Abstract
Micronekton and macrozooplankton were collected during the austral spring of 1993 in the NW Weddell Sea. Sampling was done in three areas of the marginal ice zone: pack ice, ice edge, and open water, to examine the short-term effects of the spring phytoplankton bloom on the distribution and abundance of dominant fish and invertebrate species. Significant differences were observed for several common species, including Salpa thompsoni,Euphausia superba, Electrona antarctica, Gymnoscopelus braueri,and G. opisthopterus. Increased abundance seaward of the pack ice for these species is attributed to elevated phytoplankton and zooplankton biomass at the ice edge and in the open water areas. Distribution of the hyperiid amphipods, Cyllopus lucasii and Vibilia stebbingi mirrored that of S. thompsoni. No distributional trends between the areas were observed for Thysanoessa macrura, the amphipods Cyphocaris richardi and Primno macropa, the decapod shrimp Pasiphaea scotiae, the scyphomedusae Atolla wyvilli and Periphylla periphylla, and chaetognaths, indicating a trophic independence from the ice-edge bloom for these species. Lower occurrence of the mesopelagic fish Bathylagus antarcticus and Cyclothone microdon under the ice suggested that trophic repercussions of the spring bloom can also extend to deeper living species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the Southern Ocean, seasonal change in the sea ice extent is a driving force structuring the pelagic ecosystem (Eicken 1992; Laws 1985; Smetacek et al. 1990), especially within the marginal ice zone (MIZ) where pack ice meets open water. In the austral spring, melt-water associated with the receding ice edge stabilizes the surface layer promoting the development of localized blooms of phytoplankton and protozooplankton (Garrison and Buck 1989; Smith and Nelson 1985, 1990). Although temporally and spatially constrained, ice-edge blooms are a major energy resource for zooplankton, influencing the biology and ecology of primary consumers such as krill and copepods (Atkinson and Shreeve 1995; Brierley et al. 2002; Burghart et al. 1999; Daly and Macaulay 1988, 1991; Godlewska 1993; Robins et al. 1995; Schalk 1990; Stepien 1982).
With the exception of krill, there are few studies on how ice-edge blooms influence micronekton species. Pakhomov et al. (1999) showed elevated carnivorous micronekton and macrozooplankton biomass in the MIZ and results from AMERIEZ (Antarctic Marine Ecosystem Research in the Ice-Edge Zone, 1983–1988) showed that the effects of ice cover and changes in prey distribution associated with ice-edge blooms can be manifested in the horizontal and vertical distribution of mesopelagic fish and crustaceans (Ainley et al. 1986, 1991; Daly and Macaulay 1988; Lancraft et al. 1991; Torres et al. 1984). In a study utilizing acoustics to examine the upper 100 m, Kaufmann et al. (1995) reported that while both target size and abundance were routinely greater in open water than the pack ice, a distinct diel pattern in the occurrence of larger (∼12 cm) targets was only observed under the ice. Based on day/night trawls conducted within the upper 300 m, the authors attributed the nightime increase in large targets under the ice to migrating mesopelagic fishes, notably Electrona antarctica and Gymnoscopelus braueri.
In order to further elucidate the effects of the marginal ice zone on zooplankton and micronekton, a spring cruise was conducted to sample the closed pack, ice edge, and open water areas of the MIZ sequentially as an identified bloom progressed southward with the receding ice. Results on zooplankton physiology and population dynamics, as well as micronekton physiology and biochemistry have been reported previously (Burghart 1999; Donnelly et al. 2004; Geiger et al. 2001; Kawall et al. 2001). The present study reports on the distribution and abundance of common fish and invertebrate micronekton and macrozooplankton within the 0–1,000 m water column under pre-bloom (pack ice), bloom (ice edge), and post-bloom (open water) conditions.
Materials and methods
Study area
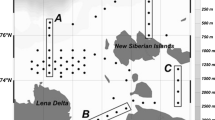
Field work was done in the Weddell Sea vic. 60°S 36°W aboard the R/V Polar Duke in late November and December 1993. To assess the location and extent of the spring bloom, two transects were run within the MIZ while continuously monitoring chlorophyll biomass as in vivo fluorescence (Fig. 1). The first was a west to east transect along the ice edge to examine the variability within the core of the bloom. The second was a north to south transect to delineate the latitudinal extent of the bloom. The north–south transect was initiated far enough north of the ice edge to be seaward of the bloom, as indicated by in-vivo fluorescence values well below those recorded in the bloom. From its most northerly point in open water, the transect proceeded through the core of the bloom at the ice edge into the pack ice until the fluorescence signal from the bloom was absent at the southern end. At that point, 5–6 day occupations were conducted in each of the three MIZ areas: pack ice (pre-bloom), ice edge (bloom), and open water (post-bloom). Day length during the south–north sampling transect ranged from 17 to 19 h with 90% of available sunlight occurring generally between 0500 and 2000 hours. The biological and physical framework of each MIZ area was provided by CTD/rosette casts, primary productivity measurements, and chlorophyll- a biomass determinations. Primary production methodology followed Cota et al (1992) and Smith and Nelson (1990); chlorophyll biomass methodology followed Holm-Hansen and Rieman (1978).
