Abstract
We conducted surrogate in-situ physiological performance measures (δ13C and δ15N) of Carex plants from 15 Eurasian Coastal Arctic sites. Leaf carbon isotope discrimination (LCID) of Carex plants exhibited significant differences between sites (populations). Additionally, LCID was inversely correlated with mean annual temperature and stomatal density, and to a lesser extent, with the depth of thaw. Leaf δ15N values of Carex plants exhibited significant differences between sites without differences among ramet age classes, and the leaf δ15N values were inversely correlated with mean annual precipitation. These ranges of Carex leaf gas exchange and mineral nutrition across the Eurasian Arctic may contribute to Carex’s dominance in coastal tundra systems. Also, the inverse correlation between LCID, precipitation, and temperature indicates that, as precipitation increases and temperatures continue to warm in Eurasia, leaf gas exchange may actually be lower in the future, leading to reductions in shoot growth and lower above-ground biomass production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding the physiological performance of arctic plants and populations is an important part of global-change research in northern latitudes (McGraw and Fetcher 1992; Callaghan and Carlsson 1996; Welker et al. 2000). Leaf gas exchange and mineral nutrition are the basis of the carbon and nitrogen cycles of arctic ecosystems (Shaver et al. 1992; Chapin et al. 1995) and may be altered by warmer temperatures, changes in precipitation, alterations of irradiance, or shifts in atmospheric N deposition (Welker et al. 1993; Wookey et al. 1993, 1995; Chapin et al. 1995; Jones et al. 1998). Most studies of leaf gas exchange and mineral nutrition in arctic plants have been conducted in Alaska, Greenland, north Sweden, and on Svalbard (Shaver et al. 1979; Jonasson 1983, 1989; Welker et al. 1997; Christensen et al. 2000; Jones et al. 2000). To date no studies exist from locations along the Eurasian Coastal Arctic, even though this region represents ~one-third of the circumarctic landscape (Bliss and Matveyeva 1992). Detailed studies of plant gas exchange and mineral nutrition of vegetation in Eurasia are especially important today as it is now clear that precipitation is increasing and temperatures are warming dramatically in this region, resulting in greater rates of river discharge (Peterson et al. 2002; Polyakov et al. 2002).
Studies of in-situ physiological performance of widely distributed arctic plant populations or taxonomically similar genera with overlapping ecological niches, such as Carex spp. and their responsiveness to the environment, have been few because of logistical and instrumental constraints, even though modern gas analyzers are portable (Vourlitis et al. 1993; Jones et al. 2000). These constraints have been overcome in Eurasia recently as a joint Swedish-Russian expedition along the Eurasian Arctic Coast occurred in 1994, with vegetation collections at 17 different sites across 140° of longitude and 10° of latitude (Hedberg et al. 1999; Jónsdóttir et al. 1999). Surrogate in-situ physiological measures were made possible at these sites by quantifying the isotopic (δ13C and δ15N) characteristics of leaves from these plants, providing an integrative measure of gas exchange and mineral nutrition (Farquhar et al. 1989; Welker et al. 1993; Nadelhoffer et al. 1996; Michelsen et al. 1998; Robinson 2001).
The leaf carbon isotope discrimination (LCID) (Δ) characteristics of plant leaves are an integrative measure of carbon fixation and CO2 diffusion (Farquhar et al. 1982, 1989; Dodd et al. 1998; Alstad et al. 1999; Gebauer and Ehleringer 2000). Leaf carbon isotope discrimination is an ecophysiological trait that is affected by soil and leaf mineral nutrition, soil and plant water status, precipitation, and irradiance (Ehleringer et al. 1991; Welker et al. 1993), and is also an indicator of population differences in habitat conditions and physiological performance (Rice et al. 1993). Typically, under abundant water supplies or higher rainfall regimes, LCID is greater than under conditions where water supplies are limited, due to higher intercellular CO2 concentrations associated with greater rates of stomatal conductance (Ehleringer and Cooper 1988; Welker et al. 1993; Dodd et al. 1998; Alstad et al. 1999). In contrast, under conditions of higher soil and plant N contents, LCID is lower than under nutrient-deficient conditions due primarily to lower intercellular CO2 concentrations associated with higher rates of photosynthesis. However, cold soil temperatures in the Arctic can limit hydraulic and stomatal conductance, and warm temperatures can increase soil and air dryness (atmospheric drought), effectively lowering the LCID of arctic plants (Dawson and Bliss 1993; Welker et al. 1993, 1995).
Similar to LCID, the δ15N values of plant leaves provide a measure of mineral nutrition that reflects a host of processes, especially differences in N sources (Michelsen et al. 1996; Nadelhoffer et al. 1996; Robinson 2001), variation in mycorrhizal infection (Michelsen et al. 1998), and differences in soil mineralization processes (Gebauer and Ehleringer 2000; Robinson 2001), but at undisturbed sites, leaf δ15N reflects the 15N abundance of available nitrogen in the soil layers where the density of roots are greatest (Gebauer et al. 1994). This measure of ecophysiological performance is especially germane to our study, as differences in the leaf δ15N values of ramet generations may indicate the degree of physiological integration of connected ramets (Welker et al. 1987, 1991; Welker and Briske 1992), as well as differences in N sources.
