Abstract
We examined the sub-ice algal community in the Chukchi Sea during June 1998 using a remotely operated vehicle (ROV). Ice algae were observed on the under-ice surface at all ten stations (from 70°29′N to 72°26′N; 162°00′W to 153°56′W) and varied in abundance and distribution from small aggregations limited to depressions in the ice to nets, curtains and strands of Melosira. There was no relationship between percent cover of sub-ice algae and physical factors at the kilometer scale, but at the scale of individual ice floes the percent cover of sub-ice algae was positively correlated with distance from the floe edge and negatively correlated with snow depth. A significant positive relationship between the concentration of sediment pigments and percent cover of sub-ice could indicate a coupling between ice algal and benthic systems. Pieces of ice algae that appeared to be Melosira were observed on the seafloor to a depth of over 100 m and cells or spores of obligate ice algal taxa were collected from sediments from 44-m to 1,000-m deep. The large biomass of sub-ice algae observed at many stations in the Chukchi Sea and the presence of ice algae on the seafloor indicates that the distribution and abundance of sub-ice algae needs to be understood if we are to evaluate the role of ice algae in the Arctic marine ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ice algae are an important component of arctic marine ecosystems. Recent studies indicate that ice algae comprise a significant portion of total primary productivity in some areas of the Arctic Ocean (Wheeler et al. 1996; Gosselin et al. 1997; Sakshaug 2004); ice algal production is estimated to average 10 gC m−2 year−1 throughout the Arctic (Legendre et al. 1992) and this may be an underestimate (Wheeler et al. 1996; Mock and Gradinger 1999), particularly for the central Arctic basin, where it may reach 50% of total primary productivity (Gosselin et al. 1997). This production contributes to pelagic and benthic food webs (Bradstreet and Cross 1982; Runge and Ingram 1988; Gulliksen and Lønne 1989; Legendre et al. 1992; Rysgaard et al. 2001; Hobson et al. 2002) and may also be important in global carbon cycling (Legendre et al. 1992; Yager et al. 1995; Pomeroy 1997; Gosselin et al. 1997). Yet, the distribution of ice algal communities has not been extensively mapped and the studies investigating the factors controlling their abundance and seasonal development have been limited in geographic extent (Legendre et al. 1992; Melnikov 1997). Furthermore, the contribution of ice algae to the structure and function of benthic communities is only beginning to be appreciated (Ambrose et al. 2001; Cooper et al. 2002).
The distribution and abundance of ice algae are highly variable (Gosselin et al. 1986; Cota and Smith 1991; Rysgaard et al. 2001), making it difficult to evaluate their role in marine ecosystems. It does appear, however, that on a large-scale ice algae are a relatively more important component of the Arctic marine ecosystem with increasing latitude (Sakshaug 2004). Small, meter-scale studies have related the distribution of algal biomass to snow depth and ice thickness (Alexander 1974; Gosselin et al. 1986; Welch and Bergmann 1989; Bergmann et al. 1991; Cota and Smith 1991; Welch et al. 1991). These results suggest that irradiance may be one of the most important environmental factors in determining ice algal abundance (Cota et al. 1991). Mesoscale studies (10s of meters to 10s of kilometers) have revealed that salinity (Gosselin et al. 1986; Legendre et al. 1996), nutrients (Maestrini et al. 1986; Cota and Smith 1991), rate of ice growth (Legendre et al. 1991) and sub-ice topography (Melnikov 1997) can be important determinants of ice algal abundance. Differences in under-ice grazer communities among areas (Gradinger and Bluhm 2004) might also contribute to mesoscale variability of ice algal abundance (Welch and Bergman 1989).
The abundance and species composition of ice algae vary throughout the ice column (Horner 1985; Syvertsen 1991; Horner et al. 1992; Melnikov 1997; von Quillfeldt et al. 2003) with the greatest accumulation of biomass usually (but not always, see Gradinger 1999) occurring in the bottom or sub-ice communities (Horner 1985; Melnikov 1997), particularly in spring (Cota and Horne 1989). While many methods have been used to sample sea ice for algae (see Horner 1990), the vast majority of studies deploy cores from the ice surface, which underestimates the abundance of ice algae in the sub-ice community (Welch et al. 1988; von Quillfeldt et al. 2003). More effective methods for sampling the under-ice algae, such as diver cores and suction pumps (Welch et al. 1988), are ineffective for determining mesoscale patterns of distribution because of the large number of cores necessary to determine patterns. Underwater cameras, remotely operated vehicles (ROVs), and autonomous underwater vehicles (AUVs) are being used with increasing frequency to investigate the under-ice community (Sasaki and Watanabe 1984; Brierley and Thomas 2002; Schnack-Schiel 2003; Gradinger and Bluhm 2004). They offer the capability of surveying large areas quickly and relating patterns of algae and animal abundance to sub-ice topography and, when equipped with sensors, a host of environmental parameters. Few studies in the Arctic, however, have used under ice images to document the distribution patterns of sub-ice algae and these studies have been limited to ROV images at a few stations in the Greenland Sea (Gutt 1995) and video images from a camera lowered through the ice at 12 stations in the Laptev Sea (Werner and Lindemann 1997).
We used an ROV equipped with a CTD to examine mesoscale patterns of abundance of sub-ice algae in the Northern Chukchi Sea in spring. These patterns were related to data on snow depth, ice thickness, and surface features collected simultaneously from the ice surface. Distribution and abundance patterns of sub-ice algae were related to environmental conditions within stations (10s meters) and among stations (10s kilometers).
