Abstract
Key message
Extensive crosstalk exists among ABA and different phytohormones that modulate plant tolerance against different abiotic stress.
Being sessile, plants are exposed to a wide range of abiotic stress (drought, heat, cold, salinity and metal toxicity) that exert unwarranted threat to plant life and drastically affect growth, development, metabolism, and yield of crops. To cope with such harsh conditions, plants have developed a wide range of protective phytohormones of which abscisic acid plays a pivotal role. It controls various physiological processes of plants such as leaf senescence, seed dormancy, stomatal closure, fruit ripening, and other stress-related functions. Under challenging situations, physiological responses of ABA manifested in the form of morphological, cytological, and anatomical alterations arise as a result of synergistic or antagonistic interaction with multiple phytohormones. This review provides new insight into ABA homeostasis and its perception and signaling crosstalk with other phytohormones at both molecular and physiological level under critical conditions including drought, salinity, heavy metal toxicity, and extreme temperature. The review also reveals the role of ABA in the regulation of various physiological processes via its positive or negative crosstalk with phytohormones, viz., gibberellin, melatonin, cytokinin, auxin, salicylic acid, jasmonic acid, ethylene, brassinosteroids, and strigolactone in response to alteration of environmental conditions. This review forms a basis for designing of plants that will have an enhanced tolerance capability against different abiotic stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike animals, plants are sessile organism and are, thus, constantly challenged by unfavorable environment, such as drought, salinity, fluctuant temperature, and metal toxicity that significantly hamper their survival capability and longevity. The negative effects of abiotic stress impact the plants at all phase of their life cycle which are evident at both molecular and physiological level. According to the report of dos Reis et al. (2012), around 90% of the cultivable land is affected by one or more abiotic stress (drought, high salinity, cold, and heat) which accounts for about 70% yield loss of the major crops (Mantri et al. 2012). Qadir et al. (2014) reported that during last two decades (1990–2013), salinity of the arable land has been increased by 37%. Additionally, enhanced evapotranspiration due to change in global precipitation patterns and global warming has increased the severity and frequency of drought stress (Dai 2011). Recently, in a meta-analysis study, Raftery et al. (2017) predicted that the average temperature of the earth is likely to be increased by 2.0 to 4.9 °C. Anthropogenic activities can further worsen the condition through discharge of the contaminated water and solid wastes in cropland that eventually leads to higher accumulation of heavy metals. Accumulation of such toxic elements above the safety level not only limits the productivity of crops, but also causes serious risk to human health (Rehman et al. 2018). Estimates based on the integration of climate change and crop yield model further suggest that in coming days, the severity of the damage due to abrupt change in environmental condition will further worsen the productivity of major crops such as maize, rice, and wheat that may lead to serious consequences for food security of such a vast and still expanding population of the world (Tigchelaar et al. 2018). To cope with such harsh conditions, major phytohormones such as abscisic acid (ABA), auxin, cytokinin, ethylene, gibberellin, jasmonic acid, salicylic acid, and melatonin cumulatively play a major role in ameliorating the negative effects of abiotic and biotic stress in plants, contributing toward their higher tolerance level (Verma et al. 2016).
All such forms of abiotic stress, as mentioned above, hinder the growth, productivity, and development of major crops. Severe abiotic stress leads to reduction in leaf area, wilting and abscission of leaf, formation of reactive oxygen species (ROS) that causes peroxidation of lipid membrane, resulting in higher leakage of electrolytes from cells and formation of cytotoxic metabolites, inactivation of enzymes, and reduction in leaf pigments which lower the overall viability of cells. Generation of tolerance level in plants against harsh environmental condition is one of the most complex mechanisms. Tolerance against abiotic stress is contributed via multiple genes, located at various loci and is, thus, a multigenic trait (Yamaguchi-Shinozaki and Shinozaki 2006). Hence, mechanisms that might induce the stress tolerance capability of plants are quite complicated and cumbersome and have long been the focus of intensive research of plant breeders and researchers.