Differences in the average δ15N values of Carex leaves between populations may be indicative of unique N source combinations (NH4 +, NO3 −, or amino acids) or site-specific soil mineralization/immobilization processes that occur across the Eurasian Coastal Arctic. For instance, if plant parts or plant populations are using a greater proportion of ammonium than nitrate, the leaf δ15N values would be slightly enriched compared to those that are using a greater fraction of nitrate. In addition, if plants are acquiring organic sources of N from the soil via mycorrhizal associations, the leaf δ15N values would be enriched compared to plants or populations that are only using inorganic N sources (Emmerton et al. 2001a, 2001b). However, because of considerable 15N fractionation associated with mycorrhizal assimilation, the leaf δ15N values of plants that are infected with ericoid or ecotomycorrhizal mycorrhiza are unlikely to directly reflect the δ15N of the original soil N sources (Emmerton et al. 2001b). Carex species are not mycorrhizal, simplifying our interpretation of δ15N values. While it is typically assumed that changes in plant δ15N values are the result of changes in the combination of natural sources (Emmerton et al. 2001a, 2001b), industrial sources of atmospheric N may influence the isotopic characteristics of vegetation (Ammann et al. 1999). However, because of the remoteness of our study sites from any anthropogenic N source, we expect the δ15N values of these plants to reflect natural sources.
It has become increasingly clear that the modular nature of clonal plants in arctic and temperate habitats confers physiological and ecological benefits (Callaghan 1976; Welker et al. 1985, 1987; Jónsdóttir and Callaghan 1988, 1989, 1990; Welker and Briske 1992; Jónsdóttir and Watson 1997; Hertefeldt and Jónsdóttir 1999). Physiologically, the ability to transport resources among interconnected ramets that may differ in age and assimilative ability (especially from mature ramets to fast-growing juveniles), allows portions of the clone, under favorable microenvironments, to provide carbon, nitrogen, or water to shaded, partially grazed ramets, or to ramets that retain active roots without leaves (Welker et al. 1985; Jónsdóttir and Callaghan 1988). This division of labor may result in a divergence of environmental sensitivity among ramet generations, especially with regard to carbon fixation and soil N acquisition. One might expect that because juvenile ramets are typically at the leading edge of clones and may be sampling the nature of the microclimate, they would be the most responsive to the environment, especially in their gas-exchange characteristics. In contrast, mature ramets that typically transport N into developing ramets (Jónsdóttir and Callaghan 1990; Welker et al. 1991) and retain established root systems, may have the most responsive mineral nutrition attributes to environmental variation.
There are no studies in the Eurasian Arctic that simultaneously examine the patterns and processes that affect LCID, δ15N, and N content of clonal arctic plants. In this study, we address three main questions. First, are there differences in the ecophysiological performance of Carex plants located across a range of sites (populations) along the Eurasian Coastal Arctic? Second, are there differences in the physiological performance of Carex ramet generations (i.e., juvenile vegetative ramets, mature ramets, and reproductive ramets) within a clone? Third, to what degree is the physiological performance of Carex ramet generations responsive to environmental parameters such as temperature, precipitation, soil organic matter, and depth of thaw? And, fourth, to what degree are ecophysiological traits of Carex plants correlated with plant growth and anatomical traits?
We recognize that our sampling involved three species of Carex across a range of sites. Thus, some of our observed differences could be attributed to species-specific traits and physiological performance. However, since two of the species are members of the circumpolar complex Carex bigelowii (Murray 1994), all three species grow clonally with very similar shoot, rhizome, and root morphology (Jonsdottir et al. 1999; Stenstrom 2000), and because these species exhibit consistent overlap in their ecological niches, we focus on general attributes. In addition, since Carex species have similar physiology (Lambers and Van Der Werf 1988; Hirose et al. 1989), sedge-dominated meadows are often characterized by genus as opposed to species (Henry et al. 1990), and sedges respond similarly to abiotic conditions (Nosko and Courtin 1995), our emphasis is on genera attributes. Species differences are given consideration where appropriate in our data interpretation.
Materials and methods
The Swedish-Russian Tundra Ecology 1994 Expedition occurred between June and August along the Eurasian Coastal Arctic from the Kola Peninsula in the west to Wrangel Island in the east (Fig. 1) with the use of the Russian ice breaker, the R/V Akademik Fedorov (Hedberg et al. 1999). The vessel was equipped with two helicopters that allowed field parties to visit tundra sites for periods of 2–3 days, performing scientific studies ranging from vegetation collections and analysis (Jónsdóttir et al. 1999) to trace gas fluxes (Christensen et al. 1999). A total of 17 field sites were visited covering 140°of longitude with a latitudinal range from 67°N to 77°N (Hedberg et al. 1999). Field parties visited most types of Arctic Eurasian environments and tundra types, ranging from the southern Arctic where dwarf birch is present to the high-Arctic, semi-polar desert-like habitats of Cape Chelyuskin. Common to all regions are mesic and wet tundra dominated by Carex species. These ecosystems often constitute >50% of the landscape and, thus, provide important forage and habitat for migratory birds, herbivores, and small rodents (Alerstam and Jonsson 1999; Danell et al. 1999; Stenstrom et al. 2002).