We also used the ROV to survey the seafloor at locations where we surveyed the under ice surface. Ice algae may serve as an early-season carbon source for benthic communities (Alexander 1980; Cary 1985, 1987; Michel et al. 1997; Legendre et al. 1992; Ambrose et al. 2001), but the few studies, which have directly addressed the presence of ice algae in sediments present conflicting results (see Horner 1985 for review). Samples of sediment were examined for the concentration of algal pigments and for cells of common ice algal taxa, allowing us to relate the concentration of pigments and the species composition in the sediment to the relative amount and species composition of sub-surface ice algae.
Materials and methods
Study area
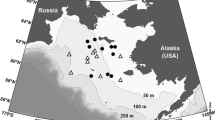
The Chukchi Sea is a shallow continental shelf area (average depth 50 m) off the northwest coast of Alaska (Fig. 1). The shelf of the Chukchi Sea is typically ice covered from November through June (Carey 1991). Water mass characteristics of the Chukchi Sea are complex (Johnson 1989; Grebmeier et al. 1995), with the southern Chukchi Sea influenced by three water masses: (1) nutrient rich Anadyr Water, which originates in the Bering Sea located on the west side of the shelf, (2) nutrient-poor Alaskan Coastal Water found along the Alaskan coasts and (3) Bering Sea Water located between these two water masses. A mixture of water types influenced by freshwater from ice melt and river runoff during the summer is found in the northern Chukchi Sea (Johnson 1989). Barrow Canyon, which begins west of Barrow Alaska and reaches a depth of 300 m before opening out onto the slope, marks the boundary between the Chukchi and Beaufort Seas.
Sampling
We used a Phantom 2 ROV to examine sub-ice algae at ten stations in the Chukchi Sea between 10 and 25 June 1998 (Fig. 1). The ROV was deployed from the United States Coast Guard Cutter Polar Sea and carried a SeaBird CTD that collected temperature and salinity data at a sample rate of once a second. Quantitative observations of ice algal cover were made with an upward looking color camera with the ROV operating approximately 1 m or less below the ice. At this distance, the resolution of the video camera was approximately 1 cm and the field of view was about 0.25 m2. The ROV was deployed for a minimum of 25 min at each station and surveyed up to 100 m from the ship.
Specific locations for deploying the ROV were selected on the basis of the ship’s ability to navigate a lead and remain stationary adjacent to an ice floe. At six stations, the ROV initially followed a transect of 2.5-cm square wooden stakes driven through the ice from the edge of an ice floe towards the floe’s center. Stakes were usually placed 5 m apart beginning 1 m from the ice edge. In some cases, stakes were placed closer together, approximately 2 m (poor water clarity, rapid change in surface features), or further apart, approximately 10 m (intervening ridge) than 5 m. Each stake protruded 1–2 m below the bottom of the ice and the end of the stake was painted with orange fluorescent paint and numbered. Snow depth (nearest 0.5 cm) and ice thickness (10 cm SIPRE ice core, nearest 0.5 cm) were measured at 5–10 locations adjacent to each stake. The locations of surface features (melt ponds, ridges) along the transect were also recorded. Transect lengths varied from approximately 20–85 m. At the four stations where a transect was not established (stations 4, 7, 8, 12), the ROV surveyed along a compass heading 90° to the ship. At all these stations except station 8, snow depth and ice thickness measurements were made from haphazard locations on the ice. We could not get off the ship at station 8 so ice thickness was determined from depth data collected by the ROV’s CTD when the ROV was in contact with the under-ice surface and correcting for the CTD’s location on the ROV (±.1 m) and snow depth was estimated.
At each station, one of the SIPRE ice cores was cut into 10 cm sections and melted slowly at temperatures just above freezing. The salinity (refractometer) and concentration of chlorophyll a (standard flurometric methods after extraction with 90% acetone; Yentsch and Mensel 1963) were determined for each section. A 50-ml sample of each section of the melted core was preserved in neutralized 4% formalin and examined for the relative abundance of algal taxa at five stations (stations 5, 7, 10, 11, 19). Here, we report only data from the bottom 10 cm of the core. For the purpose of this investigation only diatoms were counted, since they usually predominate in bottom communities. Diatoms are relatively resistant to osmotic shock and the method used (slowly melting) yields accurate cell numbers.
Sediment samples were collected from six stations (5, 7, 9, 10, 11, and 19) with a USNEL Mark III 0.25 m2 box core. Three replicate cores (9.5-cm inside diameter) were taken at least 5 cm from the edge of each box and divided into 1 cm sections, which were homogenized before subsampling. One 1 cm3 of homogonized sediment was analyzed immediately for chlorophyll a and phaeopigments with a fluorometer using standard methods (extraction with acetone, methanol and distilled water, 45:45:10). Only data from the top 1 cm are used in the comparisons described below.
Surface water samples were collected from 30-l Niskin bottles mounted on a rosette. Usually, 1 l of water was filtered through a GF/F filter, which was briefly frozen before the pigments were extracted with 90% acetone and concentrations of chlorophyll a and phaeopigments determined flurometrically (see Yager et al. 2001 for details and for additional data collected on the cruise). A 50 ml sample of water was preserved and analyzed for algal taxa as described above for ice.
Image and data analysis
The distribution and abundance of ice algae recorded on video were examined both qualitatively and quantitatively. Four different morphological types of sub-ice algae could be characterized and their distribution qualitatively compared within and among stations. Quantitative analysis consisted of determining the percent cover of sub-ice algae in 20 video images in the vicinity of each stake or, for stations without stakes, from at least 20 images collected a minimum of 20 m from the floe edge. Images for analysis were selected by haphazardly stopping the videotape. The image displayed was selected for analysis if it was from the color, upward viewing, camera and it was not over exposed. Images were saved as TIFF images using Flashpoint FPG 32 and analyzed for percent cover with Sigma Scan.