ABA is the key phytohormone, which regulates a wide range of physiological process (starting from seed germination to stomatal regulation) and simultaneously controls plant growth and development by regulating the formation of various protective metabolites that enable the plants to combat the damaging effects of stressed environment, and hence accordingly designated as ‘universal stress hormone’ (Singh et al. 2022). Initial studies revealed that ABA plays a pivotal role in leaf abscission and seed dormancy and was, thus, named as abscisin II and dormin (Hewage et al. 2020). However, further studies confirmed that ABA is not directly involved in senescence; rather, it regulates several processes that lead to senescence (Xu et al. 2020). With the progress of time, various studies focused on the crucial role played by ABA in the mitigation of negative effects of abiotic stress in plants by gene expression modulation, stomatal closure, formation of protective metabolites, and other adaptive biochemical processes (Bulgakov and Koren 2022; Kumar et al. 2019; Vishwakarmaet al. 2017). The level of ABA varies within plant species and tissues that contribute toward controlling fruit ripening, growth inhibition, root structure organization, and seedling development (Kozaki and Aoyanagi 2022). Although ABA controls a wide range of physiological processes, the growth, development, and tolerance against harsh condition are not the result of single hormonal action. Abiotic stress response pathways are mostly connected via some common elements generally referred to as ‘nodes’ or connections of crosstalk (Kundu and Gantait 2017). The term ‘crosstalk’ is generally used to denote situation where different signaling pathways have one or more common intermediates/components or common outputs. Physiological response of plants against stressed environment via alteration of various morphological, anatomical, cytological, and biochemical processes arises due to antagonistic or synergistic interaction among various phytohormones (Muller and Munne-Bosch 2021). Thus, tolerance in plants toward abiotic stress is an intricate phenomenon that involves various cellular, molecular, and metabolic aspects (Ronen et al. 2019). Traditional breeding techniques are not efficient enough in developing abiotic stress tolerance in plants. In addition, continuous rise in global temperature further intensifies the effects of such harsh conditions which again limits the success of breeding techniques. Therefore, to improve the plant performance and economic returns and to fulfill the food demand of the continuous growing population of the world, it is important to investigate new avenues for inducing the yield of crops amidst the onslaughts of rapid changing climate. However, we still have limited knowledge on complex regulatory networks where multiple hormone pathways interact and influence plant defence responses. The present review aims to present an updated snapshot of the signaling crosstalk operated by ABA for generating abiotic stress tolerance in plants. ABA acts as an endogenous messenger in regulating the water status of plants that helps the plants to respond accordingly in stressed conditions like salinity, drought, and cold. Plants respond to harsh conditions by mechanisms which include both ABA-dependent and ABA-independent processes (Swamy and Smith 1999). The regulation of homeostasis and transportation of ABA along with its signaling pathway has also been highlighted to decode the effective mechanisms lying behind these crosstalk pathways during plant stress response.
Homeostasis and transportation of ABA in plants
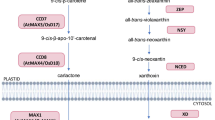
ABA is a major sesquiterpenoid (C15H20O4) hormone biosynthesised in plants. ABA homeostasis is mostly controlled in plants by its formation, transportation, catabolism, and conjugation with other metabolites (Roychoudhury et al. 2013). There are at least two pathways for the biosynthesis of ABA: fungi directly produce ABA from farnesyl diphosphate, whereas plants indirectly produce ABA from β-carotene (Finkelstein 2013). In most phytopathogenic fungi, cyclization of farnesyl diphosphate into 2Z,4E-α-ionylidene ethane marks the beginning of formation of ABA. Recently, Izquierdo-Bueno et al. (2018) showed via gene inactivation, complementation, and chemical analysis, that BcStc5/BcAba5 is the key enzyme responsible for ABA biosynthesis in fungi. Plants biosynthesize ABA in response to abiotic stress mainly by upregulating the activity of enzymes responsible for the formation of ABA from β-carotene (Roychoudhury and Basu 2012). In Arabidopsis, the biosynthesis of ABA begins with the epoxidation of zeaxanthin to produce all-trans-violaxanthin by the catalytic activity of the enzyme, zeaxanthin epoxidase (encoded by AtABA1) (Nambara and Marion-Poll 2005). North et al. (2007) reported that the conversion of violaxanthin to neoxanthin is performed by AtABA4. Next, the cleavage of cis-isomers of neoxanthin and violaxanthin to a C-15 compound is catalyzed by nine-cis-epoxycarotenoid dioxygenase (NCED) enzyme which controls the entire ABA biosynthesis process by regulating the rate-limiting step. All the steps mentioned above occur in plastids, while the last two steps, i.e., conversion of xanthine to abscisic aldehyde by the catalytic activity of AtABA2, followed by oxidation of abscisic aldehyde to ABA by the enzymatic action of abscisic aldehyde oxidase, occur in cytosol (Tan et al. 2003; Seo et al. 2004). In addition to that of de novo pathway, ABA biosynthesis can also occur in endoplasmic reticulum (ER) and vacuole by the hydrolysis of ABA glucosyl ester (ABA-GE) via the action of AtBG1 and AtBG2 (β-glucosidase homolog), respectively (Xu et al. 2012). During stressed condition, plants rapidly enhance the level of ABA via hydrolysis of ABA-GE to ABA rather than the lengthy de novo biosynthesis pathway, since it would be a challenging task for the plants to enhance the level of so many proteins by regulating their transcription and translation (Fig. 1).
