Abstract
A diet analysis of the Patagonian toothfish Dissostichus eleginoides, trawled in the South Georgia Islands area in March–April 1996, was carried out by frequency of occurrence (F%) and coefficient “Q” (%) methods. The samples consisted chiefly of immature specimens, with predominant length ranges of 30–70 cm (TL). Fish was by far the main food on the shelves of Shag Rocks and South Georgia, accounting for about 70% of prey. Krill appeared as secondary food, although its importance was overestimated by the frequency of occurrence method. Cephalopods and mysids were infrequent in the stomachs, and only at Shag Rocks and South Georgia, respectively. Lepidonotothen kempi, Champsocephalus gunnari and Chaenocephalus aceratus constituted the main fish prey and their variability between Shag Rocks and South Georgia depended on their local abundance. The large proportion of fish exhibiting stomachs full or close to fullness (together 62%) suggests that feeding intensity of the species was high.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Patagonian toothfish, Dissostichus eleginoides, is a large, long-lived nototheniid widespread in deep waters of the Southern Hemisphere, mainly in the sub-Antarctic region of the Atlantic and Indian Oceans near the Antarctic Convergence and along the southern coasts of Argentina and Chile (Fischer and Hureau 1985; Gon and Heemstra 1990; Yukhov 1997; Pilling et al. 2001). The congener Antarctic toothfish, Dissostichus mawsoni, is endemic to the Antarctic continental shelf, south of latitude 65°S (Goldsworthy et al. 2002).
Both toothfish species are commercially important and of interest to the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR). In the Atlantic sector of the Antarctic Ocean, D. eleginoides has been caught mainly around South Georgia and Shag Rocks as by-catch in otter-trawls and, since 1989, targeted in the longline fishery. Total allowable catches of this resource have been established annually by CCAMLR from 1991 onwards resulting in reported catches in the range of 650–7,600 tonnes (SC-CAMLR 2003). The general knowledge of the biology of this species is useful for the development of stock assessment methods necessary for fishery management.
In the Atlantic and Indian Antarctic sectors the distribution in depth of D. eleginoides ranges from 70 m to 1,500 m (Fischer and Hureau 1985). Juveniles are more pelagic and are mainly distributed down to a depth of 400–500 m ; adults are demersal and inhabit deeper waters, down to maximum depth. The larger specimens caught reached around 215 cm total length (DeWitt et al. 1990). Sexual maturity in males and females is reached mainly at total lengths of 70–90 cm (7–11 years) and 90–100 cm (9–12 years), respectively (Zhivov and Krivoruchko 1990). Spawning takes place during autumn–winter (Duhamel 1981; Zhivov and Krivoruchko 1990). Around South Georgia, early stages of D. eleginoides from eggs to post-larvae were caught in summer between December and January (Efremenko 1983).
From a number of publications on the diet of D. eleginoides in its area of distribution, several are referred to the South Georgia and Shag Rocks region (Tarverdiyeva 1972; McKenna 1991; García de la Rosa et al. 1997; Pilling et al. 2001; among others). This study aims to provide new information on the diet of Patagonian toothfish in these regions, focusing the attention on juvenile stages.
Materials and methods
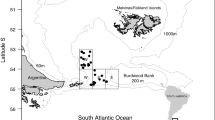
Samples were obtained during the survey of the R/V Dr Eduardo L. Holmberg around South Georgia and Shag Rocks from 20 March 1996 to 9 April 1996 (Fig. 1). The survey was organised and sponsored by the Instituto Antártico Argentino in conjunction with the Instituto Nacional de Investigación y Desarrollo Pesquero. It was planned principally to estimate the biomass of Champsocephalus gunnari in the area; sampling was conducted during daylight from 7:00 h to 19:00 h along random transects. The net used is a copyright design of Engel Netze. Its main dimensions are: headline, 35.3 m; mesh sizes: wings, 200 mm; body, 100 mm and codend 25 mm. The target was a 15-min haul on the bottom. Complete data of the stations and fishing operations are given in Marschoff et al. (1996). The entire catch of 353 D. eleginoides specimens was processed. The stomach were frozen on board at −30°C and later examined at the Instituto Antártico Argentino. The gonad maturation stage was determined immediately after capture, according to a five-point scale (Kock and Kellermann 1991). Basic information on sampling stations and fish examined is summarised in Table 1.