Water salinity usually showed little change along a transect and was only used for comparisons among stations. Salinity data were averaged from 60 measurements (1 min) taken from the middle of a transect.
Percent cover data and environmental parameters were analyzed on two spatial scales, among stations (10s kilometers) and within stations (10s meters). Measurements made at stakes were averaged across all stakes at a station for comparison among stations. Data from stations without stakes were also used in the among station comparison. Pearson’s correlation coefficient (Sokal and Rohlf 1969) was used to examine relationships among latitude, longitude, ice thickness, snow depth, water salinity, ice salinity, ice chlorophyll, sediment chlorophyll, sediment phaeopigments, total sediment pigments, and water chlorophyll, phaeopigments and total pigments. Following the correlation, the relationships between percent cover of algae and the concentration of total pigments in the sediment and water were examined with linear regressions. Percent coverage data were transformed using an arcsine transformation so they conformed to a normal distribution prior to correlation and regression analyses.
It was possible to make within station comparisons at stations where stakes were established. For these analyses, Pearson’s correlation coefficient was again used to examine the relationships among percent cover of sub-ice algae, distance from the floe edge, snow depth and ice thickness. The correlation analysis was followed by a multiple linear regression of snow depth, ice thickness, and distance from floe edge on percent cover of algae. These analyses were performed for all distances from the ice edge and only for locations 20 m and greater from the edge. Positioning the ship adjacent to a floe was sometimes difficult and we felt the disturbance from the ship, particularly its bow thrusters, might have disturbed loosely attached algae near the ice edge.
The ROV was also used to survey the seafloor at the same locations ice was examined except for at station 19, which was too deep for the ROV. For bottom surveys, the color camera was mounted pointing directly down and the ROV operated so that the camera was about 20 cm from the sediment surface. At this distance, the camera could resolve objects as small as 5 mm. Bottom dives were 1–2 h in duration, during which time the ROV moved at the speed of ship drift that varied between 1 knots and 4 knots.
Results
All of the stations were shallow, less than 50-m deep, except for station 13, which was slightly deeper at 104 m and station 19, which was at the mouth of Barrow Canyon and 1,061-m deep (Table 1, Fig. 1). The ice map (SSMI 85 GHz) for 10 and 11 June 1998 indicated 90–100% ice cover north of about 71°N, except immediately along the coast which was generally ice free. All stations were covered by at least 80% and most stations by 90% or more ice at the time of sampling. All of the ice encountered was first year ice.
We were able to identify four growth types of algae on the underside of the ice: strands/curtains, nets, patches, and film. Long strands and curtains were common at four stations (7, 8, 9, 10, Table 2). These were up to 3-m long and clearly were Melosira assemblages as described by Apollonio (1985), Gutt (1995), and Melnikov (1997). Melosira also grew as nets, sometimes coalescing into mats at several of these stations (7 and 10) and at station 19. Ice algae grew as small patches at many stations, sometimes in depressions in the ice. The patches were less than a meter in their greatest dimension, ranging in size down to the order of 0.20 m. Patches and film were loosely attached because they were easily dislodged by the ROV. Finally, a thin film of loosely attached ice algae was observed as a layer on the top side of blocks (1–2 m in all dimensions) under an ice ridge at station 4 and on the underside of ice at many of the other stations (Table 2).
The percent cover of ice algae, both among and within stations, was highly variable (Table 1). With the exception of station 4, the average algal coverage was greater than 40% and there was no apparent qualitative relationship between algae and sub-ice features. The range of coverage among images at the same station, even among images at the same location on the floe, however, was typically very large (Table 1). At station 13 the two locations with the lowest coverage of algae along the transect (30% and 60%) were the only locations under a melt pond.
We observed pieces of algae on the seafloor at every station where we surveyed the bottom except stations 4 and 5, the first two stations surveyed. Pieces varied in size up to a few centimeters in length and could be seen moving along the bottom with the current as well as trapped in depressions. These pieces were easily disturbed by the movement of the ROV. Although we were not able to collect any of these pieces, we surmised they were pieces of the strands and curtains of Melosira, we observed under the ice at many stations.
The taxonomic diversity of algae in the ice cores from this region has been reported to be extraordinarily high, with over 250 taxa recorded from one entire core and the bottom 20 cm of another at a station slightly further south than our southern most station (von Quillfeldt et al. 2003). A total of 46 taxa were identified from sediment at station 7. The relative abundances of the nine most common taxa in the ice and sediment demonstrate that sediment contains spores or cells of typical phytoplankton and ice algal taxa (Table 3). Phytoplankton taxa or taxa that live facultatively in ice and plankton were proportionally more common in the sediment than typical ice algal taxa, but ice algal taxa (Melosira arctica, Nitzschia frigida, N. cf. promare) were present in the sediment. Synedropsis hyperborea is an epiphytic species, particularly on M. arctica, but also on Fossula arctica, Fragilariopsis oceanica, and N. frigida, (Horner et al. 1988; Syvertsen 1991; von Quillfeldt 1997) and was recorded from the ice and the sediment. The percent composition of the obligate ice algae taxa (M. arctica, N. frigida, N. frigida cf. promare) and the most common facultative ice/phytoplankton taxa (Fragilariopsis oceanica, Fossula arctica) make up 36% of the cells in the sediment, very close to the 32% they represent in the ice.