Route map of the Tundra Ecology-1994 Expedition across the Eurasian Coastal Arctic and the spatial distribution of the three Carex species used in this study. For our study, no samples were used from site 7 and only samples from site 13.1 were used. Note that the eastern sites, such as NE Taymyr Peninsula (13), were actually sampled earlier in the summer (~ Julian day 160) compared to the Kanin Peninsula (2) that was sampled on Julian day 213). The specific sites are listed in Table 1
At each site, samples of one or two dominating Carex taxa were collected from mesic tundra for later analysis in the laboratory. The targeted taxa were Carex ensifolia Krecz. ssp. arctisibirica Yurtsev (here called C. ensifolia), C. lugens Holm, and C. stans Drej. Along 80- to 120-m transects, ten randomly chosen clonal segments were carefully removed from the soil, and preserved in a plant press. Clonal segments consisted of interconnected ramets, including vegetative juvenile (<2 years old) and mature vegetative ramets along with reproductive ramets (as indicated by the presence of an inflorescence). The material was allowed to air-dry and stored under cool temperatures.
In the autumn of 1999, replicate clonal segments were examined from all sites, and interconnected juvenile, mature, and reproductive ramets were severed. C. ensifolia samples available for laboratory analysis were from sites 2, 3, 4, 5, 6, 8, 9, 10, and 12a (Fig. 1, Table 1). C. stans samples were collected at sites 12b, 13, 14, and 16a, and C. lugens samples from sites 16b and 17 (Fig. 1, Table 1). Our sample size (N) was equal to 5 for all sites except sites 3, 4, 6, and 10, where N was equal to 4. Intact plant material with discernible ramet sequences was not available from all ten replicates because the material had been used in companion studies or was broken during storage and transport (Stenstrom 2000).
Green leaf material was separated from dead leaves and oven-dried at 60°C for 48 h, ground to a fine powder, and analyzed by combustion for total C and N on a Carlo Erba CHN analyzer. The combusted samples were then passed into an Isoprime Stable Isotope Mass Spectrometer under continuous flow, and the 13C/12C and 15N/14N ratios of the CO2 and N2 gas, respectively, were measured (Farquhar et al. 1989; Welker et al. 1993; Hogberg 1997; Robinson 2001). The isotopic ratios were then converted to “delta units” (δ) in parts per thousand (‰) following the formula of Farquhar et al. (1982, 1989):
where R is the molar ratio of heavy to light isotopes (13C/12C, 15N/14N). The standard was PDB for carbon and atmospheric air for nitrogen. Leaf carbon isotope discrimination (Δ, LCID) (Welker et al. 1993; Alstad et al. 1999; Gebauer and Ehleringer 2000) values were calculated from the leaf carbon isotope ratios using the formula of Farquhar and Lloyd (1993) with the δ13C of the atmosphere assumed to be −8.0‰.
In this study, we used a General Linear Model (GLM) (SAS 1999) to test whether LCID, total leaf N and leaf δ15N values were significantly (P<0.05) different between sites and ramet age class, and their interaction. We also used GLM procedures to test whether there was a significant (P<0.05) covariant (sample date) effect on LCID, total leaf N, and leaf δ15N values since we were not able to simultaneously sample all the sites. We used a least square means separation test to evaluate significant (P<0.05) differences in ecophysiological traits when there was a main effect of site (e.g., for LCID and leaf δ15N), and when there was a site by ramet age class interaction (e.g., for % N). We used a correlation analysis (Pearson’s r) (SAS 1999) to examine whether Carex plants or Carex ramet age classes responded to the range of abiotic and soil properties that exist along the natural gradient of environmental conditions found among our sampling locations. The abiotic parameters used in our analysis were mean annual temperature (MAT), mean July temperature (MJT), mean annual precipitation (MAP) (Jónsdóttir et al. 1999), soil temperature at the time of our plant collections (ST, 10 cm), soil organic carbon (SOC), and depth of thaw at time of collection (DOT) (Goryachkin et al. 1994; Christensen et al. 1999). Because the same plants were used in the evaluation of growth (shoot height—Sheight), and anatomical traits (stomatal size—Stsize and stomatal density—Stdensity), we were also able to examine whether LCID, N content, and δ15N values were correlated with these parameters (Stenstrom 2000).
Results
Environmental conditions
The environmental conditions across the study sites ranged from being relatively warm and wet in the southern tundra sites (Kanin Peninsula, site 2 and Pechora Bay, site 4) (Table 1) to being colder and drier at sites that were farther east and north, such as Taymyr Peninsula (site 10) and Wrangel Island (site 17). These abiotic parameters have been measured at these sites for >10 years, and have been reported and published elsewhere by Goryachkin et al. (1994), and are thus suitable for our analysis. In addition to long-term abiotic conditions, a series of measurements were taken at the time of plant-material collection and trace gas measurements (Christensen et al. 1999; Jonsdottir et al. 1999). While these measurements are only single point-in-time, they are our best estimates of site-site variation in conditions and appear to reflect general trends that would be expected based on latitude and longitude.