Cruise track in NW Weddell Sea. (filled circle) Pack ice stations, (filled triangle) ice edge stations, (filled square) open water stations, (open diamond) transitional stations. Dashed line represents approximate position of ice edge at the beginning and end of the cruise. Dashed boxes show trawling locations during multi-day occupations within each MIZ area
Collection of specimens
Sampling was done by oblique tows in the upper 1,000 m of the water column using two different-sized Tucker trawls, one with a 2.3 m2 mouth area, the second with a 9.3 m2 mouth area. Both trawls had a 4 mm mesh main net tapering to a 1 mm mesh meter net. Each net terminated in a bucket-type cod-end with a 1 mm mesh liner. Trawls were towed at an average speed of 2 knots. While fishing, trawling depth was estimated by wire angle triangulation and continuously recorded with a time-depth recorder mounted on the trawl frame. The volume of water filtered was estimated using tow speed, effective mouth area, and distance covered. Effective mouth area was calculated assuming a 35° net angle. Distance covered was calculated from duration of tow, ship’s distance traveled, and depth of tow. A 0.5 m (2.3 m2 trawl) or 0.75 m (9.3 m2 trawl), 163 μm-mesh plankton net was nested within the mouth of the trawl. Micronekton specimens captured in the plankton net were included in trawl catch summaries. Tows in the pack ice were conducted in leads created by the ship’s wake. Ice cover within the pack ice area of the study was substantial, usually 8/10 cover or greater. However, owing to the time of year, the ice was fairly soft and did not impede the ship’s forward way. Trawls conducted within the ice had a speed of 2 knots. Trawls were executed with the A-frame in, to bring the wire closer to the back of the vessel and minimize the chance of hooking the wire on ice floes.
Forty-five successful oblique tows were conducted in the three MIZ areas: 14 in the pack ice, 20 at the ice edge, and 11 in open water (Table 1). Five ice-edge tows and three open water tows occurred during daylight hours.
Sample analysis
All trawl catches were preserved in a buffered 5–10% formaldehyde solution, shipped to the laboratory for analysis, and then stored in 50% isopropanol. For some tows, subsampling of the catch prior to preservation was required due to the large salp (Salpa thompsoni) biomass. Salp numbers were estimated volumetrically assuming 1 l∼500 individuals ∼40 g dry mass (gDM). All fish, megaplankton, and large micronekton specimens (e.g., decapods, scyphomedusae) were removed from the trawl catch prior to subsampling. Abundance and biomass totals for species not sorted prior to subsampling were corrected to reflect total catch volume. Sorted micronekton and macrozooplankton were identified to the lowest possible taxon, enumerated, measured [(mm standard length (SL)], and weighed. Chaetognaths were considered collectively. Previous studies indicated that the principally occurring species were Sagitta gazellae,S. marri, and Eukrohnia hamata (Hopkins and Torres 1988; Lancraft et al. 1989, 1991; Siegel et al. 1992).
Amphipod SL was measured from the front of the head to the tip of the telson. For euphausiids and the decapod shrimp Gennadas kempi, SL was measured from the tip of the rostrum to the tip of the telson. For the decapods Nematocarcinus lanceopes and Pasiphaea scotiae, SL was measured from the middle of the eye to the tip of the telson. Fish SL was measured from the front of the head to the base of the caudal fin. Sphere diameter in the hydromedusa Calycopsis borchgrevinki was measured parallel to the suture axis.
For C. borchgrevinki and S. thompsoni, dry mass (mgDM) was calculated from length–weight regressions generated from the present data set; for the chaetognaths, dry mass was either measured directly or calculated from a previously generated regression. Wet mass (mgWM) for C. borchgrevinki,S. thompsoni, and chaetognaths was estimated by multiplying dry mass values by 20 (=95% water level; Donnelly et al. 1994; Huntley et al. 1989; Lancraft et al. 1989, 1991). Biomass (mgWM, mgDM) for all the other species was determined from regressions compiled from multiple Antarctic data sets (J. Donnelly and J. J. Torres, unpublished data).
Integrated abundance (# m−2) was calculated for each species by dividing their number in the catch by the water volume filtered for the tow and multiplying that quotient by the depth range of the tow. Total integrated abundance within the 0–200 m and 0–1,000 m layers was determined by averaging calculated values for all individual tows within each depth layer. Tows in which a species was not caught were included in the average as zeroes. Integrated biomass (mgWM m−2, mgDM m−2) values were determined using an identical protocol.
Mean integrated abundance and biomass values from the different MIZ areas were compared using one-way analysis of variance (ANOVA) or, when variances were not homogeneous, a Kruskal–Wallis (KW) non-parametric test (Zar 1974). The level of significance for all statistical tests was P≤ 0.05. Potential temperature versus salinity (T–S) plots were generated using Ocean Data View software (Schlitzer 2005).
Results
Hydrographic setting
Three water column layers can be discerned from representative CTD profiles taken during the south–north trawling transect (Fig. 2). A surface layer roughly 40 m thick overlaid a colder, previous-winter water layer. The winter water layer was centered at 75 m and extended from 40 to 200 m. Moving from open water southward, the upper boundary of the cold winter water layer became progressively shallower, reaching the surface under the pack ice. A third layer of warm deep water extended from below 200 m to the maximum depths sampled. Salinity was reduced in the surface layer at the ice edge stations in conjunction with the local melt-water lens.