There were few significant correlations among biological and physical variables at the large, among station, scale (Table 4). No physical variables were correlated with percent coverage of ice algae on the under ice surface or with pigments in the bottom 10 cm of ice. There was a significant positive relationship, however, between the percentage coverage of ice algae and the concentrations of all pigments in the sediment, with coverage of ice algae explaining 79.7% (linear regression, P<0.02) of total sediment pigments (Fig. 2). Ice algal cover was negatively correlated with water chlorophyll a, phaeopigments, and total pigments (Table 4) and explained 63.7% of the variability in total water pigments (P<0.02, Fig. 2).
There was little variation in ice thickness or snow depth at the scale of individual ice floes (Table 5). Nevertheless, percent coverage of ice algae was positively correlated with distance from the edge of the ice floe and negatively correlated with snow depth at the 10-m scale within stations (Table 6). This analysis was restricted to locations 20 m or farther from the floe edge to reduce possible effects of mooring the boat on algal coverage near the ice edge, but if locations less than 20 m are included the results are nearly identical. Using just data from greater than 20 m from the floe edge, snow depth and distance from the edge explained 32.2% of the variability in ice algal coverage (multiple linear regression, P<0.001, percent coverage=0.998+0.008 distance 4.39 snow depth).
Discussion
Ice algal distribution and abundance
The large aggregations of Melosira we observed at many stations are in contrast to earlier observations in the Barents Sea and central Arctic Ocean where these assemblages were reported to typically be restricted to multi-year ice (Horner et al. 1988, 1992; Melnikov 1997; Syvertsen 1991); but in agreement with observations from Northeast Greenland (Gutt 1995; von Quillfeldt 1997), the central Arctic Basin (Gosselin et al. 1997), and the Laptev Sea (Werner and Lindemann 1997) where similar aggregations have been recorded from first year ice. Whether he observed these diatom aggregations on multi-year or less commonly first year ice in the central Arctic Basin, Melnikov (Melnikov 1997; Melnikov and Bondarchuk 1987) always found them in areas protected from strong currents. These zones or ‘hydrodynamic shadows’ were formed by under ice structures that allowed these assemblages to persist for several weeks (Melnikov 1997). While we sometimes observed small patches of ice algae in depressions (station 5) or on tops of ice blocks under a ridge (station 4) the long strands, ropes and curtains ofMelosira, we observed at many stations were not associated with any under ice structures such as cracks or hummocks, which might provide protection from currents. A relationship between under ice structure and large accumulations of Melosira may be more common on multi-year than first year ice or in areas with strong currents where large aggregations of algae cannot develop in unprotected areas. Nevertheless, Melnikov’s (1997) estimate that such assemblages occupy 2% of arctic sea ice based on an estimate of the area of hummocks is likely very low. Our observations suggest that on the Chukchi Sea shelf, large accumulations of Melosira and associated diatoms are common over large areas of the under ice surface.
The distribution of sub-ice algae was marked by variability at both large, among station, and small, within-station, scales (Tables 1, 5). Light is the single most important factor in determining abundance of ice algae on large- and small-scales in both the Arctic (Gosselin et al. 1986, 1990; Hsiao 1988; Welch and Bergmann 1989; Cota et al.1991; Rysgaard et al. 2001) and Antarctic (Brierley and Thomas 2002). Snow depth and ice thickness were probably the major determinants of light in our study and neither varied tremendously among stations (Table 1). Low variability likely accounts for the lack of correlation among these factors and percent cover of sub-ice algae at a mesoscale (Table 4). The lack of significant relationships may also be due to sampling late in the season when snow and ice melt was well underway. Variability in ice structure caused in part by variability in ice salinity is also a major determinant of algal abundance (Gosselin et al. 1986, 1997; Robineau et al. 1997), but again there was not much variability in ice or water salinity among our stations (Table 1). High variability is a hallmark of ice algal distribution (Alexander 1980; Gosselin et al. 1986; Cota and Smith 1991; Rysgaard et al. 2001; Brierley and Thomas 2002), but the physical factors we measured were unable to explain this variability on a mesoscale.
Distance from the floe edge and apparently irradiance appeared to be important in determining the small scale, within station, distribution of ice algae. The pattern of increased abundance of sub-surface ice algae with distance from the floe edge held for distances greater than 20 m from the floe edge, so the pattern is probably not related to ship disturbance. Although, we noted no differences in current flow with distance from the ice edge, it is also possible that current flow decreases with distance from the floe edge resulting in less algae being dislodged with distance from the edge. Disturbance from waves would decrease with distance from the floe edge and could explain the pattern we observed. The percent cover of ice algae was negatively correlated with snow depth (Table 6), a relationship that has been well documented throughout the Arctic and is a consequence of snow reducing light (Gosselin et al. 1986; Smith et al. 1988; Welch and Bergman 1989). Together snow depth and distance from the floe edge explain a little over a third of the variability in percent coverage of sub-surface ice algae, so other factors are clearly important. Near Resolute, snow thickness and light explained 77% of the variability in under-ice algal biomass (Welch and Bergmann 1989). Small-scale variation in the under-ice surface can also affect algal growth and biomass (Krembs et al. 2002). More studies, using nondestructive means, of algae abundance on the scale of 10s of meters are needed before we will understand variability in sub-ice algal abundance at this scale.