Soil temperatures were warmest at Kanin Peninsula and Kotelny Island, averaging ~8°C when the field party visited the sites, while temperatures were coldest at Wrangel Island, NE Taymyr, and Chelyuskin, averaging ~0.5°C (see Christensen et al. 1999). The soil organic-matter contents were almost 100% at the Kanin Peninsula site, ~ 60% at the Kolyma site, and <10% at the Chelyuskin site. The depth of thaw ranged from 100 cm at Kolguyev Island to <30 cm at the NE Taymyr location.
Leaf Carbon Isotope Discrimination
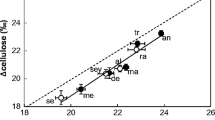
The LCID of Carex plants exhibited significant (F=2.11, P<0.022) site differences even when considering the significant (F=6.2, P<0.018) sampling date covariant (Table 2). Carex plants at NE Taymyr Peninsula (10), NW Taymyr Peninsula (8), Faddeyevsky Island (13), and Indigirka Delta (14) all had LCID values that were generally higher (21.8‰ average) than those of plants from the other populations, especially populations at the Yana Delta (12) that had values of 19.6‰ (Fig. 2A).
Population differences in the leaf carbon isotope discrimination (LCID) (A), total leaf N (B) content, and leaf δ15N values (C) of Carex plants sampled along the Eurasian Coastal Arctic. The LCID and the leaf δ15N values were significantly different between populations, even when the covariant (date of sampling) was considered, though the leaf N content exhibited a significant (P<0.05) population by ramet age class interaction and, thus, a mean separation test was not performed to distinguish population differences. Each value represents the mean and one standard error. N=5 for all sites, except for sites 3, 4, 6, and 10 where N=4
Leaf N content
The leaf N content of Carex plants exhibited a significant (F=1.92, P<0.036) site by ramet age class interaction, and when the covariant (sample date) was considered, only the N content of juvenile and mature ramets was significantly (P<0.02) different between sites (Figs. 2B, 3). Juvenile ramets from the NW Taymyr (8), Chelyuskin (9), Faddeyevsky (13), and Ayon Island (16a, 16b) sites had the highest amount of leaf N, while plants from the West Yamal (5) and North Yamal (6) sites had the lowest (Fig. 3A). The leaf N content of mature ramets was consistently highest in the Carex plants from the Chelyuskin (9) and the NW Taymyr Peninsula (10) sites (Fig. 3B).
Leaf N content of Carex populations for juvenile (A), mature (B), and reproductive (C) ramet age classes collected along the Eurasian Coastal Arctic. Sites are the same as in Fig. 1
Leaf δ15N values
The leaf δ15N values of Carex plants were significantly (F=8.2, P<0.001) different between sites (Table 2). The leaf δ15N values of plants at the Yana Delta (12a), Faddeyevsky (13), and Indigirka Delta (14) were significantly higher (4.2‰ average) than those of plants from other sites (Fig. 2C). The leaf δ15N values were most depleted in plants from Kanin Peninsula (2), Chelyuskin (9), and Ayon Island (16a) sites where the values averaged 0.8‰. These similarities in N mineral nutrition between C. ensifolia and C. stans further substantiate the ecological and physiological similarity across the Carex species sampled in this study.
Ecophysiological traits and responses to the Eurasian environmental gradient
Overall response
Carex LCID was inversely (P<0.01) correlated with mean annual temperature and stomatal density (Table 3), and to a lesser extent, with the depth of thaw. Leaf carbon isotope discrimination was typically enriched in Carex leaves of plants from colder sites and was depleted at sites where temperatures were warmer and possibly drier. We also found that LCID was significantly (P<0.05) correlated with shoot height, indicating that under conditions of higher rates of gas exchange, plant growth is greater, and the converse.
Correspondingly, Carex leaf δ15N values were inversely (P<0.05) correlated with mean annual precipitation. Under wetter conditions, leaf δ15N values were depleted compared to plants that were found under lower rainfall conditions across the Eurasian Coastal Arctic. In addition, lower leaf δ15N values were found in Carex leaves of plants growing in soil where organic matter content was higher. Plant N content was inversely correlated with mean July temperatures (P<0.05) and with stomatal density.
Ramet age class responses
Only in juvenile vegetative ramets did LCID appear to be responsive to the environment (Table 4). Leaves of juvenile ramets had consistently higher LCID under colder conditions, and their LCID was significantly (P<0.01) correlated with stomatal size and inversely correlated with stomatal density. Neither the N content nor the leaf δ15N values of juvenile ramets were correlated with any of our environmental, growth, or anatomical traits. In contrast, the leaf N content of mature ramets was inversely correlated with air temperature, depth of thaw, and stomatal density.