Metabolism of ABA in higher plants; β-carotene serves as the precursor of ABA biosynthetic pathway. The biosynthesis of ABA begins with the epoxidation of zeaxanthin (formed from β-carotene) to produce all-trans-violaxanthin by the catalytic activity of the enzyme, zeaxanthin epoxidase, which is further converted into 9′-cis-violaxanthin and 9′-cis-neoxanthin. The cleavage of cis-isomers of neoxanthin and violaxanthin to a C-15 compound (xanthoxin) is catalyzed by nine-cis-epoxycarotenoid dioxygenase (NCED) enzyme which controls the entire ABA biosynthesis process by regulating the rate-limiting step. Xanthoxin is next converted to abscisic aldehyde by the action of ABA2. Finally, abscisic aldehyde is converted to ABA by abscisic aldehyde oxidase. ABA can also be formed from ABA glucosyl ester present in endoplasmic reticulum and vacuole by BG1 and BG2, respectively. Again hydroxylation of ABA (catalyzed by CYP707A) and conjugation with glucose, catalyzed by ABA-glucosyltransferase (ABA-GT) also occur to maintain the optimum level of ABA in plants. [Solid arrow denotes the anabolic pathway and broken arrow denotes the catabolic pathway]
To maintain the level of ABA, plants catabolize ABA via glucose conjugation and hydroxylation. In the conjugation pathway, ABA is linked with glucose by the action of ABA-glucosyltransferase. Saito et al. (2004) reported that of all the three methyl groups (C-7′, C-8′ and C-9′), hydroxylation reaction catalyzed by the protein encoded by AtCYP707A gene occurs predominantly at C-8′ position. Previously, it was thought that ABA-GE is the physiologically inactive by-product of ABA stored in lytic vacuoles; however, recent studies have shown that ABA-GE is the stored form of ABA (Fig. 1).
Earlier it was presumed that ABA is produced in almost all the tissues of plants including roots and leaves; however, recent studies have shown higher localization of ABA-producing proteins in vascular parenchyma cells which suggest that ABA is generally transported from one tissue to the other through vascular system. Various studies have shown long-distance translocation of ABA from root to leaf tissues and from xylem to guard cells for stomatal closure. Seo and Koshiba (2011) hypothesized that the protonated form of ABA is able to penetrate the lipid bilayer due to its lower pKa (4.7) which enable the long-distance translocation of ABA, i.e., ABA formed in root tissues can transport to the apoplastic space of leaf from where it can easily diffuse into the cytoplasm of leaf cells in absence of any transport. In contrast to such simple mechanism, the apoplastic pH increases significantly during stressed condition which inhibits the diffusion of ABA from apoplast to cytoplasm, suggesting the presence of an ABA transporter. Kanno et al. (2012), Kang et al. (2010) and Kuromori et al. (2010) reported the presence of three different ABA transporters in plants. Low affinity nitrate transporter AtNRT1.2 has been shown to act as ABA transporter (Kanno et al. 2012). In addition, it was observed that atait1/atnrt1.2 mutant plants showed enhanced sensitivity to exogenously applied ABA during dehydration stress, whereas contrasting observation was noted during seed germination and post-germination phases. Previously, Kang et al. (2010) reported that AtABSG40/AtPDR12 is responsible for ABA transportation which lies in concurrent to the observation of phenotype exhibited by atabcg40/atpdr12 mutants where defective stomatal closure was noted along with severe water loss. In a similar work, Kuromori et al. (2010) reported the presence of ATP-binding cassette transporter (AtABCG25) that harbors the ATP-dependent ABA efflux activity.
ABA-GE, the stored form of ABA, is also involved in long-distance translocation. Under stressed condition, the level of ABA-GE rises significantly in xylem sap which suggests its long-distance movement. Jiang and Hartung (2008) stated that the limited ability of ABA-GE to diffuse across cell membrane makes it an ideal compound to be transported to long distances.
Crosstalk of ABA with other phytohormones
Crosstalk with gibberellin
Abscisic acid and gibberellin are one pair of classic hormones that act antagonistically during regulation of several plant developmental processes such as seed dormancy, seed germination and maturation, root growth and flowering time control (Shu et al. 2016a, b; Yang et al. 2014; Wang et al. 2013). Due to such contrasting behavior, crosstalk between ABA and gibberellin is a major field of research for plant biologist. Numerous key regulators such as DELLAs and AP2-domaining containing transcription factors have been widely studied in the past. Generally, gibberellin is considered as a phytohormone which primarily regulates flowering time, developmental processes, and stem elongation; however, recent studies have demonstrated the role of gibberellin in mediating abiotic stress response in plants by controlling molecular and cellular processes (Hyun et al. 2016; Urano et al. 2017; Wang et al. 2017).