The diet analysis was done using the frequency of occurrence of each prey item expressed as a percentage of all the stomachs with food (F%) and by the dietary coefficient “Q”, which is the product of the percentage by number and the percentage by weight of each prey type (Hureau 1970).
The use of frequency of occurrence presents shortcomings and advantages, but usually this procedure is used because it allows quick comparison among the results of similar diet studies (see Kock et al. 1994). However, this method does not discriminate among the number or weight of organisms of single prey items, indicating only “presence” or “absence” and therefore is very sensitive to small amounts of prey. The coefficient “Q” gives a more consistent measure of the importance of each prey type in the diet, as it combines numeric and weight data. According to this method, prey are classified as main (Q>200), secondary (200>Q>20) or occasional food (Q<20).
The index of stomach fullness was evaluated according to a five-point scale: 0 (empty), 1 (1/4 full), 2 (1/2 full), 3 (3/4 full), 4 (full).
The length compositions (Total length=TL) of the fish are expressed in 5-cm intervals.
Results and discussion
The length composition of D. eleginoides caught during the survey is summarised in Fig. 2. Almost 90% of the fish were caught around Shag Rocks, where the length distribution showed a predominance of sizes in the range 30–50 cm with a peak at 40–45 cm. The mean length was 44 cm and only 1.2% of the specimens were larger than 70 cm. From the point of view of sexual maturity, most of the fish (94%) were immature or at an early stage of development (stages 1 and 2), whereas the remaining (6%) was at stage 3. The ratio between males and females was close to 1:1. The specimens from South Georgia (24 females, and 11 males) predominated in the sizes 60–70 cm with a mean of 64 cm. Eight fish (23%) were larger than 70 cm. Similarly to Shag Rocks, most fish (68%) were at maturity stages 1 and 2, 32% at stage 3.
The known bathymetric distribution of D. eleginoides indicates that, in general, fish lengths increase with increasing depth. The dominance of smaller sizes in the samples from Shag Rocks might be due to differences in fishing depth, since in this region the stratum from 250 m to 500 m was not sampled due to the difficulty to find suitable grounds for trawling.
All these data showing that the material collected consisted chiefly of small immature individuals are in agreement with reported information on length at maturity of the species at South Georgia, i.e. 70–100 cm (Zhivov and Krivoruchko 1990; Konforkin and Kozlov 1992). It is to be expected that mainly the juveniles are caught on the shelves.
The results of the stomach content analysis by frequency of occurrence and dietary coefficient “Q” show that demersal fish was by far the main food at Shag Rocks and South Georgia (Fig. 3). Fish occurred in about 70% of the stomachs analysed, and in 63% as sole prey. Krill (Euphausia superba) appeared as secondary prey, although its importance was overestimated by the frequency of occurrence method. This problem was evident in the analysis of data of a previous Holmberg survey in 1994, in which the importance of krill in the diet of the mackerel icefish C. gunnari was overestimated by the frequency of occurrence, when compared with Hureau’s method (Barrera-Oro et al. 1998).
The occurrence of cephalopods (both, octopods and squids) and mysids in the diet was very low and only at Shag Rocks and South Georgia, respectively. The importance of these items appeared as negligible by the index of Hureau (1970). The presence of other taxa was found as negligible (F% <0.5) and therefore not considered in the following discussion.
Among the fish prey, the species identified included Nototheniidae [Lepidonotothen kempi, Gobionotothen gibberifrons and Gobionotothen marionensis (=N. angustifrons)], Channichthyidae (C. gunnari and Chaenocephalus aceratus) and Muraenolepididae (Muraenolepis microps). In 49% of the stomachs containing fish material, this was too digested to be identified as to the species level.
Lepidonotothen kempi, C. gunnari and C. aceratus constituted the main fish prey of D. eleginoides and their variability between Shag Rocks and South Georgia depended on their local abundance (Fig. 4). An example is C. aceratus, with greater consumption at South Georgia reflected in its higher presence in the catches at this area (88% and 9% of the stations at South Georgia and Shag Rocks, respectively). Gobionotothen marionensis occurred in the stomachs occasionally at Shag Rocks and G. gibberifrons and M. microps secondarily at South Georgia. These last fish preys appeared as less important or even negligible according to the coefficient “Q”.