Discussion
The isotopic attributes we measured in Carex plants along the Eurasian Coastal Arctic are within the range of values reported for Carex and other tundra species in the Arctic (Welker et al. 1993; Michelsen et al. 1996, 1998; Nadelhoffer et al. 1996). However, we discovered several important findings regarding Carex site (population) and ramet generation differences in leaf isotopic traits across 140° of longitude and 10° of latitude. Carex plants from different sites along the Eurasian Coastal Arctic from mesic habitats did not have identical gas exchange attributes as measured by LCID. An LCID range of ~3.0‰ represents a difference of ~45 ppm in the intercellular CO2 concentrations of Carex leaves between our two extreme populations (NE Taymyr and Yana Delta—both C. ensifolia). The differences in physiology between these Carex populations may be due to differences in photosynthetic capacity, their rates of stomatal conductance, or a combination of both (Ehleringer et al. 1991; Rice et al. 1993; Alstad et al. 1999). Since we found that Carex LCID values were positively correlated with leaf N content (r=0.30, P<0.09—data not shown), it is likely that photosynthetic processes (carboxylation) are partially involved in the differences between the populations we observed. However, Carex LCID is reduced under warmer temperatures (negative correlation—P<0.01), and this may be the result of decreases in stomatal conductance in response to drier soils, larger vapor pressure deficits, or increases in the rates of transpiration. Decreases in stomatal conductance in C. stans have been reported by Nosko and Courtin (1995) over the course of the summer as soil and air drought progressed on Devon Island, NWT (latitudes similar to those we sampled from in this study). Thus, differences in the rates of stomatal conductance likely account for the majority of population (site) variations in LCID across the Eurasian Coastal Arctic.
We found the range of leaf δ15N values (4.8‰–0.05‰) to be almost identical to those for Carex species in the sub-arctic of Sweden, low-arctic in Alaska, high-arctic on E. Greenland, and for other graminoid species in Siberia (Nadelhoffer et al. 1996; Michelsen et al. 1998). The leaf δ15N values of Carex plants from all sites were positive, which is indicative of non-mycorrhizal species that use inorganic nitrogen as their N source (Michelsen et al. 1996, 1998). The Eurasian plants do, however, exhibit an inverse relationship between leaf δ15N values, mean annual precipitation, and soil organic carbon content. This correlation suggests that when water is in greater abundance, leaf δ15N values are depleted, indicating either a change in the proportion of ammonium and nitrate used by Carex plants or a change in the ratio of microbial immobilization to mineralization, and a subsequent shift in the δ15N values of the soil N pool used by Carex plants (Handley and Raven 1992).
The responsiveness of Carex LCID and plant and soil nitrogen processes (δ15N values) to precipitation and temperature are especially germane to the changes being observed in Eurasian climatology (Peterson et al. 2002; Polyakov et al. 2002). It is now apparent that Eurasia is getting wetter in summer and warmer throughout the year which, based on our analysis, will translate into different rates of leaf gas exchange, as well as plant N sources and/or soil mineralization/immobilization processes (Schimel et al. 2004). Warmer temperatures will likely reduce LCID during the growing season, but this effect may be counteracted by greater precipitation; the net effect of these climate scenarios are uncertain and merit further investigation with field manipulations (Welker et al. 1993). Simultaneously, greater rainfall in summer will result in increased availability of nitrate and its use by Carex plants. This increase would ameliorate, to some degree, the inherent nutrient limitations that curtail growth, biomass production, and carbon sequestration in sedge-dominated systems (Shaver et al. 1998). Collectively, the warming and wetter conditions developing in Eurasia will have cascading affects on Carex ecosystem function and structure, though field-based manipulation studies along with long-term monitoring will be necessary to fully elucidate the changes in this important vegetation type (Serreze et al. 2000; Shaver et al. 2000).
The modular nature of clonal organisms is expressed in our findings through the differential sensitivity of each ramet age class to the environment. Juvenile vegetative ramets exhibited the strongest changes in gas exchange (LCID) along this Eurasian environmental gradient. This response indicates that the youngest portions of Carex clones that are undergoing the greatest rates of growth have the ability to significantly alter photosynthesis and/or stomatal conductance in response to environmental fluctuations. Juvenile ramets of Carex are typically at the leading edge of individual clones and thus, are most likely to encounter variance in soil and canopy microclimate (Jónsdóttir and Callaghan 1988). Consequently, effective control of carbon and water exchange in juvenile ramets provides a means through which Carex clones can retain their resource exploitation capacity, even in the face of competition with other species or adjacent clones and with microclimatic variations in soil moisture and canopy conditions.
In contrast, gas exchange (LCID) attributes of mature vegetative or reproductive ramets were largely insensitive to the environmental gradient, maintaining a high degree of buffering capacity within Carex clones in the face of daily, seasonal, and annual changes in temperature and precipitation. This dichotomy of gas exchange patterns within a Carex clone may confer several ecological advantages. For instance, the mature and reproductive ramets provide consistency in carbon acquisition and water use, securing the existing space from competitors. However, juvenile ramets may be opportunistic in their carbon acquisition and water use when conditions are favorable, facilitating the expansion of clones. These traits are physiologically and ecologically important, as mature vegetative ramets in Carex clones are the primary carbon-assimilating generation. They supply carbon to both juvenile vegetative ramets and older, non-assimilating generations that no longer have active leaf tissue, but retain roots that may supply the clone with water and nutrients (Jónsdóttir and Callaghan 1988, 1989; Welker et al. 1995; Brooker et al. 1999).