In this region, Weddell Sea waters are separated from the warmer Scotia Sea by the Weddell Scotia Confluence (WSC), a transitional area that extends eastward from the Antarctic Peninsula to the South Sandwich Islands (Gordon 1967). Characteristic WSC hydrography attenuates quickly east of the South Orkney Plateau and by 40°W, the WSC is a diffuse transitional boundary containing meso-scale eddies (Foster and Middleton 1984; Paterson and Sievers 1980). Foster and Middleton (1984) used the −0.8°C isotherm at 50 dbar to mark the northern boundary of Weddell Sea water while Mountain and Huber (1985) used the salinity of the deep −1°C isotherm as an index for distinguishing WSC water (salinity at −1°C <34.35‰). Applying these same criteria in the present study, hydrographic data (Fig. 2, 3) indicate that our pack ice and ice edge sampling areas were in Weddell Sea water, and our open water sampling area was in the southern portion of the WSC.
Bloom characterization
Measurements of chlorophyll- a concentration and carbon production indicate that the study area conformed to the classical model of primary productivity following a receding ice edge (Sullivan et al. 1988). During the initial north–south transect, maximum chlorophyll concentration (2.8 μg l−1, Fig. 4a) coincident with a melt-water lens (σt≤27.3, Fig. 4b) was detected in association with the ice edge between 59°S and 60°S. At the southern end of the rapid N–S transect (Station 23, 62°S), chlorophyll was low (0.4 μg l−1, integrated 0–40 m) and primary production was low relatively to the ice edge. During the occupation in the pack ice, net primary production (integrated 0–40 m) increased from 250 mg C m−2 day−1 to 1,000 mg C m−2 day−1 and chlorophyll biomass increased, characteristic of a developing bloom. Continuing south to Station 38 at 63°S, chlorophyll was again low (0.4 μg l−1) and primary production was 500 mg C m−2 day−1. We then headed north to the multi-day occupation within the core of the bloom, which was now displaced roughly 150 km southward with the receding ice edge (61.2°S, Fig. 1). Mean chlorophyll concentration at the core of the bloom (Stations 42–55) was 3.5 μg l−1 with primary production around 1,000 mg C m−2 day−1. Mean chlorophyll concentration in the open water (Stations 61–67) was 1.7 μg l−1 ; primary production was also intermediate, ranging from 500 to 750 mg C m−2 day−1.
Abundance and biomass
Mean integrated abundance and biomass values for 29 species within the three MIZ areas are shown in Table 2 (0–200 m) and Table 3 (0–1,000 m). The euphausiid Thysanoessa macrura and chaetognaths were most ubiquitous, being caught in every tow, followed by Euphausia superba, which occurred in all but two tows in the pack ice area. Eight of the 29 species examined were not collected in particular MIZ areas. The hyperiid amphipod Vibilia stebbingi and the ostracod Gigantocypris mulleri were not captured within the pack ice. The hyperiid amphipod Themisto gaudichaudii, the euphausiid E. triacantha, and the fish Cyclothone kobayashii, Gymnoscopelus nicholsi, and Protomyctophum bolini only occurred in the open water area.
Species that did not occur in 0–200 m tows in any MIZ area included the gammarid amphipod Cyphocaris richardi, the decapods G. kempi and P. scotiae, the euphausiid Euphausia triacantha, the fishes B. antarcticus, C. kobayashii, and P. bolini, the scyphomedusa Periphylla periphylla, and the giant ostracod G. mulleri. The fishes Cyclothone microdon (open water) and Gymnoscopelus opisthopterus (ice edge) were only represented in shallow layer tows by one small individual each.
Catch densities
Catch densities were considerably reduced in the three shallow daytime tows (1 ice edge, 2 open water), particularly for S. thompsoni, E. superba, Thysanoessa macrura, and E. antarctica. No differences in specimen abundance or catch assemblage were evident in the five deep daytime tows (4 ice edge, 1 open water). Reduced catches during the day in epipelagic waters can result from diel changes in vertical distribution or an increased level of net avoidance. To avoid potential bias, daytime tows shallower than 500 m were not included in abundance and biomass totals (cf. Lancraft et al. 1989).
Catch densities were further examined for the euphausiids E. superba and T. macrura as these species can form large aggregations which may result in very high variability between samples. Maximum catch densities were 0.059 individuals m−3 for E. superba (station 25, tow 3) and 0.026 individuals m−3 for T. macrura (station 65, tow 38). Ratios of median to mean catch densities (individuals m−3) for both species were close to unity (0.6–1.1), suggesting a relatively uniform sampling success. The only exception to this trend occurred with E. superba in the pack ice where a single, disproportionately large catch resulted in a median/mean ratio value of 0.05 for the 0–1,000 m layer. Mean catch densities for all the three MIZ areas ranged from 0.001–0.017 individuals m−3 for E. superba and from 0.004–0.017 individuals m−3 for T. macrura.
Comparisons between the MIZ areas
Integrated abundance and biomass values for the 24 species present in two or more MIZ areas were compared. Only those species exhibiting changes are shown (Table 4). Among crustaceans, Cyllopus lucasii and V. stebbingi were significantly more abundant in the open water. However, biomass was significantly higher only for V. stebbingi. In contrast, the gammarid amphipod Eusirus antarcticus and larval N. lanceopes were more abundant in the pack ice. E. superba showed a very significant (P<0.01) increase in both abundance and biomass moving from pack ice to ice edge to open water only in the 0–200 m layer. Over the 0–1,000 m layer, the trend was similar but differences were not significant primarily due to increased variability in the pack ice area.