In previous studies, piscivory has also been indicated as predominating in juveniles and adults D. eleginoides (Duhamel and Pletikosic 1983; McKenna 1991; Garcia de La Rosa et al. 1997; Pilling et al. 2001; Goldsworthy et al. 2002; Arkhipkin et al. 2003) and D. mawsoni (Yukhov 1971; Pakhomov and Tseytlin 1992; Fenaughty et al. 2003) at all depths in their entire area of occurrence. Although demersal fish was reported as the main prey of D. eleginoides in the South Georgia region, the presence in the diet of pelagic prey species of the family Myctophidae was also indicated in samples taken from around a depth of 200 m (Zhivov and Krivoruchko 1990; Konfortin and Kozlov 1992; García de la Rosa et al. 1997). In this study, myctophiids were not represented in the diet of D. eleginoides . This could be at least in part due to the high percentage of stomach contents with fish in advanced stage of digestion (49%), which made taxonomic identification difficult, resulting in a considerable number of fish remaining unidentified.
The high percentage of stomachs full or in degree 3 of repletion (together 62%) indicates that feeding intensity of D. eleginoides was high (Fig. 5). This finding agrees with the observation of García de la Rosa et al. (1997) on juvenile specimens caught at similar depths (around 200 m) in the South Georgia region (83% of full stomachs).
The stomach fullness analysis showed 29% of empty stomachs (30% and 26% at Shag Rocks and South Georgia, respectively) (Fig. 5). These proportions are very low in comparison to those observed in samples collected by longlines, which are normally higher than 90% and are indicated as being caused by the sampling method (Konforkin and Kozlov 1992; Pilling et al. 2001). The scarce amount of stomachs in degrees 1–2 of repletion (together 9%) in comparison with the large amount of stomachs full (38%) or in degree 3 (24%), raises some doubts on the proportion of empty stomachs estimated in this study. As it happens in large scale in the longline fishery, it is possible that a fraction of the fish caught by trawls vomits due to pressure changes or stress during net operations.
Although the present study deals mostly with juvenile D. eleginoides specimens and the diet analysis was done on sexes combined, information from trawling samples in the literature indicate no differences in diet and feeding intensity between males and females in the same area (García de la Rosa et al. 1997).
Conclusion
The stomach content analysis of D. eleginoides by frequency of occurrence and coefficient “Q” methods simultaneously gave a more complete picture of the importance of prey items in the diet. Present results agree with those reported in the literature for similar depth ranges around Shag Rocks and South Georgia, indicating that fish was the main prey, followed by krill. Differences between areas in the fish species consumed depend on their local availability. Not unexpectedly, other invertebrates like cephalopods, mysids, shrimps, crabs, etc. as well as mesopelagic fish of the family Myctophidae were not or scarcely eaten, since they are common preys at depths deeper than 500–600 m (Konforkin and Kozlov 1992; Zaitsev 1995).