The total leaf N content of mature vegetative ramets responded to our environmental gradient, with leaf N contents being highest where temperatures were coldest. This inverse relationship indicates that under cold temperatures, growth may be lower and leaf N dilution less. Previous studies by Körner (1989) indicated that at higher altitudes and latitudes, where growing seasons were shorter and colder than lower altitudes and latitudes, leaf N was significantly higher in herbaceous and graminoid species. Thus, even along the temperature gradient that exists across the Eurasian Coastal Arctic, portions of Carex clones are exhibiting fundamental plant nutritional patterns that have been found at the global scale. However, this relationship is ramet generation-specific.
References
Alerstam T, Jonsson PE (1999) Ecology of tundra birds: patterns of distribution, breeding and migration along the Northeast Passage. Ambio 28:212–224
Alstad KP, Welker JM, Williams S, Trilica MJ (1999) Carbon and water relations of Salix monticola in response to winter browsing and changes in surface water hydrology: an isotopic study using δ13C and δ18O. Oecologia 120:375–385
Ammann M, Siegwolf R, Pichlmayer F, Suter M, Saurer M, Brunold C (1999) Estimating the uptake of traffic-derived NO2 from 15N natural abundance in Norway spruce needles. Oecologia 118:124–131
Bliss LC, Matveyeva NV (1992) Circumpolar arctic vegetation. In: Chapin FS, Jeffries RL, Reynolds JF, Shaver GR, Svoboda J (eds) Arctic ecosystems in a changing climate: an ecophysiological perspective. Academic, San Diego, pp 59–90
Brooker RW, Callaghan TV, Jonasson S (1999) Nitrogen uptake by rhizomes of the clonal sedge Carex bigelowii: a previously overlooked nutritional benefit for rhizomatous growth. New Phytol 142:35–48
Callaghan TV (1976) Growth and population dynamics of Carex bigelowii in an alpine environment. Strategies of growth and population dynamics of tundra plants 3. Oikos 27:402–413
Callaghan TV, Carlsson BA (1996) Impacts of climate change on demographic processes and population dynamics in arctic plants. In: Oechel WC, Callaghan TV, Gilmanov T, Holten JI, Maxwell B, Molau U, Svinbjornsson B (eds) Global change and Arctic terrestrial ecosystems. Springer, Berlin Heidelberg New York, pp 129–153
Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA (1995) Responses of arctic tundra to experimental and observed changes in climate. Ecology 76:694–711
Christensen TR, Jonasson S, Callaghan TV, Havstrom M, Livens FR (1999) Carbon cycling and methane exchange in Eurasian Tundra Ecosystems. Ambio 38:239–244
Christensen TR, Friborg T, Sommerkorn M, Kaplan J, Illeris L, Soegaard H, Nordstoem C, Jonasson S (2000) Trace gas exchange in a high-arctic valley: variations in CO2 and CH4 flux between tundra vegetation types. Global Biogeochem Cycles 14:701–714
Danell K, Erlinge S, Hogstedt G, Hasselquist D, Olofsson EB, Seldal T, Svesson M (1999) Tracking past and ongoing leming cycles on the Eurasian Tundra. Ambio 28:225–229
Dawson TE, Bliss LC (1993) Plants as mosaics: leaf-, ramet-, and gender-level variation in the physiology of the dwarf willow, Salix arctica. Funct Ecol 7:293–304
Dodd MB, Lauenroth WK, Welker JM (1998) Differential water resource use by herbaceous and woody plants in a shortgrass steppe community. Oecologia 117:504–512
Ehleringer JR, Cooper TA (1988) Correlations between the carbon isotope ratio and microhabitat in desert plants. Oecologia 76:562–566
Ehleringer JR, Phillips SL, Schuster SF, Sandquist DR (1991) Differential utilization of summer rains by desert plants. Oecologia 88:430–434
Emmerton KS, Callaghan TV, Jones HE, Leake JR, Michelsen A, Read DJ (2001a) Assimilation and isotopic fractionation of nitrogen by mycorrhizal fungi. New Phytol 151:503–511
Emmerton KS, Callaghan TV, Jones HE, Leake JR, Michelsen A, Read DJ (2001b) Assimilation and isotopic fractionation of nitrogen by mycorrhizal and nonmycorrhizal subarctic plants. New Phytol 151:513–524
Farquhar GD, Lloyd J (1993) Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In: Ehleringer J, Hall A, Farquhar G (eds) Stable isotopes and plant carbon-water relations. Academic, San Diego, pp 47–70
Farquhar GD, Ball MC, Caemmerer S, Roksadic Z (1982) Effect of salinity and humidity on the δ13C value of halophytes: evidence for diffusional isotope fractionation determined by the ratio of intercellular/atmospheric partial pressure of CO2 under different environmental conditions. Oecologia 52:121–124
Farquhar GD, Ehleringer JR, Hubick K (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Gebauer G, Giesemann A, Schultze E-D, Jager HJ (1994) Isotope ratios and concentrations of sulfur and nitrogen in needles and soils of Picea abies stands as influenced by atmospheric deposition of sulfur and nitrogen compounds. Plant Soil 164:267–281