Few studies have recorded the presence of ice algae on the seafloor or in sediments (Alexander and Chapman 1981; Pomeroy et al. 1990; Ambrose et al. 1994) and direct observation of ice algae on the bottom has only been made by divers in shallow water (Alexander and Chapman 1981; Pomeroy et al. 1990). Our observation of ice algae at the sediment water interface at seven of the nine stations where the bottom was surveyed indicates that ice algae commonly reach the bottom over a large area and range of depths in the Chukchi Sea. Furthermore, the documentation of obligate ice algal taxa in the sediment (Table 3) demonstrates that this material is incorporated into the sediment. The obligate ice taxa and the facultative ice/phytoplankton taxa make up 36% of the cells in the sediment, very close to the 32% they represent in the ice. There is no correspondence at the species level, however, between the percent composition of dominant algae in the ice and sediment at the five stations where both were sampled (Table 2). The obligate ice algal taxon Melosira arctica, is proportionally more abundant in the sediment compared to the overlying ice (Table 3), but this species is typically found in the sub-ice community, which is under represented by surface ice coring, so our ice abundance numbers may be low. The sediment also integrates material deposited from ice and water column communities over a long period of time (weeks to months), so lack of correspondence in species composition between ice and sediment is not surprising.
The significant positive relationship between percent cover of ice algae in the sub-ice community and sediment pigment concentrations (Fig. 2), suggests a coupling between the two communities. Ice algae fall rapidly to the seafloor with the onset of ice melt in spring (Tremblay et al. 1989; Reibesell et al. 1991; Ambrose and Renaud 1995; Michel et al. 1997; Bauerfeind et al. 1997). Consequently, benthos under areas of high algal cover might expect to have high sediment pigments, reflecting fairly recently sedimented material. The shallow depth of the Chukchi Sea increases the likelihood that benthic pigments are a reflection of dynamics in the overlying ice and water. The fact that percent cover of ice algae can explain nearly 80% of the variability in total sediment pigments in the underlying sediment is remarkable. The relationship between algal cover and sediment pigments suggests that ice algae reaches the seafloor quickly because the benthos accumulates pigments over a period of time and ice is continually moving. Conversely, ice and associated algal cover reduces light available to water column algae; possibly explaining the negative relationship between algal cover and water column pigments (Fig. 2). The relationships between ice algal cover and pigments should be interpreted cautiously, however, because they are based on limited data. It is possible that these relationships have nothing to do with vertical processes and are all a reflection of advection of material to the water and benthos from other areas. Taken together, the pigment and taxonomic data suggest that ice algae reach the seafloor and consequently might be an important food source for benthic organisms, particularly early in the season, as suggested by others (Dunbar 1977; Bradstreet and Cross 1982; Carey 1985, 1987; Ambrose et al. 2001).
Methodological considerations
Remote sensing is a common method of quantifying patterns of distribution and abundance of plants and animals in marine systems where physical sampling is difficult, prohibitively time consuming, biased, or dangerous (Auster et al. 1989). Under-ice communities in the Arctic and Antarctic are notoriously difficult to sample because of the very patchy distribution of the flora and fauna (Schnack-Schiel 2003), the bias of surface coring, and the hazards of diving. Remote methods of observing sub-ice communities have proven successful in the Arctic (Alexander 1980; Gutt 1995; Werner and Lindemann 1997; Gradinger and Bluhm 2004) and Antarctic (Brierley and Thomas 2002) at revealing patterns of ice algal distribution and abundance.
The inability of surface ice cores to adequately sample the sub-ice algal community has been recognized for a long time (Alexander 1980; Welch et al. 1988; Horner et al. 1992) and has undoubtedly led to a biased view of the distribution, abundance and importance of ice algae in the Arctic ecosystem. Underestimation of ice algae may be particularly severe late in the season; before ice melt but when the sub-ice community is well developed. Late in the season in Resolute, in the Canadian archipelago, SIPRE cores underestimate ice algal chlorophyll a by as much as 90% compared to subsurface cores (Welch and Bergmann 1989). This was also evident in our investigation in which the ROV showed long strands of Melosira arctica, but few cells of the species were found in the ice (Tables 2, 3). The high percent coverage of sub-ice algae we observed (Table 1), indicates that this community constitutes a large portion of the total biomass of ice algae in the spring. Primary production studies based on ice cores, even when carefully collected to avoid loss of the bottom portion (Hsiao 1988; Smith et al. 1988; Mock and Gradinger 1999), do not adequately measure the contribution of the sub-ice community to total primary productivity. Only those studies which measure primary productivity in situ, without disturbing the sub-ice algal community or collect cores from the under-ice surface provide an accurate view of the contribution of this assemblage to primary productivity (Herman et al. 1993; McMinn et al. 2000; Kühl et al. 2001; Rysgaard et al. 2001).
Percent cover is commonly used to quantify the abundance of sessile plants and animals in marine communities. To our knowledge it has never been used to quantify the abundance and distribution of under-ice algae. However, quantifying the abundance of under-ice algae using percent cover is not without bias. Percent cover was most easily determined for discrete patches of algae because the border of the patch was easily discernable and the algae were largely two dimensional. In contrast, meter long strands and curtains of Melosira were not easily quantified by our measure of percent cover. Their abundance was underestimated because they sometimes hung down beyond the ROV camera and because they are three dimensional. Thin films of diatoms were also difficult to quantify as the borders of the patches were often vague and it was sometimes hard to distinguish a thin film from interstitial algae visible through the ice. In the only other study to quantify the abundance of sub-ice algae, divers counted aggregates of ice algae in replicate areas of under ice surface (Melnikov 1997). ROV imagery provides a fast and safe means of surveying large areas of ice and images can be used for measurement of percent cover or density. Given the variety of growth forms, we encountered we decided that percent cover, despite biases, was a better measure of sub-ice algal abundance than density. Several of the patterns in sub-surface ice algal abundance, we documented are in agreement with other studies conducted on smaller scales, which suggests that percent cover can be used to assay the abundance of under-ice algae.