In normal situation, ABA signaling is downregulated by ubiquitination of PSY family of proteins which is regulated by gibberellin signaling pathways (Vishal and Kumar 2018). According to Tuan et al. (2018), gibberellin promotes seed germination by inhibiting dormancy, while contrasting response is carried out by ABA in promoting seed dormancy. Recently, Ali et al. (2022) reported that antagonistic behavior between ABA and gibberellin is regulated by transcription factors containing ABI4, AP2, FUS3, and LEC1 domains. ABI4 upregulates the function of NCED6 and GA2ox7 which results in higher formation of ABA and inactivation of gibberellin, respectively, that eventually results in prolonged dormancy of seeds (Shu et al. 2018). Along with this, high temperature during spring and summer decreased the dormancy of seeds by upregulating gibberellin synthesis (GA3ox1) and ABA catabolism (CYP707A2). Conversely, higher dormancy of seeds during winter season is facilitated by higher synthesis of ABA (NCED6) and gibberellin catabolism (GA2ox2) (Footitt et al. 2011). During stressed condition, the bioactive form of gibberellin is inactivated due to upregulation of GA2ox6 and GA2ox7 which results in 2β-hydroxylation, leading to DELLA repressor activation in Arabidopsis (Magome and Kamiya 2018). Guilfoyle et al. (2015) showed earlier that RGL2 (DELLA repressor of gibberellin signaling) stimulates ABI3 and ABI5 activities and ABA formation via XERICO protein. Conjugation between ABI5 and DELLA proteins is often involved in the regulation of several genes like EXPA1, MFT, and SOM that control various physiological processes in plants. Vaistij et al. (2018) reported that based on environmental condition, a negative feedback loop operates between ABI2 and DELLA proteins (RGA and RGL2) and MFT expression. RGL2 and RGA enhance the expression of MFT which in turn downregulates ABI5 expression, thus ensuring proper germination of seeds in presence of suitable environmental factors. Under heat stress, proper germination of seeds is controlled by modulating the expression of SOM genes via ABI5 and RGA interaction. Binding of RGA or ABI5 to specific motifs at the promotor region of SOM inhibits or enhances its expression. Thus, SOM protein eventually enhances or lowers the formation of ABA or gibberellin, respectively (Skubacz et al. 2016). The ABA–gibberellin interaction not only controls the dormancy, germination, and development of seeds, but also contributes to other developmental processes which are mostly promoted or suppressed by a specific set of transcription factors, controlled by ABA/gibberellin ratio. According to Shu et al. (2016a, b), the stability of ABI4 protein is directly controlled by ABA and gibberellin by modulating the activity of transcription factors for NCED6 and GA2ox7 genes, which suggest that ABI4 plays a pivotal role in ABA and gibberellin antagonism behavior during post-germination stage. In addition to ABI4, Yaish et al. (2010) reported that OsAP2-39 is also involved in regulating the ABA–gibberellin interaction in various physiological processes such as flowering time, pollen grain morphology, and size of root systems. Thus, it can be stated that seed germination is controlled by various factors that ultimately results in a complicated crosstalk network between ABA and gibberellin. The studies focusing on the interaction between ABA and gibberellins is, however, still in very early stage and requires further exploration.
Crosstalk with melatonin
Another major phytohormone which acts antagonistic to ABA is melatonin. Initially, it was just considered as an antioxidant, but recent studies have proved its role in plant growth and development and stress tolerance regulation which leads us to consider it as a master regulator similar to ABA (Arnao and Hernandez-Ruiz 2019). Melatonin is an indole compound which is derived from 5-hydroxytryptamine. Initially, tryptophan is converted into tryptamine by the catalytic activity of tryptophan decarboxylase. Again tryptamine is converted to 5-hydroxytryptamine by tryptamine 5-hydroxylase, commonly known as serotonin, which is again N-acetylated by serotonin N-acetyltransferase. N-acetyl serotonin is finally converted to melatonin by the catalytic action of acetylserotonin methyl transferase (Arnao and Hernández-Ruiz 2018). Li et al. (2019) reported that during salt stress, the level of melatonin along with gibberellin was induced and the level of ABA was reduced in Limonium bicolor which modulates the tolerance level in plants. They further concluded that the level of gibberellin was induced due to its higher biosynthesis and level of ABA was lowered due to upregulation in the expression of ABA-catabolizing genes (LbCYP707A2 and LbCYP707A1) and reduced expression of ABA biosynthesis genes (LbNCED2 and LbNCED1). In another study, Li et al. (2020a) also reported that melatonin-mediated seed germination is controlled by higher efflux of Ca2+ ions and H2O2 biosynthesis via upregulation of CAX3 (H+/Ca2+ antiporter) which results in higher gibberellin formation and ABA catabolism. Melatonin-mediated delay in leaf senescence and fruit ripening by restricting the biosynthesis of ABA was also reported earlier by Tan et al. (2019) and Liu et al. (2020a, b), respectively. Recent studies have also suggested that melatonin modulates the level of ABA which eventually induces the tolerance capability of plants. Wang et al. (2021) reported that during drought stress, melatonin regulates the level of NCED1, PYL4, AAO, and PP2Cs which in turn lowers ABA level in loquat, resulting in delayed leaf senescence. In a similar work, Li et al. (2015) showed that during drought stress, ABA-catabolising genes, viz., MdCYP707A2 and MdCYP707A1 and ABA anabolising gene, viz., MdNCED3 were upregulated and