Gebauer RLE, Ehleringer JR (2000) Water and nitrogen uptake patterns following moisture pulses in a cold desert community. Ecology 81:1415–1424
Goryachkin SV, Zlotin RI, Tertitsky GM (1994) Diversity of natural ecosystems in the Russian Arctic: a guidebook. Reprocentralen, Lund University
Handley LL, Raven JA (1992) The use of natural abundances of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ 15:965–985
Hedberg D, Hjort C, Sonesson M (1999) The Northwest Passage: an ecological approach. Ambio 38:210–211
Henry GHR, Svoboda J, Freedman B (1990) Standing crop and net production of sedge meadows on an ungrazed polar desert oasis. Can J Bot 68:2660–2667
Hertefeldt TD, Jónsdóttir IS (1999) Extensive physiological integration in intact clonal systems of Carex arenaria. J Ecol 87:258–264
Hirose T, Lambers H, Konings H, Van Der Werf A (1989) Modelling of respiration: effect of variation in respiration of plant growth in two Carex species. Funct Ecol 3:655–665
Hogberg P (1997) 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Jonasson S (1983) Nutrient content and dynamics in north Swedish tundra areas. Holarct Ecol 6:295–304
Jonasson S (1989) Implications of leaf longevity, leaf nutrient reabsorption, and translocation for the resource economy of five evergreen species. Oikos 56:121–131
Jones MH, Fahnestock JT, Walker DA, Walker MD, Welker JM (1998) Carbon dioxide fluxes in moist and dry arctic tundra during the snow-free season: responses to increases in summer temperature and winter snow accumulation. Arct Alp Res 30:373–380
Jones MH, Fahnestock JT, Stahl PD, Welker JM (2000) A note on summer CO2 flux, soil organic matter and soil microbial biomass from different high arctic ecosystem types in northwestern Greenland. Arct Antarct Alp Res 32:104–106
Jónsdóttir IS, Callaghan TV (1988) Interrelationships between different generations of interconnected tillers of Carex bigelowii. Oikos 52:120–128
Jónsdóttir IS, Callaghan TV (1989) Localized defoliation stress and the movement of 14C photoassimilates between tillers of Carex bigelowii. Oikos 54:211–219
Jónsdóttir IS, Callaghan TV (1990) Interclonal translocation of ammonium and nitrate nitrogen in Carex bigelowii Torr. Ex Schwein. Using 15N and nitrate reductase assays. New Phytol 114:419–428
Jónsdóttir IS, Watson MA (1997) Extensive physiological integration: an adaptive trait in resource-poor environments? In: De Kroon H, van Groenendale J (eds) The ecology and evolution of clonal plants. Backhuys, Leiden, pp 109–136
Jónsdóttir IS, Virtanen R, Karnefelt I (1999) Large-scale differentiation and dynamics in tundra plant populations and vegetation. Ambio 38:230–238
Körner Ch (1989) The nutritional status of plants from high altitudes. A worldwide comparison. Oecologia 81:379–391
Lambers H, Van Der Werf A (1988) Variation in the rate of root respiration of two Carex speices: a comparison of four related methods to determine the energy requirements for growth, maintenance, and ion uptake. Plant Soil 111:207–211
McGraw JB, Fetcher N (1992) Response of tundra plant populations to climatic change. In: Chapin FS, Jeffries RL, Reynolds JF, Shaver GR, Svoboda J (eds) Arctic ecosystems in a changing climate: an ecophysiological perspective. Academic, San Diego, pp 359–376
Michelsen A, Schmidt IK, Jonasson S, Quarmby C, Sleep D (1996) Leaf 15N abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal, and non-and arbuscular mycorrrhizal species access different sources of soil N. Oecologia 105:53–63
Michelsen A, Quarmby C, Sleep D, Jonasson S (1998) Vascular plant 15N natural abundance in heath and forest tundra ecosystems is closely correlated with presence and type of mycorrhizal fungi in roots. Oecologia 115:406–418
Murray DF (1994) Floristics, systematics, and the study of arctic vegetation—a commentary. J Veg Sci 5:777–780
Nadelhoffer K, Shaver GR, Fry B, Giblin A, Johnson L, McKane R (1996) 15N natural abundances and N use by tundra plants. Oecologia 107:386–394
Nosko P, Courtin GM (1995) The water relations of Carex stans in wet sedge-moss tundra at a high arctic oasis, Devon Isl., NWT Canada. Arct Alp Res 27:137–145
Peterson BJ, Holmes RM, McClelland JW, Vörösmarty CJ, Shiklomanov AI, Lammers RB, Rahmstorfs S (2002) Increasing river discharge to the Arctic Ocean. Science 298:2171–2173
Polyakov I, Akasofu I, Bhatt U, Colony R, Ikeda M, Makshtas A, Swingley C, Walsh D, Walsh J (2002) Trends and variations in the arctic climate system. EOS 83:547–548
Rice KJ, Gordon DR, Hardison JL, Welker JM (1993) Phenotypic variation in seedlings of a “keystone” tree species (Quercus douglasii): the interactive effects of acorn source and competitive environment. Oecologia 96:536–547
Robinson D (2001) 15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
SAS Institute (1999) SAS/STAT User’s Guide version 6, 4th edn. SAS Institute, Cary, N.C.
