode genus Dichromadora in the Weddell Sea and adjacent areas. Deep-Sea Res Pt II 51:1643–1664
Verschelde D, Gourbault N, Vincx M (1998) Revision of Desmodora with descriptions of new desmodorids (Nematoda) from hydrothermal vents of the pacific. J Mar Biol Assoc UK 78:75–112
Vincx M (1996) Meiofauna in marine and fresh water sediments. In: Hall GS (ed) Methods for the examination of organismal diversity in soils and sediments. IUBS Series of Methodology Handbooks, CAB International, University Press, Cambridge, pp 214–248
Wenthworth CK (1922) The Wenthworth scale of grain size for sediments. J Geol 30:381
Wieser W (1959) The effect of the grain size in the distribution of small invertebrates inhabiting the Beaches of Puget Sound. Limnol Oceanogr 4:181–194
Acknowledgements
We are very much indebted to the Alfred-Wegener Institute for Polar and Marine Research and the captain and crew members of the RV Polarstern for their expertise and professionalism. We would like to thank Dr. W. Bonne and Dr. R. Herman for their sampling efforts during the LAMPOS-campaign, Drs. S. Derycke for molecular work and results, and Prof. Dr. M. Vincx for the use of research facilities. This research was performed during the M.Sc. course Marelac at the University of Ghent and under the auspices of the Scientific Research Program on Antarctica from the Belgian Science Policy (BIANZO) and the concerted actions of Ghent University (GOA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ingels, J., Vanhove, S., De Mesel, I. et al. The biodiversity and biogeography of the free-living nematode genera Desmodora and Desmodorella (family Desmodoridae) at both sides of the Scotia Arc. Polar Biol 29, 936–949 (2006). https://doi.org/10.1007/s00300-006-0135-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-006-0135-4