downregulated, respectively, resulting in higher catabolism of ABA in Malus species. Similarly, during heat stress, melatonin suppressed the expression of ABA-related genes (LpABI5, LpABI3, and LpNCED3) which delayed leaf senescence in Lolium perenne (Zhang et al. 2017). Another interesting study was conducted by Singh et al. (2022) where they reported that melatonin lowered the level of ABA which in turn abrogated fluoride-induced damages in rice seedlings. In contrast to the above mentioned studies, several studies have also showed positive crosstalk between melatonin and ABA, allowing alleviation of the damaging effects of abiotic stress. Based on transcriptomics study, Li et al. (2019) showed that melatonin induced the formation of ABA via enhancing the level of ABA biosynthetic genes and also inhibiting the transcription of ABA-catabolising gene which resulted in wax biosynthesis, thus conferring higher tolerance level against drought stress in watermelon. Similarly, Fu et al. (2017) also showed that exogenous application of melatonin triggers the level of ABA in Elymus nutans. ABA–melatonin synergistic effects was also reported by Banerjee and Roychoudhury (2019), where they showed that during fluoride toxicity, the level of ABA was induced on exogenous application of melatonin in rice seedlings. They observed that induced ABA level was attributed to upregulation of ABA biosynthesizing gene like NCED3 or downregulation of ABA-catabolizing gene, viz., ABA8ox1. Likewise, during salt stress, melatonin enhanced the level of ABA in Fragaria ananassa that resulted in higher tolerance level of plants (Zahedi et al. 2020). Thus, ABA–melatonin crosstalk in plants highly varies from species to species under different stressed conditions. Based on the available reports, both synergistic and antagonistic relations are believed to exist between melatonin and ABA.
Crosstalk with auxin
Unlike melatonin and gibberellin, auxin acts synergistically with ABA which eventually controls vital processes of plants during abiotic stress (Emenecker and Strader 2020). ABA controls auxin signaling pathway mainly by regulating the expression of auxin response factors ARF5, ARF6, and ARF10 via ubiquitination reactions (Li et al. 2020b). According to Sun et al. (2018a, b), higher formation of ABA under stressed condition represses the expression of genes encoding transporters involved in translocation of auxin, whereas during non-stressed situation, lower ABA level promotes transportation of auxin, thereby initiating auxin signaling pathway. During salt stress, higher accumulation of ABA resulted in induced expression of ABI5 which in turn inhibited the expression of auxin transporter, PIN1. Based on this observation, Liu et al (2015a, b) reported that reduced expression of PIN1 gene leads to reduced size of root meristem by stabilizing AXR3/IAA17 due to lower auxin levels. Similarly, Ding et al. (2015) also reported that under salt stress, WRKY46 transcription factor regulates auxin homeostasis in plants along with higher ABI4 activity that promotes the emergence of lateral roots. Sirko et al. (2021) demonstrated that ibr5 mutants showed resistance to both ABA and auxin that highlighted the ambiguous role of IBR5 during ABA and auxin crosstalk. Recently, Perez-Alonso et al. (2021) reported that AMIDASE1, an enzyme involved in the biosynthesis of indole-3-acetic acid from indole-3-aceteamide, regulates auxin homeostasis via modulating ABA signaling by promoting ABA accumulation. Additionally, various studies have also reported the role of ABA–auxin crosstalk in regulating seed development in plants. Transcriptome study conducted by Promchuea et al. (2017) revealed that ARF2 promotes primary root growth and seed development in Arabidopsis by suppressing ABA-responsive genes. Breitel et al. (2016) revealed that auxin controls the expression of ARF2 which leads to the reprogramming of hormonal signals of ABA that eventually results in anthocyanin formation, delayed fruit ripening, and increment of berry size.
Crosstalk with cytokinin
Another hormone showing antagonistic relation with ABA during regulation of bud dormancy, leaf senescence, seed germination, and abiotic stress response in plants like rose, rice, maize, and soybean is cytokinin (Sah et al. 2016; Corot et al. 2017; Sirko et al. 2021). Mutant studies of ARABIDOPSIS HISTIDINE KINASEs (AHKs) helped in deciphering the connectivity between ABA and cytokinin, since AHKs serve as cytokinin receptors during salt and drought stress. AHK1 functions as positive regulator of ABA signaling, while AHK2 and AHK3 act as negative regulator of ABA during above mentioned stressed condition (Skalak et al. 2021; Tran et al. 2007). According to Huang et al. (2018), crosstalk between ABA and cytokinin is regulated by interaction between type A response regulator (ARR) and SnRK2s. ABA negatively regulates cytokinin signaling via SnRK2-mediated phosphorylation of ARR5 which in turn enhances its stability in Arabidopsis, resulting in the repression of cytokinin signaling pathway. Earlier reports also showed that overexpression of ARR5 leads to hypersensitivity of ABA that confers drought tolerance in plants (Huang et al. 2017). Along with type A response regulators, role of type B response regulators was also reported during ABA–cytokinin crosstalk. Upon interaction with ARR1, ARR11, and ARR12, the activity of SnRK2 was inhibited (Huang et al. 2018). Moreover, Nguyen et al. (2016) demonstrated that triple mutant (arr1arr10arr12) Arabidopsis plants showed higher tolerance toward drought stress via suppression of cytokinin signaling and higher ABA formation. ABI4 also helps in attenuating cytokinin signaling pathway that negatively regulates cotyledon greening and seed germination (Chandrasekaran et al. 2020).