Among the fishes, B. antarcticus showed a trend of increased abundance and biomass at the ice edge. C. microdon, E. antarctica, G. braueri and G. opisthopterus all had conspicuously lower occurrences in the pack ice area. None of the four species were collected in tows shallower than 200 m, although seven of the 35 E. antarctica caught under the ice came from a 0–290 m tow. Only two C. microdon, one G. braueri,and one G. opisthopterus were caught in deep tows under the ice, resulting in significantly lower integrated abundance and biomass for all three species in the pack ice area compared to the ice edge or open water. E. antarctica showed a marginally significant (P=0.052) trend of lower abundance under the ice but no significant difference in biomass. Neither C. microdon nor the three myctophid species showed any significant changes in abundance or biomass between the ice edge and open water. Notolepis coatsi was the only fish species to have higher abundance and biomass in the 0–200 m layer in the pack ice area. Over the 0–1,000 m layer, higher abundance in the pack ice was marginally non-significant (P=0.053) and differences in biomass were not significant.
Among gelatinous species, C. borchgrevinki showed a marginally non-significant (P=0.058) trend of increasing abundance from pack ice to edge to open water in the 0–200 m layer, but no change in biomass. For S. thompsoni, both abundance and biomass were significantly lower in the pack ice than at the ice edge and in open water. This pattern was also exhibited in the 0–200 m layer but differences were not significant due to the high variability in catches from open water. For the polychaete Tomopteris carpenteri, both abundance and biomass were significantly higher in the open water.
Size frequencies of Cyllopus lucasii, V. stebbingi, E. superba, T. macrura, E. antarctica, and C. borchgrevinki were tabulated for the different MIZ areas (Table 5). Mean and median SL of C. lucasii were significantly lower in the open water in both the 0–200 and 0–1,000 m layers. For E. superba in the 0–200 m layer, mean and median SL were significantly higher at the ice edge than in the pack ice or open water. In the 0–1,000 m layer, mean and median values showed a significant increase moving from pack ice to ice edge to open water. In both depth layers, C. borchgrevinki mean and median sphere diameter decreased significantly from pack ice to ice edge to open water. No significant changes in size frequency of V. stebbingi, T. macrura, and E. antarctica were observed.
Discussion
Hydrographic considerations
The marginally recognizable hydrographic signature of WSC water and the consistently low chlorophyll levels at the open-water stations at both the beginning and end of the cruise suggest that the local phytoplankton bloom was not the result of allochthonous input from warmer water to the north. Furthermore, the presence of Themisto gaudichaudii, E. triacantha, C. kobayashii, G. nicholsi, and P. bolini at the open water stations was not unexpected and does not reflect a significant water mass change. These species all occur south of the WSC in Weddell Sea water even though their maximum distributions are centered around the Polar Front region (Gon and Heemstra 1990; Kirkwood 1982; McGinnis 1982; Miya 1994; Nemoto and Yoo 1970). More telling from an assemblage point of view was the complete absence of E. carlsbergi, G. bolini, and Krefftichthys anderssoni, common warm water species that do not occur in Weddell Sea water (Gon and Heemstra 1990; McGinnis 1982).
Since both the physical and biological characteristics of the study area indicated a predominantly Weddell Sea water influence, we considered any observed differences in the species’ abundance among the MIZ areas to be primarily related to the dynamics of the ice-edge bloom and not due to sampling different water masses.
General abundance and biomass
The micronekton/macrozooplankton assemblage examined in this study is a typical Antarctic pelagic community (Lancraft et al. 1989, 1991). Total integrated abundance and biomass values over the upper 200 m in the open water are similar to those reported by Lancraft et al. (1989) for the spring 1983 AMERIEZ cruise (∼20 individuals m−2, ∼1,000 mgDM m−2). Over the 0–1,000 m layer, however, their numbers were much higher primarily due to considerably larger catches of S. thompsoni. Piatkowski et al. (1994) also found a predominance of salps in the open waters of the Scotia Sea, as well as a micronekton assemblage exhibiting a greater contribution from sub-Antarctic species.
Abundance and biomass totals for mesopelagic fishes in the open water area are also similar to that reported by Lancraft et al. (1989). High percentage contributions from B. antarcticus, E. antarctica, G. braueri and G. opisthopterus, especially in the 200–1,000 m layer, support their statement on the importance of this faunal component to the total pelagic biomass.
Our values from the pack ice area for the 0–1,000 m layer are considerably lower than those reported by Lancraft et al. (1991) for the ice-covered Scotia Sea in winter. Most notably, their samples contained larger collections of E. superba, mesopelagic fishes, and coelenterates. However, this difference could simply reflect a greater sampling effort under the ice as well as an influence of the WSC. The low fish abundances in shallow net tows from the present study as well as in those conducted by Kaufmann et al. (1995) support the finding of Lancraft et al.(1991) that peak abundances for mesopelagic fishes under the ice are well below the near-freezing surface water layer. The primary exception was juveniles of N. coatsi, which are common in shallow under-ice tows. Data from Kaufmann et al. (1995) also suggested that non-juvenile specimens of E. antarctica and G. braueri may be underestimated in shallow net tows under the ice. These species were the proposed sources for large acoustic targets recorded shallower than 100 m.