Conclusion
Ice algae are an undeniably important component of polar marine systems, but are only rarely included in ecosystem models (Welch et al. 1992). In the Arctic, most studies investigating the distribution and abundance of ice algae have largely relied on surface coring to collect ice algae and collected few replicate cores per station despite ice algae’s well documented spatial variability. Divers (Melnikov and Bondarchuk 1987) and subsurface coring (Welch and Bergmann 1989) have collected valuable observations and data, but the spatial extent of these studies is limited. Remote imaging has the potential to survey large areas of the sub-ice algal community; exploring factors controlling the distribution and abundance of this important component of the ice algal community. Our survey of the under-ice community in the Chukchi Sea not only extends the imaging work of Gutt (1995) and Werner and Lindemann (1997) to the western arctic, but it also revealed patterns of ice algal abundance under the ice and on the seafloor at scales ranging from 10s of meters to 10s of kilometers. The large biomass of sub-ice algae observed at many stations in the Chukchi Sea, and the presence of ice algae over large areas of an Arctic shelf indicate that sub-ice algae likely play an important role in the function of the Arctic marine ecosystem.
References
Alexander V (1974) Primary productivity regimes of the nearshore Beaufort Sea, with reference to the potential roles of ice biota. In: Reed JC, Sater JE (eds) The coast and shelf of the Beaufort Sea. Arctic Institute of North America, Arlington, pp 609–632
Alexander V (1980) Interrelationships between the seasonal sea ice and biological regimes. Cold Regions Sci Tech 2:157–178
Alexander V, Chapman T (1981) The role of epontic algae communities in Bering Sea Ice. In: DW Hood DW, Calder DW (eds) Bering sea shelf: oceanography and resources, vol 2. University of Washington Press, Seattle, pp 773–780
Ambrose WG Jr, Renaud PE (1995) Benthic response to water column productivity patterns: evidence for benthic-pelagic coupling in the Northeast Water Polynya. J Geophys Res 100:4411–4421
Ambrose WG Jr, Ahrens M, Brandt A, Dimmler W, Graf G, Gutt J, Herman R, Jensen P, Piepenburg D, Queisser Q, Renaud P, Ritzrau R, Scheltz A, Thomsen L (1994) Benthos. Ber Polarforsch 142:106–112
Ambrose WG Jr, Clough LM, Tilney PR, Beer L (2001) Role of echinoderms in benthic remineralization in the Chukchi Sea. Mar Biol 139:937–949
Apollonio S (1985) Arctic marine phototrophic systems: functions of sea ice stabilization. Arctic 38:167–173
Auster PJ, Stewart LL, Sprunk H (1989) Scientific imaging with ROVs: tools and techniques. Mar Technol Soc J 23:16–20
Bauerfeind E, Garrity C, Krumbholz M, Ramseier RO, Voß M (1997) Seasonal variability of sediment trap collections in the Northeast water polynya. Part 2 Biochemical and microscopic composition of sedimenting matter. J Mar Sys 10:371–389
Bergmann MA, Welch HE, Butler-Walker JE, Sifred TD (1991). Ice algae photosynthesis at Resolute and Saqvaqjuac in the Canadian Arctic. J Mar Sys 2:43–52
Bradstreet MS, Cross WE (1982) Trophic relationships at high Arctic ice edges. Arctic 35:1–12
Brierley AS, Thomas DN (2002) Ecology of the southern ocean pack ice. Adv Mar Biol 43:171–277
Carey AG Jr (1985) Marine ice fauna: Arctic. In: Horner RA (ed) Sea ice biota. CRC, Boca Raton, pp 173–190
Carey AG Jr (1987) Particle flux beneath fast ice in the shallow southwestern Beaufort Sea, Arctic Ocean. Mar Ecol Prog Ser 40:247–257
Carey AG Jr (1991) Ecology of North American Arctic continental shelf benthos: a review. Cont Shelf Res 11:865–883
Clough LM, Ambrose WG Jr, Cochran JK, Barnes C, Renaud PE, Aller RC (1998) Infaunal density, biomass, and bioturbation in the sediments of the Arctic Ocean. Deep Sea Res II 11:865–883
Cooper LW, Grebmeier JM, Larsen IL, Egorov VG, Therdorakis C, Kelly HP, Lovvorn JR (2002) Seasonal variation in sedimentation of organic materials in the St. Lawrence Island polynya region, Bering Sea. Mar Ecol Prog Ser 226:13–26
Cota GF (1985) Photoadaptation of high arctic ice algae. Nature 315:219–222
Cota GF, Horne EPW (1989) Physical control of arctic ice algal production. Mar Ecol Prog Ser 52:111–121
Cota GF, Smith REH (1991) Ecology of bottom ice algae: II. Dynamics, distributions and productivity. J Mar Sys 2:279–295
Cota GF, Legendre L, Gosselin M, Ingram RG (1991) Ecology of bottom ice algae: I. Environmental controls and variability. J Mar Syst 2:257–277
Dunbar M (1977) The evolution of polar ecosystems. In: Llano GA (ed) Adaptations with Antarctic Ecosystems. In: Proceedings of the Third SCAR Symposium on Antarctic Biology. Smithsonian Institute, Washington, pp 1063–1076
Gosselin M, Legendre L, Therriault J-C, Demers S, Rochet M (1986) Physical control of the horizontal patchiness of sea-ice microalgae. Mar Ecol Prog Ser 29:289–298