References
Arkhipkin A, Brickle P, Laptikhovsky V (2003) Variation in the diet of the Patagonian toothfish with size, depth and season around the Falkland Islands. J Fish Biol 63:428–441
Barrera-Oro ER, Casaux R, Marschoff E (1998) Analysis of the diet of Champsocephalus gunnari at South Georgia in late summer from 1994 to 1997, Dr. Eduardo L. Holmberg surveys. CCAMLR Sci 5:103–123
DeWitt H, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Duhamel G (1981) Caractéristiques biologiques des principales espèces de poissons du plateau continental des Iles Kerguelen. Cybium 5:19–32
Duhamel G, Pletikosic M (1983) Données biologiques sur les Nototheniidae des Iles Crozet. Cybium 7:43–57
Efremenko VN (1983) Atlas of fish larvae of the Southern Ocean. Cybium 7:1–74
Fenaughty JM, Stevens DW, Hanchet SM (2003) Diet of the Antarctic toothfish (Dissostichus mawsoni) from the Ross Sea, Antarctica (Subarea 88.1). CCAMLR Sci 10:113–123
Fisher W, Hureau JC (eds) (1985) FAO species identification sheets for fishery purposes. Southern Ocean (Fishing areas 48, 58 and 88)(CCAMLR Conservation Area). Prepared and published with the support of the Commission for the Conservation of Antarctic Marine Living Resources, Vol 2. Rome, FAO, pp 233–470
García de la Rosa SB, Sánchez F, Figueroa D (1997) Comparative feeding ecology of Patagonian toothfish (Dissostichus eleginoides) in the southwestern Pacific. CCAMLR Sci 4:105–124
Goldsworthy SD, Lewis M, Williams R, He X, Young JW, van den Hoff J (2002) Diet of Patagonian toothfish (Dissostichus eleginoides) around Macquarie Island, South Pacific Ocean. Mar Freshwater Res 53:49–57
Gon O, Heemstra PC (eds) (1990) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown
Hureau JC (1970) Biologie comparée de quelques Poissons antarctiques (Nototheniidae). Bull Inst Oceanogr (Monaco) 68:244
Kock K-H, Kellermann A (1991) Reproduction in Antarctic notothenioid fish (review). Antarctic Sci 3:125–150
Kock K-H, Wilhelms S, Everson I, Gröger J (1994) Variations in the diet composition and feeding intensity of mackerel icefish Champsocephalus gunnari at South Georgia (Antarctic). Mar Ecol Prog Ser 108:43–57
Konforkin IN, Kozlov AN (1992) Pre-spawning and spawning biology of the Patagonian toothfish, D. eleginoides, around South Georgia (Subarea 48.3). Document WG-FSA 92/13, CCAMLR, Hobart, Australia, pp. 14
Marschoff E, González B, Calcagno J, Shandikov G, López F, Madirolas A, Reta R (1996) Results of Dr Eduardo L Holmberg 1996 fish survey in Subarea 48.3. Document WG-FSA 96/27, CCAMLR, Hobart, Australia, pp. 25
McKenna JE Jr (1991) Trophic relationships within the Antarctic demersal fish community of South Georgia island. Fish Bull 89:643–654
Pakhomov YA, Tseytlin VB (1992) Diet of seven species of Antarctic fishes and estimation of their daily rations. J Ichthyol 32:31–41
Pilling GM, Purves MG, Daw TM, Agnew DA, Xavier JC (2001) The stomach contents of Patagonian toothfish around South Georgia (South Atlantic). J Fish Biol 59:1370–1384
SC-CAMLR (2003) Report of the Working Group on Fish Stock Assessment. In: Report of the 22nd meeting of the Scientific Committee (SC-CAMLR-XXII), Annex 5. CCAMLR, Hobart, Australia, pp. 289–547
Tarverdiyeva MI (1972) Daily food consumption and feeding pattern of the South Georgian cod (Notothenia rossii marmorata) and the Patagonian toothfish (Dissostichus eleginoides Smitt) (fam. Nototheniidae) in the South Georgia area. J Ichthyol 12:684–692
Yukhov VL (1971) The range of Dissostichus mawsoni Norman and some features of its biology. J Ichthyol 11:8–18
Yukhov VL (1997) Toothfishes of the genus Dissostichus-geographic range of distribution. Document WG-FSA-97/18, CCAMLR, Hobart, Australia, pp 8
Zaitsev AK (1995) Brief biological characteristics of patagonian toothfish (D. eleginoides) in Subarea 48.3 according to the results of the SRTMK ITKUL fishing trip in May 1995. Document WG-FSA-95/12, CCAMLR, Hobart, Australia, pp 6
Zhivov VV, Krivoruchko VM (1990) On the biology of the Patagonian toothfish, Dissostichus eleginoides, of the Antarctic Part of the Atlantic. J Ichthyol 30:142–146
Acknowledgements
We thank the scientific staff on board the R/V Dr Eduardo Holmberg 1996 for the collection of the samples, and the crew members for the logistic support. Carlos Bellisio provided technical assistance. We are grateful to Prof. J. Eastman and to Dr M. La Mesa, who critically commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barrera-Oro, E.R., Casaux, R.J. & Marschoff, E.R. Dietary composition of juvenile Dissostichus eleginoides (Pisces, Nototheniidae) around Shag Rocks and South Georgia, Antarctica. Polar Biol 28, 637–641 (2005). https://doi.org/10.1007/s00300-005-0723-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-005-0723-8