The numerical importance of T. macrura and chaetognaths under the pack ice was not unexpected and has been reported in previous studies (Fisher et al. 2004; Kaufmann et al. 1995; Siegel et al. 1992; Stepien 1982). E. superba is most prevalent in the upper 100 m while T. macrura and chaetognaths have deeper peak abundances (Hopkins and Torres 1988; Lancraft et al. 1989, 1991; Marr 1962). Several studies focusing on the upper 50 or 100 m water column in the northern Weddell Sea during the spring and summer have reported E. superba to be the dominant euphausiid in the under-ice assemblage (Daly and Macaulay 1988; Kaufmann et al. 1995; Siegel et al. 1992; Sprong and Schalk 1992). In a winter study integrating the 0–200 m water column under the ice, Lancraft et al. (1991) also found E. superba to be exceedingly dominant. In contrast, a net-based study integrating the upper 200 and 500 m layers in the Lazarev Sea during late winter–early spring (Stepien 1982) found T. macrura to be the most abundant euphausiid under the ice. A more recent net-based study (Fisher et al. 2004) in the northwest Weddell Sea during September–October that sampled much shallower (0–50 and 50–100 m) also found T. macrura to be more abundant than E. superba under the ice.
The patchy nature of the pelagic environment complicates the study of distribution and abundance patterns, but it is of particular importance for understanding the distribution of species such as E. superba that form large, dense swarms. T. macrura is more uniformly distributed but concentrated aggregations have also been reported for this species (Daly and Macaulay 1988). Daly and Macaulay (1988) also noted that large fluctuations in krill density (up to 45-fold) can occur over a short-time period (30–60 min). The low maximum and mean catch densities encountered in the present study for both E. superba and T. macrura were indicative of “background” or dispersed abundance levels (Daly and Macaulay 1988; Nast 1982), rather than those expected within a swarm.
Distribution across the marginal ice zone
Of the 24 species examined for distributional patterns across the MIZ, 12 exhibited differences in abundance and/or biomass between one or more areas. Patterns for ten of the 12 species can be related either directly or indirectly to the dynamics of the ice edge and the spring bloom. E. superba and S. thompsoni, the two most abundant species at the ice edge and in the open water, have direct trophic connections to the bloom, with each responding to elevated phytoplankton production via different means. The increased occurrence of E. superba from pack ice to open water reflects their seasonal transition from an under-ice habitat in the winter to one of open water in the spring/summer (Daly and Macaulay 1988, 1991; Macintosh 1972; Siegel 1988; Sprong and Schalk 1992). Observed changes in size class between the different MIZ areas are also consistent with the scenario of a maturing E. superba population moving from under the ice to take advantage of an increasing pelagic food supply (Daly and Macaulay 1991; Sprong and Schalk 1992). In contrast, large numbers of S. thompsoni at the ice edge and in the open water are not likely to be the result of horizontal redistribution so much as a rapid population increase through asexual reproduction. Salps are highly efficient particle feeders that are able to quickly respond to favorable changes in food supply (Perissinotto and Pakhomov 1998). The near absence of S. thompsoni under the ice is consistent with findings from previous studies (Fisher et al. 2004; Siegel et al. 1992) and is a consequence of not only the reduced food supply but lower water temperatures as well (Foxton 1966; Mackintosh 1934; Piatkowski 1985).
Tomopteris carpenteri is linked trophically to the ice-edge bloom as it feeds on phytoplankton, either directly or secondarily through the ingestion of S. thompsoni (Hopkins 1985). Piatkowski (1989), however, found that T. carpenteri occurrence was notably reduced in cold Weddell Sea water so hydrographic conditions under the ice may be a factor in the distribution of this species. Diet analysis has not been reported for the hydromedusa C. borchgrevinki. However, this species is likely to be a small-zooplankton grazer and as such, would benefit from higher prey biomass seaward of the ice edge. The higher number of small C. borchgrevinki individuals in the epipelagic layer of the open water relative to the ice edge and pack ice areas suggests distributional response to improved trophic conditions.
The observed trends in V. stebbingi distribution were directly related to S. thompsoni abundance, the close association of Vibilia spp. with salps being well documented (Harbison et al. 1977; Madin and Harbison 1977; Vinogradov 1966). The distribution pattern of C. lucasii is also most likely to be linked to salp abundance, even though a direct association between C. lucasii and a particular gelatinous host has not been reported as yet. C. lucasii may have a lifestyle similar to that of T. gaudichaudii: generally free-living as an adult but associated with gelatinous hosts as a juvenile (Sheader and Evans 1975; Madin and Harbison 1977). The higher number of small C. lucasii individuals in the open water where salp densities were highest is consistent with this scenario. A larger percentage of small individuals in the open water also explains the observed lack of significant difference in C. lucasii biomass with its increased abundance.
The fact that T. macrura did not increase in abundance at the ice edge or in the open water suggests that its life history is not tied to the spring bloom in the same way that E. superba’s is. T. macrura has a widespread and uniform distribution (Brinton 1985; Nordhausen 1994) and is omnivorous throughout the year (Hopkins 1985, 1987, Hopkins and Torres 1989; Mayzaud et al. 1985). Consequently, T. macrura is not as energetically dependent on the seasonal phytoplankton bloom as is E. superba. The absence of discernible trends in distribution for the amphipods C. richardi and P. macropa, the decapods G. kempi and P. scotiae, the scyphomedusae Atolla wyvilli and P. periphylla, the squid G. glacialis, and the chaetognaths also implies a trophic independence from the bloom. The majority of these species are deeper living carnivores and are less common members of the mesopelagic assemblage.