Gosselin ML, Legendre L, Demeres S, Terriault J-C (1990) Light and nutrient limitation of sea-ice microalgae (Hudson Bay, Canadian Arctic). J Phycol 26:220–236
Gosselin M, Levasseur M, Wheeler PA, Horner RA, Booth B (1997) New measurements of phytoplankton and ice algae production in the Arctic Ocean. Deep-Sea Res II 44:1623–1644
Gradinger R (1999) Vertical fine structure of the biomass and composition of algae communities in Arctic pack ice. Mar Biol 133:745–754
Gradinger RR, Bluhm BA (2004) In-situ observations on the distribution and behavior of amphipods and Arctic cod (Boregadus saida) under the sea ice of the High Arctic Canadian Basin. Polar Biol 27:595–603
Grebmeier JM, Smith WO Jr, Conover RO (1995) Biological processes on Arctic continental shelves: ice-ocean-biotic interactions. In: Smith WO, Grebmeier JM (eds) Arctic oceanography: marginal ice zone and continental shelves (coastal and estuarine studies 49). American Geophysical Union, Washington, pp 231–261
Gulliksen B, Lønne OJ (1989) Distribution, abundance and ecological importance of marine sympagic fauna in the Arctic. Rapp v Reu. Cons Perm Int Exp Mer 188:133–138
Gutt J (1995) The occurrence of sub-ice algae aggregations off northeast Greenland. Polar Biol 15:247–252
Herman AW, Knox DF, Conrad J, Mitchell MR (1993) Instruments for measuring subice algae profiles and productivity in situ. Can J Fish Aquat Sci 50:359–369
Hobson KA, Fisk A, Karnovsky N, Holst M, Gagnon J-M, Fortier M (2002) A stable isotope (δ13C, δ15N) model for the North Water food web: implications for evaluating trophodynamics and the floe of energy contaminants. Deep-Sea Res II 49:5131–5150
Horner RA (1985) Ecology of sea ice microalgae. In: RA Horner (ed) Sea Ice Biota. CRC, Boca Raton, pp 84–103
Horner RA (1990) Techniques for sampling ice algae. In: Medlin LK, Priddle J (eds) Polar marine diatoms. British Antarctic Survey, Cambridge, pp 19–23
Horner R, Syvertsen EE, Thomas DP, Lange C (1988) Proposed terminology and reporting units for sea ice algal assemblages. Polar Biol 8:249–253
Horner RA, Ackley SF, Dieckmann GS, Gulliksen B, Hoshiai T, Legendre L, Melnikov IA, Reeburgh WS, Spindler M, Sullivan C (1992) Ecology of sea ice biota I. Habitat, terminology, and methodology. Polar Biol 12:417–427
Hsiao SIC (1988) Spatial and seasonal variations in primary production of sea ice microalgae and phytoplankton in Frobisher Bay, Arctic Canada. Mar Ecol Prog Se 44:275–285
Johnson WR (1989) Current response to wind in the Chukchi Sea: a regional upwelling event. J Geophys Res 94:2057–2064
Krembs C, Tuschling K, Juterzenka KV (2002) The topography of the ice-water interface-its influence on the colonization of sea ice algae. Polar Biol 25:106–117
Kühl M, Glud RN, Borum J, Roberts R, Rysgaard S (2001) Photosynthetic performance of surface-associated algae below sea ice as measured with a pulse-amplitude-modulated (PAM) fluorometer and O2 microsensors. Mar Ecol Prog Ser 223:1–14
Legendre L, Aota M, Shirawaws K, Martineay M-J, Ishikawa M (1991) Crystallographic structure of sea ice along a salinity gradient and environmental control of microalgae in the brine cells. J Mar Syst 2:347–357
Legendre L, Ackley SF, Dieckmann GS, Gulliksen B, Horner R, Hoshiai T, Melnikov IA, Reeburgh WS, Spindler M, Sullivan CW (1992) Ecology of sea ice biota 2. Global significance. Polar Biol 12:429–444
Legendre L, Robineau B, Gosselin M, Michel C, Ingram RG, Fortier L, Therriault J-C, Demers S, Monti D (1996) Impact of freshwaters on a subarctic coastal ecosystem under seasonal ice (southeastern Hudson Bay, Canada) II. Production and export of microalgae. J Mar Syst 7: 233–250
Maestrini SY, Rochet M, Legendre L, Demers S (1986) Nutrient limitation of the bottom microalgae biomass (southeastern Hudson Bay, Canadian Arctic). Limnol Oceanogr 31:969–982
McMinn A, Ashworth C, Ryan KG (2000) In situ net primary productivity of an Antarctic fast ice bottom algal community. Aquat Microb Ecol 21:177–185
Melnikov IA (1997) The Arctic Sea Ice Ecosystem. Gordon and Breach Science Publishers, Amsterdam
Melnikov IA, Bondarchuk LL (1987) Ecology of mass aggregations of colonial diatom algae under drifting Arctic sea ice. Oceanology 27:233–236
Michel D, Legendre L, Taguchi S (1997) Coexistence of microalgal sedimentation and water column recycling in a seasonally ice-covered ecosystem (Saroma-Ko Lagoon, Sea of Okhotsk, Japan). J Mar Syst 11:133–148
Mock T, Gradinger R (1999) Determination of Arctic ice algae production with a new in situ incubation technique. Mar Ecol Prog Ser 177:15–26
Pomeroy LR (1997) Primary production in the Arctic Ocean estimated from dissolved oxygen. J Mar Sys 10:1–8
Pomeroy LR, Macko SA, Ostrom PH, Dunphy J (1990) The microbial food web in the Arctic seawater: concentration of dissolved free amino acids and bacterial abundance and activity in the Arctic Ocean and Resolute Passage. Mar Ecol Prog Ser 61:31–40