Schimel JP, Bilbrough CB, Welker JM (2004) The effect of changing snow cover on year-round soil nitrogen dynamics in Arctic tundra ecosystems. Soil Biol Biochem (in press)
Serreze MC, Walsh JE, Chapin FS, Osterkamp T, Dyurgerov M, Romanovsky V, Oechel WC, Morison J, Zhang T, Barry RG (2000) Observational evidence of recent change in the northern high-latitude environment. Clim Change 46:159–207
Shaver GR, Chapin FS, Billings DW (1979) Ecotypic differentiation of Carex aquatilis in relation to ice-wedge polygons in the Alaskan coastal tundra. J Ecol 67:1025–1046
Shaver GR, Billings DW, Chapin FS, Giblin AE, Nadelhoffer KJ, Oechel WC, Rastetter EB (1992) Global change and the carbon balance of arctic ecosystems. BioScience 42:433–441
Shaver GR, Johnson, LC, Cades DH, Murrary G, Laundre JA, Rastetter EB, Nadelhoffer KJ, Giblin AE (1998) Biomass and CO2 flux in wet sedge tundra: responses to nutrients, temperature, and light. Ecol Appl 68:75–97
Shaver GR, Canadell J, Chapin FS, Gurevitch J, Harte J, Henry G, Ineson P, Jonasson S, Melillo J, Pitelka L, Rustad L (2000) Global warming and terrestrial ecosystems: a conceptual framework for analysis. BioScience 50:871–882
Stenstrom A (2000) Morphological variation in clonal sedges (Carex) in the Eurasian arctic: effects of taxonomy, ecotypes, lemmings, and climate. From pollination to variation-reproduction in arctic clonal plants and the effects of simulated climate change. PhD Thesis, Botanical Institute, Goteborg University
Stenstrom A, Jónsdóttir IS, Augner M (2002) Genetic and environmental effects on morphology in clonal sedges in the Eurasian Arctic. Am J Bot 89:1410–1421
Vourlitis GL, Oechel WC, Hastings SJ, Jenkins MA (1993) A system for measuring in situ CO2 and CH4 flux in unmanaged ecosystems: an arctic example. Funct Ecol 7:369–379
Welker JM, Briske DD (1992) Clonal biology of the temperate, caespitose, graminoid Schizchyrium scoparium: a synthesis with reference to climate change. Oikos 63:357–365
Welker JM, Rykiel EJ, Briske DD, Goeschl JD (1985) Carbon import among vegetative tillers within two bunchgrasses: assessment with carbon-11 labeling. Oecologia 67:209–212
Welker JM, Briske DD, Weaver RW (1987) Nitrogen-15 partitioning within a three generation tiller sequence of the bunchgrass Schizachyrium scoparium: response to selective defoliation. Oecologia 24:330–334
Welker JM, Briske DD, Weaver RW (1991) Intraclonal nitrogen allocation in the bunchgrass Schizachyrium scoparium: an assessment of the physiological individual. Funct Ecol 5:433–440
Welker JM, Wookey PA, Parsons AN, Callaghan TV, Press MC, Lee JA (1993) Leaf carbon isotope discrimination and demographic responses of Dryas octopetala to water and temperature manipulations in a high arctic polar semi-desert, Svalbard. Oecologia 95:463–470
Welker JM, Heaton THE, Sprio B, Callaghan TV (1995) Indirect effects winter climate on the δ13C and the δD characteristics of annual growth segments in the long-lived, arctic clonal plant Cassiope tetragona. Palaeoclim Res 15:105–120
Welker JM, Molau U, Parsons AN, Robinson C, Wookey PA (1997) Response of Dryas octopetala to ITEX manipulations: a synthesis with circumpolar comparisons. Global Change Biol 3:61–73
Welker JM, Fahnestock JT, Jones MH (2000) Annual CO2 flux from dry and moist arctic tundra: field responses to increases in summer temperature and winter snow depth. Clim Change 44:139–150
Wookey PA, Parsons AN, Welker JM, Potter JC, Callaghan TV, Lee JA, Press MC (1993) Comparative responses of subarctic and high arctic ecosystems to simulated climate change. Oikos 67:490–502
Wookey PA, Robinson CH, Parsons AN, Welker JM, Press MC, Callaghan TV, Lee JA (1995) Environmental constraints on the growth and performance of Dryas octopetala ssp. at a high arctic polar semi-desert. Oecologia 102:478–489
Acknowledgements
We would like to thank all those who contributed to the implementation and success of Swedish-Russian Tundra Ecology-94, including the Swedish Polar Research Secretariat, the Swedish Natural Science Research Council, and the Russian Academy of Sciences. We also wish to thank those that contributed to the Eurasian Carex Project, including M. Augner, T. Fagerström, and A. Stenström. We also acknowledge the support of the National Science Foundation (NSF), Office of Polar Programs (OPP-9907356). This research is also a contribution to the International Tundra Experiment (ITEX) and its goal of understanding the ecology and biology of arctic plants and ecosystems. We also wish to thank Dr. James Zumbrunnen of the Colorado State University Statistics Department for statistical advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Welker, J.M., Jónsdóttir, I.S. & Fahnestock, J.T. Leaf isotopic (δ13C and δ15N) and nitrogen contents of Carex plants along the Eurasian Coastal Arctic: results from the Northeast Passage expedition. Polar Biol 27, 29–37 (2003). https://doi.org/10.1007/s00300-003-0562-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-003-0562-4