The higher occurrences of E. antarcticus and N. lanceopes in the pack ice area were a consequence of habitat preference and were not likely to be related to the ice-edge bloom. E. antarcticus is cryopelagic and is commonly collected by scuba divers from interstitial spaces beneath the pack ice where it feeds on euphausiid larvae (Hopkins and Torres 1988, 1989). Larval N. lanceopes occur primarily in epipelagic waters (Lancraft et al. 1989) and feed on phytoplankton and protozoans (Hopkins and Torres 1989), likely utilizing the underside of the pack ice as both a refuge and a more concentrated source of food, an association similar to that seen in larval krill (Daly 1990; Daly and Macaulay 1991; Bergström et al. 1990). The higher number of N. coatsi in the pack ice area may also reflect a greater affinity to the under-ice environment during larval and early juvenile periods. However, more data on specimen length and depth of capture is needed. Catch records from previous studies show N. coatsi to have a wide depth distribution (Lancraft et al. 1989, 1991; Piatkowski et al. 1994; White and Piatkowski 1993) but data on specimen lengths are lacking. In the present study, N. coatsi specimens ranged from 10 to 107 mm SL with smaller individuals more prevalent in shallow tows (median SL [0–200 m tows] ∼25–30 mm; [0–1,000 m tows] ∼35–50 mm).
The reduced occurrences of B. antarcticus, C. microdon, E. antarctica, G. braueri and G. opisthopterus under the ice imply distributional responses to changing conditions within the marginal ice zone. All five species are metazoan plankivores, with B. antarcticus and C. microdon primarily taking small-sized copepods (e.g., Metridia, cyclopoids). The myctophid species also feed heavily on krill (Hopkins 1985; Hopkins and Torres 1989; Pusch et al. 2004). Data on zooplankton collected during the same time as this micronekton study indicated that abundance of the dominant species was highest in the open water with early copepodite stages dominating in all stations seaward of the pack ice (Burghart 1999). Together with the greater krill abundance observed in our data set, it means that feeding conditions for mesopelagic fishes would be clearly better out from under the ice. Four of the five fish species vertically migrate (Lancraft et al. 1989; Torres and Somero 1988) and as a consequence, would encounter maximal prey densities in the epipelagic waters seaward of the ice. Furthermore, the fact that C. microdon, a non-migrating species, was also less abundant under the ice suggests that trophic repercussions of the ice-edge bloom can extend well below the epipelagic layer.
References
Ainley DG, Fraser WR, Sullivan CW, Torres JJ, Hopkins TL, Smith WO Jr (1986) Antarctic mesopelagic micronekton: evidence from seabirds that pack ice affects community structure. Science 232:847–849
Ainley DG, Fraser WR, Smith WO Jr, Hopkins TL, Torres JJ (1991) The structure of upper level pelagic food webs in the Antarctic: Effect of phytoplankton distribution. J Mar Sys 2:111–122
Atkinson A, Shreeve RS (1995) Response of the copepod community to a spring bloom in the Bellingshausen Sea. Deep Sea Res II 42:1291–1311
Burghart SE, Hopkins TL, Vargo GA, Torres JJ, (1999) Effects of a rapidly receding ice edge on the abundance, age structure, and feeding of three dominant calanoid copepods in the Weddell Sea, Antarctica. Polar Biol 22:279–288
Brierley AS, Fernandez PG, Brandon MA, Armstrong F, Millard NW, McPhail SD, Stevenson P, Pebody M, Perrett J, Squires M, Bone DG, Griffiths G (2002) Antarctic krill under the sea ice: elevated abundance in a narrow band just south of the ice edge. Science 295:1890–1892
Brinton E (1985) The oceanographic structure of the eastern Scotia Sea—III. Distributions of euphausiid species and their developmental stages in 1981 in relation to hydrography. Deep Sea Res 32:1153–1180
Cota GF, Smith WO Jr, Nelson DM, Muench RD, Gordon LI (1992) Nutrient and biogenic particulate distributions, primary productivity and nitrogen uptake in the Weddell-Scotia Sea marginal ice zone during winter. J Mar Res 50:155–181
Daly KD, Macaulay MC (1988) Abundance and distribution of krill in the ice edge zone of the Weddell Sea, austral spring 1983. Deep Sea Res 35:21–41
Daly KD, Macaulay MC (1991) Influence of physical and biological mesoscale dynamics on the seasonal distribution and behavior of Euphausia superba in the Antarctic marginal ice zone. Mar Ecol Prog Ser 79:37–66
Donnelly J, Kawall H, Geiger SP, Torres JJ (2004) Metabolism of Antarctic micronektonic crustacea across a summer ice-edge bloom: respiration, composition, and enzymatic activity. Deep Sea Res II 51:2225–2245
Donnelly J, Torres JJ, Hopkins TL, Lancraft TM (1994) Chemical composition of Antarctic zooplankton during austral fall and winter. Polar Biol 14:171–183