Crosstalk with ethylene
Similar to that of ABA, ethylene is also considered as a classic stress phytohormone. Both positive and negative interaction occurs between ABA and ethylene, depending on the developmental process involved. ABA affects the synthesis of ethylene by controlling the expression of ethylene biosynthetic genes such as ethylene response factor 11, NCED, and acyl-CoA synthetase. At the molecular level, ABA regulates the transcription of DREB which belongs to ethylene responsive family that is induced in presence of ethylene (Jogawat 2019). During various physiological processes such as fruit ripening, leaf senescence, and photoperiodic induction, ABA enhances the formation of ethylene (Jakubowicz et al. 2020; Zaharah et al. 2013; Wilmowicz et al. 2008a, b). In contrast, ABA downregulates the formation of ethylene via ABI4-mediated lowering of the transcriptional level of ethylene biosynthetic genes, ACO or ACS (Lee and Yoon 2018; Lee et al. 2017). Similarly, Tosetti et al. (2021) reported that ethylene-mediated induction in the activity of ABA 8’-hydroxylase occurs in the onion bulb that eventually results in higher degradation of ABA and accumulation of the end product, viz., phaseic acid. Based on electrophoretic mobility shift and yeast one-hybrid assays, Wang et al. (2019a) reported that ethylene downregulates the transcriptional level of ABA biosynthetic genes (NCED2 and NCED3) during fruit ripening in Prunus persica. Novikova et al. (2020), on the other hand, reported that a positive interaction occurs between ethylene and ABA signaling pathway to couple the cell division and cell differentiation during tissue culture. Proteomics and genomics studies revealed that ABA and ethylene crosstalk occurs through a common set of responsive proteins and genes via enhancement of glutathione content during abiotic stress (Kumar et al. 2016). In another study, triple interaction between ethylene, ABA, and gibberellin was reported by Sun et al. (2020) where they demonstrated that ethylene mainly regulates ABA sensitivity by modulating the expression of ABA metabolizing genes such as TaNCED2 and TACYP707A1 along with ABA signaling genes (TaABI3). Gibberellin sensitivity was regulated by altering the expression levels of gibberellin metabolizing genes (TaGA20ox1, TaGA3ox2, and TaGA2ox6) and its signaling gene (TaGAMYB) which modulated ABA/GA ratio. During stressed condition, ethylene enhances the expression of EIN3 which represses the level of ABI4 that eventually results in the higher expression of VITAMIN C DEFECTIVE 2, leading to higher formation of ascorbic acid which acts as potent antioxidant by scavenging the reactive oxygen species (Yu et al. 2019). In addition, positive interaction was noted between ethylene and ABA during flower senescence, where both the hormones accelerate the senescence of potted flowering plants and cut flowers (Ferrante et al. 2015). Chang et al. (2014) reported that exogenous application of ABA and ethylene increased the transcript of PhHD-Zip that eventually accelerated the flower senescence in petunia. Along with flower senescence, ethylene also acts as an inhibitor of flowering in short day plants when applied in the second half of the inductive light (Wilmowicz et al. 2008a, b). Wilmowicz et al. (2014) reported that exogenous application of ABA just before the beginning of 16-h long dark period inhibited flowering which was partially reversed in presence of amino vinylglycine suggesting that ABA directly influences the formation of ethylene, thus inhibiting flowering. Riboni et al. (2013) demonstrated that ABA-deficient mutants (aba2-1 and aba1-6) showed late flowering phenotype which can be directly linked with the enhanced level of floral suppressing hormone, ethylene, in aba mutant plants.