Quillfeldt CH von (1997) Distribution of diatoms in the Northeast Water Polynya, Greenland. J Mar Syst 10:211–240
Quillfeldt CH von, Ambrose WG Jr, Clough LM (2003) High numbers of diatom species in first year ice from the Chukchi Sea. Polar Biol 26:806–818
Reibesell U, Schloss I, Semtacek V (1991) Aggregation of algae released from melting sea ice: implications for seeding and sedimentation. Polar Biol 11:239–248
Robineau B, Legendre L, Kishino M, Kudoh S (1997) Horizontal heterogeneity of microalgae biomass in the first-year ice of Saroma-Ko Lagoon (Hokaido, Japan). J Mar Syst 11:81–91
Runge JA, Ingram RG (1988) Underice grazing by planktonic, calanoid copepods in relation to a bloom of microalgae in southeastern Hudson Bay. Limnol Oceanogr 32:280–286
Rysgaard S, Kühl M, Gludd RN, Hansen HJW (2001) Biomass, production and horizontal patchiness of sea ice algae in a high-Arctic fjord (Young Sound, NE Greenland). Mar Ecol Prog Ser 223:15–26
Sakshaug E (2004) Primary and secondary production in the Arctic Sea. In: Stein R, Macdonald RW (eds) The organic carbon cycle in the Arctic Ocean. Springer, Berlin Heidelberg New York, pp 57–81
Sasaki H, Watanabe K (1984) Underwater observation of ice algae in Lützow-Holm Bay, Antarctica. Antarctica Rec 81:1–8
Schnack-Schiel SB (2003) The macrobiology of sea ice. In: Thomas DN, Dieckmann GS (eds) Sea ice: an introduction to its physics, chemistry, biology and geology. Blackwell, Oxford, pp 211–239
Smith REH, Anning J, Clément P, Cota G (1988) Abundance and production of ice algae in Resolute Passage, Canadian Arctic. Mar Ecol Prog Ser 48:251–263
Sokal RR, Rohlf FJ (1969) Biometry. WH Freeman and Company, San Francisco
Syvertsen EE (1991) Ice algae in the Barents Sea: types of assemblages, origin, fate, and role in the ice-edge phytoplankton bloom. Polar Res 10:277–288
Tremblay C, Runge JA, Legendre L (1989) Grazing and sedimentation of ice algae during and immediately after a bloom at the ice-water interface. Mar Ecol Prog Ser 56:291–300
Welch HE, Bergmann MA (1989) The seasonal development of ice algae and its prediction from environmental factors near Resolute, N.W.T., Canada. Can J Fish Aquat Sci 46:1793–1804
Welch HE, Bergmann MA, Jorgenson JK, Burton W (1988) A subice suction corer for sampling epontic algae. Can J Fish Aquat Sci 45:562–588
Welch HE, Bergmann MA, Sifred TD, Amarualik PS (1991) Seasonal development of ice algae near Chesterfield Inlet, N.W.T. Canada. Can J Fish Aquat Sci 48:2395–2402
Welch HE, Bergmann MA, Sifere TD, Martin KA, Curtis MF, Crawford RE, Conover RJ, Hopp H (1992) Energy flow through the marine ecosystem of the Lancaster Sound Region, Arctic Canada. Arctic 45:343–357
Werner I, Lindemann F (1997) Video observations of the underside of arctic sea ice-features and morphology on medium and small scales. Polar Res 16:27–36
Wheeler PA, Gossellin M, Sherr E, Thilbault D, Kirchmans DL, Benner R, Whitledge TE (1996) Active carbon cycling of organic carbon in the central Arctic Ocean. Nature 380:697–699
Yager PL, Wallace DWR, Johnson KM, Smith WO Jr, Minnett PJ, Deming JW (1995) The northeast water polynya as an atmospheric carbon sink: a seasonal rectification hypothesis. J Geophys Res 100:4389–4398
Yager PL, Connelly TL, Mortazavi B, Wommack KE, Bano N, Bauer JE, Opshal S, Holligaugh JT (2001) Dynamic bacterial and viral response to an algal bloom at sub-zero temperatures. Limnol Oceanogr 46:790–801
Yentsch CS, Mensel DW (1963) A method for determination of phytoplankton chlorophyll a and phaeophytin by fluorescence. Deep Sea Res 10:221–231
Acknowledgements
Our work was supported by grants from the National Oceanographic Atmospheric Administration National Undersea Research Program, West Coast and Polar Regions Center and the Howard Hughes Medical Institute through a grant to Bates College. Ship support was provided by the United States Coast Guard. Our work would not have been successful without the tremendous efforts of the officers and crew of the USCGC Polar Sea. We are also very grateful to Lance Horn from the University of North Carolina National Undersea Research Center for expertly managing all aspects of the ROV, Tim Buckley and Aaron Putman under the direction of Deb Meese for analyzing the ice samples for pigments and Jon Reierstand and Dan Robarts for making the map. We are also indebted to Paul Renaud for his comments on earlier versions of this manuscript and to three reviewers whose comments and suggestions improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ambrose, W.G., Quillfeldt, C.v., Clough, L.M. et al. The sub-ice algal community in the Chukchi sea: large- and small-scale patterns of abundance based on images from a remotely operated vehicle. Polar Biol 28, 784–795 (2005). https://doi.org/10.1007/s00300-005-0002-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-005-0002-8