Eicken H (1992) The role of sea ice in structuring Antarctic ecosystems. Polar Biol 12:3–13
Foster TD, Middleton JH (1984) The oceanographic structure of the eastern Scotia Sea, I, Physical Oceanography. Deep Sea Res 31:529–550
Fisher EC, Kaufmann RS, Smith KL Jr (2004) Variability of epipelagic macrozooplankton/micronekton community structure in the NW Weddell Sea, Antarctica (1995–1996). Mar Biol 144:345–360
Foxton P (1966) The distribution and life-history of Salpa thompsoni Foxton with observations on a related species, Salpa gerlachei Foxton. Discov Rep 34:1–116
Garrison DL, Buck KR (1989) Protozoolankton in the Weddell Sea, Antarctica: abundance and distribution in the ice edge zone. Polar Biol 9:341–351
Geiger SP, Kawall HG, Torres JJ (2001) The effect of the receding ice edge on the condition of copepods in the northwestern Weddell Sea: results from biochemical assays. Hydrobiologia 453/453:79–90
Godlewska M (1993) Acoustic observations of krill (Euphausia superba) at the ice edge (between Elephant I. and South Orkney I., Dec.1988/Jan.1989). Polar Biol 13:507–514
Gon O, Heemstra PC (eds) (1990) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, South Africa, 462 pp
Gordon AL (1967) Structure of Antarctic waters between 20°W and 170°W. Am Geogr Soc, Antarctic Map Folio Ser 6:1–10, pls 1–14
Harbison GR, Biggs DC, Madin LP (1977) The associations of Amphipoda Hyperiidea with gelatinous zooplankton- II. Associations with Cnidaria, Ctenophora and Radiolaria. Deep Sea Res 24:465–488
Holm-Hansen O, Riemann B (1978) Chlorophyll-a determination: Improvements in methodology. Oikos 30:438–447
Hopkins TL (1985) Food web of an Antarctic midwater ecosystem. Mar Biol 89:197–212
Hopkins TL (1987) Midwater food web in McMurdo Sound, Ross Sea, Antarctica. Mar Biol 96:93–106
Hopkins TL, Torres JJ (1988) The zooplankton community in the vicinity of the ice edge, western Weddell Sea, March 1986. Polar Biol 9:79–87
Hopkins TL, Torres JJ (1989) Midwater food web in the vicinity of a marginal ice zone in the western Weddell Sea. Deep Sea Res 36:543–560
Huntley ME, Sykes PF, Marin V (1989) Biometry and trophodynamics of Salpa thompsoni Foxton (Tunicata: Thaliacea) near the Antarctic Peninsula in austral summer, 1983–1984. Polar Biol 10:59–70
Kaufmann RS, Smith Jr RL, Baldwin RJ, Glatts RC, Robison BH, Reisenbichler KR (1995) Effects of seasonal pack ice on the distribution of macrozooplankton and micronekton in the northwestern Weddell Sea. Mar Biol 124:387–397
Kawall HG, Torres JJ, Geiger SP (2001) Effects of the ice-edge bloom on the metabolism of copepods in the Weddell Sea, Antarctica. Hydrobiologia 453/454:67–77
Kirkwood JM (1982) A guide to the Euphausiacea of the Southern Ocean. Aust Nat Antarct Res Exped, ANARE Res Notes, 44 pp
Lancraft TM, Torres JJ, Hopkins TL (1989) Micronekton and macrozooplankton in the open waters near Antarctic ice edge zones (AMERIEZ 1983 and 1986). Polar Biol 9:225–233
Lancraft TM, Hopkins TL, Torres JJ, Donnelly J (1991) Oceanic micronektonic/ macrozooplanktonic community structure and feeding in ice covered Antarctic waters during the winter (AMERIEZ 1988). Polar Biol 11:157–167
Laws RM (1985) The ecology of the Southern Ocean. Am Sci 73:26–40
Macintosh NA (1972) Life cycle of Antarctic krill in relation to ice and water conditions. Discov Rep 36:1–94
Madin LP, Harbison GR (1977) The associations of Amphipoda Hyperiidae with gelatinous zooplankton. I. Associations with Salpidae. Deep Sea Res 24:449–463
Marr JWS (1962) The natural history and geography of the Antarctic krill (Euphausia superba Dana). Discov Rep 32:33–464
Mayzaud P, Färber Lorda J, Corre MC (1985) Aspects of the nutritional metabolism of two Antarctic euphausiids, Euphausia superba and Thysanoessa macrura. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic Nutrient Cycles and Food Webs (Proceedings of the 4th SCAR symposium on antarctic biology). Springer, Berlin Heidelberg New York, pp 330–338
McGinnis RF (1982) Biogeography of lanternfishes (Myctophidae) south of 30°S. In: Pawson DL (ed) Antarctic research series, vol 35. Biology of the Antarctic Seas XII. American Geophysical Union, Washington DC, 110 pp
Miya M (1994) Cyclothone kobayashii, a new gonostomatid fish (Teleostei: Stomiiformes) from the Southern Ocean, with notes on its ecology. Copeia 1994:191–204
Mountain DG, Huber BA (1985) Water properties and chlorophyll distribution near the Scotia Sea ice edge, November 1983 (abstract). Eos Trans AGU 66:1270
Nast F (1982) The assessment of krill (Euphausia superba Dana) biomass from a net sampling programme. Meeresforschung 29:154–165
Nemoto T, Yoo KI (1970) An amphipod, (Parathemisto gaudichaudii) as food of the Antarctic Sei whale. Sci Rep Whales Res Inst 22:153–158