Crosstalk with salicylic acid
Salicylic acid acts synergistically with ABA in various environmental stressed conditions. Shakirova et al. (2017) and Munoz-Espinoza et al. (2015) reported that exogenous application of salicylic acid enhances the formation of ABA in drought- and cadmium-stressed tomato and wheat seedlings, respectively, that confers their tolerance. Another instance of positive feedback interaction between salicylic acid and ABA was reported by Lopez-Orenes et al. (2020) where they showed that priming of Zygophyllum fabago with salicylic acid enhanced the formation of ABA which in turn conferred plant tolerance against lead toxicity. Similarly, ABA accumulation in salt and Pseudomonas-infected cucumber seedlings also enhanced the formation of salicylic acid (Chojak-Kozniewskaet al. 2017). Protective role of exogenous application of salicylic acid in cold-stressed wheat seedling via the accumulation of ABA and its interaction with H2O2 was also reported by Wang et al. (2018). Prodhan et al. (2020) reported that salicylic acid biosynthesis defective mutant and npr1 mutants showed effective ABA-regulated stomatal closure. Earlier, Prodhan et al. (2018) also reported that the mutant for ABA biosynthesis exhibited effective salicylic-acid-induced stomatal closure. Salicylic acid and ABA signaling pathways act independently of each other; however, various molecular studies have shown that following Ca2+ accumulation and reactive oxygen species formation during stress condition, salicylic acid and ABA signaling is integrated by MPK12 and MPK9-mediated mitogen-activated protein kinase (MAPK) pathway and CPK9 and CPK3-mediated calcium-dependent protein kinase pathway (Prodhan et al. 2018; Jammes et al. 2009). In addition, various reports have also proved the efficacy of interaction between salicylic acid and ABA and mitigation of biotic stress. Gong et al. (2014) reported that ABA suppresses plant defence against pathogen attack by repressing salicylic-acid-mediated jasmonic acid/ethylene immune response. Earlier, Audenaert et al. (2002) reported that ABA might inhibit the formation of salicylic acid by hampering phenylalanine ammonia lyase (PAL) activity. Ueno et al. (2015) also showed that ABA hampered salicylic acid signaling by inactivating the MAPK cascade. Similarly, Manohar et al. (2017) reported that salicylic acid antagonizes ABA action by binding to PP2Cs which serve as repressor of ABA signaling pathway. During induction of systematic acquired resistance in tomato plants, a negative interaction was observed between ABA and salicylic acid, where ABA pre-treated plants reduced the formation of salicylic acid (Kusajima et al. 2017). Thus, it can be stated that a complex crosstalk (either synergistic or antagonistic) occurs between salicylic acid and ABA under different abiotic and biotic stressed environment.
Crosstalk with jasmonic acid
Similar to ABA, jasmonic acid also plays a pivotal role in the amelioration of negative effects of abiotic stress. According to Per et al. (2018), jasmonic acid and ABA interaction regulates major processes in plants such as leaf senescence, stomatal closure, and adaptation to a wide range of abiotic stress via JAZ, MYC, and COI1 proteins of methyl jasmonate signaling pathway. Most of the work focusing the biosynthesis of jasmonic acid in plants has been undertaken in the model plants like Arabidopsis and tomato. In Arabidopsis, three pathways has been described occurring in chloroplast, cytoplasm, and peroxisomes. Hexadecatrienoic acid (16:3) and α-linolenic acid (18:3) serve as the starting substrate for the hexadecane pathway and octadecane pathway, respectively (Chini et al. 2018). The unsaturated fatty acids are converted to 12-oxo-phytodienoic acid that is eventually converted to jasmonic acid in chloroplast (Ruan et al. 2019). Recent studies based on biomolecular fluorescence complementation and yeast two hybrid assays suggest that a complex interaction between jasmonic acid signaling molecules (MYC2, JAZ1, JAZ5, and TIFY10B) and ABA signaling molecules (ABF3, PYL4, PYL5, AHG3, and ABI2) occurs in plants (Liu et al. 2018). Ruan et al. (2019) suggested that jasmonic acid signaling occurs by the release of MYC transcription factors from inactivated JAZ-MYC complex in presence of jasmonate. Proteomics and immunotechnical analyses performed by Aleman et al. (2016) provided a direct link between ABA and jasmonic acid signaling pathway via co-interaction of MYC2 and PYL6 during abiotic stress. MED25 takes part in ABA–jasmonic acid signaling pathway by negatively regulating the ABA-linked genes (ABI5, EM1, EM2, and RAB18) and jasmonic acid-linked genes (MYC2, JAZ6, JAZ8, LOX2, and VSP1) (Skubaczet al. 2016). Interestingly, Wang et al. (2020) reported that jasmonic acid confers drought tolerance in wheat plants by acting upstream to that of ABA. In contrast, jasmonic acid acts downstream of ABA signaling to enhance plant survival against cold stress (Wang et al. 2016a). Additionally, Ding et al. (2019) reported that a synergistic interaction occurs between ABA and jasmonic acid that can be supported from the modulation of ICE1 that regulates the transcription of cold responsive CBF genes by both ABA and jasmonic acid. Furthermore, synergistic interaction between jasmonic acid and ABA can also be proved by the previous works of Wang et al. (2016b) and Wang et al. (2015) where they reported that exogenous application of n-propyl dihydrojasmonate (a jasmonic acid derivative) or α-keto linolenic acid activates the expression of VvNCED1 and VvCYP707A1 in Glomerella cingulata-infected grapes which eventually maintained higher endogenous level of both ABA and jasmonic acid. Positive interaction between jasmonic acid and ABA can be explained by SAPK10-WRKY72-AOS1 model. Hou et al. (2019) explained that WRKY72 represses the AOS1 activity that eventually ceases the formation of jasmonic acid. ABA can effectively activate SAPK10 (a SnRK2 kinase) which in turn phosphorylates and deactivates WRKY72 and, thus, restores the level of jasmonic acid by rescuing the activity of AOS1. However, few studies also showed that ABA negatively affects the signaling pathways of jasmonic acid. Peian et al. (2020) reported that exogenously applied ABA represses the level of endogenous jasmonic acid in Botrytis cinerea-infected strawberry that resulted in higher damage during pathogen attack via upregulation of two pathogenesis-related (PR) proteins (Histone deacetylase 19 and Topless-related protein 3). Thus, both synergistic and antagonistic interaction occurs between jasmonic acid and ABA; however, very few reports suggest the antagonistic crosstalk so that this area needs to be explored further.