Nordhausen W (1994) Distribution and diel vertical migration of the euphausiid Thysanoessa macrura in Gerlache Strait, Antarctica. Polar Biol 14:219–229
Pakhomov EA, Perissinotto R, Froneman PW (1999) Predation impact of carnivorous macrozooplankton and micronekton in the Atlantic sector of the Southern Ocean. J Mar Syt 19:47–64
Paterson SL, Sievers HA (1980) The Weddell-Scotia confluence. J Phys Oceanogr 10:1584–1610
Perissinotto R, Pakhomov EA (1998) Contribution of salps to carbon flux of marginal ice zone of the Lazarev Sea, southern ocean. Mar Biol 131:25–32
Piatkowski U (1985) Distribution, abundance and diurnal migration of macro-zooplankton in Antarctic surface waters. Meeresforsch 30:264–279
Piatkowski U, Rodhouse PG, White MG, Bone DG, Symon C (1994) Nekton community of the Scotia Sea as sampled by the RMT 25 during austral summer. Mar Ecol Prog Ser 112:13–28
Pusch C, Hulley PA, Kock K-H (2004) Community structure and feeding ecology of mesopelagic fishes in the slope waters of King George Island (South Shetland Islands, Antarctica). Deep Sea Res I 51:1685–1708
Robins DB, Harris RP, Bedo AW, Fernandez E, Fileman TW, Harbour DS, Head RN (1995) The relationship between suspended particulate material, phytoplankton and zooplankton during the retreat of the marginal ice zone in the Bellingshausen Sea. Deep Sea Res II 42:1137–1158
Schalk PH (1990) Biological activity in the Antarctic zooplankton community. Polar Biol 10:405–411
Schlitzer R (2005) Ocean Data View. http://www.awi-bremerhaven.de/GEO/ODV
Sheader M, Evans F (1975) Feeding and gut structure of Parathemisto gaudichaudii (Geurin) (Amphipoda, Hyperiidea). J Mar Biol Assoc UK 55:641–656
Siegel V (1988) A concept of seasonal variation of krill (Euphausia superba) distribution and abundance west of the Antarctic Peninsula. In: Sahrage D (ed) Antarctic ocean and resources variability. Springer, Berlin Heidelberg New York, pp 219–230
Siegel V, Skibowski A, Harm U (1992) Community structure of the epipelagic zooplankton community under the sea-ice of the northern Weddell Sea. Polar Biol 12:15–24
Smetacek V, Scharek R, Nöthig E-M (1990) Seasonal and regional variation in the pelagial and its relationship to the life history cycle of krill. In: Kerry KR, Hempel G (eds) Antarctic ecosystems. Ecological change and conservation. Springer, Berlin Heidelberg New York, pp 103–114
Smith WO Jr, Nelson DM (1985) Phytoplankton bloom produced by a receding ice edge in the Ross Sea: spatial coherence with the density field. Science 227:163–166
Smith WO Jr, Nelson DM (1990) Phytoplankton growth and new production in the Weddell Sea marginal ice zone in the austral spring and summer. Limnol Oceanogr 35:809–821
Smith WO Jr, Sakshaug E (1990) Polar phytoplankton. In: Smith WO Jr (ed) Polar oceanography, part B: chemistry, biology, and geology. Academic, San Diego, pp 477–525
Sprong I, Schalk PH (1992) Acoustic observations on krill spring-summer migration and patchiness in the northern Weddell Sea. Polar Biol 12:261–268
Stepien JC (1982) Zooplankton in the Weddell Sea, October-November 1981. Antarct J US 17:109–111
Sullivan CW, McClain CR, Comiso JC, Smith WO Jr (1988) Phytoplankton standing crops within an Antarctic ice edge assessed by satellite remote sensing. J Geophys Res 93:12487–12498
Torres JJ, Lancraft TM, Weigle BL, Hopkins TL (1984) Distribution and abundance of fishes and salps in relation to the marginal ice zone of the Scotia Sea, November and December 1983. Antarct J US 19:117–119
Torres JJ, Somero GN (1988) Vertical distribution and metabolism in Antarctic mesopelagic fishes. Comp Biochem Physiol 90B:521–528
Vinogradov ME (1966) Hyperiid amphipods (Amphipoda, Hyperiidea) of the world oceans. Smithsonian Institution Libraries, Washington DC, 632 pp
White MG, Piatkowski U (1993) Abundance, horizontal and vertical distribution of fish in eastern Weddell Sea micronekton. Polar Biol 13:41–53
Zar JH (1974) Biostatistical analysis. Prentice Hall, Englewood Cliffs, 620 pp
Acknowledgements
We thank the captain and crew of the R/V Polar Duke for their expert field assistance. Thanks are also due to Renee Bishop, Scott Burghart, Liz Clarke, and Teresa Greely for their considerable help with sample collection and processing during the cruise. This study was supported by NSF OPP 9220493 and NSF OPP 9910100, both to J.J. Torres.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donnelly, J., Sutton, T.T. & Torres, J.J. Distribution and abundance of micronekton and macrozooplankton in the NW Weddell Sea: relation to a spring ice-edge bloom. Polar Biol 29, 280–293 (2006). https://doi.org/10.1007/s00300-005-0051-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-005-0051-z