Crosstalk with brassinosteroids
During unfavorable conditions, brassinosteroid crosstalk with ABA is crucial for survival and development of plants. Molecular studies revealed that cold stress enhances the transcription of DWF4 and DET2 genes that are responsible for brassinosteroid formation that eventually leads to inactivation of ABA biosynthesis, thus releasing the ABA-mediated dormancy of seedlings (Kimet al. 2019). Ha et al. (2018) showed that ABA–brassinosteroid interaction is necessary for stomatal closure. They stated that at higher concentration, brassinosteroids hamper the ABA-mediated stomatal closure by downregulating the expression of ABA biosynthetic genes which results in reduced ABA formation; however, ABA positively regulates the stomatal closure action of brassinosteroids, since both the hormones regulate open stomata 1 (OST1) activity that plays a pivotal role in stomatal closure. Similarly, during primary root development, the antagonistic relation between ABA and brassinosteroids can also be explained by the triple (bin2-3 bil 1 bil2) mutant study that exhibited higher ABA insensitivity and double (bin2-1 and brl1-9) mutant exhibiting brassinosteroid insensitivity and ABA hypersensitivity (Xue et al. 2009; Lopez-Ruiz et al. 2020). In a similar study, Yang et al. (2016) showed that Brassinazole-Resistant2 transcription factor directly targets ABI5, thus deactivating its expression which eventually results in ABA insensitivity during primary root growth. A complex interaction upon exogenous application of 24-epibrassinolide was reported in drought-stressed Vitis vinifera where application of 24-epibrassinolide upregulates the transcriptional level of VvNCED1, VvNCED2, and VvZEP (ABA biosynthetic genes) and VvaBF1, VvaBF2, VvPP2C4, and VvSnRK2.6 (ABA signaling genes) (Wang et al. 2019b). Setsungnern et al. (2020) reported that the loss of function of brassinosteroid signaling mutant bes1 (BRI1-EMS-SUPPRESSOR 1) showed higher accumulation of ABA after 1 day of heat exposure. Hu and Yu (2014) suggested brassinosteroid–ABA crosstalk model based on molecular analysis. They observed that in presence of lower ABA level, ABI1 and ABI2 are active and dephosphorylate BIN2, releasing its inhibitory action on brassinosteroid signaling through inhibition of BIN2 kinase action on brassinosteroid signaling receptors BES1/BZR1, resulting in brassinosteroid-mediated responses. In presence of ABA, BIN2 is the inactive form, and phosphorylates and activates the kinase action of SnRK2s and stabilizes ABI5, leading to ABA-mediated response. Another report by Gui et al. (2016) also effectively demonstrated the antagonistic behavior between brassinosteroid and ABA by reporting that BRI1-SERK family complex plays a crucial role in brassinosteroid signaling pathway which is inhibited by ABA-mediated remorin protein in rice. Connection between brassinosteroid and ABA signaling pathway can also be demonstrated by a set of WRKY transcription factors. Chen et al. (2017) reported that a triple (wrky64 wrky54 wrky70) mutant showed brassinosteroid-deficient phenotype along with ABI5 expression under dehydration as well as non-stressed conditions, while single (wrky64) mutant showed ABI4 upregulation under both salt and drought stress in the lateral roots (Chen et al. 2017; Ding et al. 2015).
Crosstalk with strigolactones
Both ABA and strigolactone share a common precursor, i.e., β-carotene and, thus, share a mutual regulation in their biosynthetic pathway (Yoneyama and Brewer 2021). Lopez-Raez et al. (2010) reported that the repression of LeCCD7 and LeCCD8 (strigolactone biosynthesising genes) in tomato plants led to the reduced formation of ABA, thus suggesting the possible role of strigolactone during ABA formation. In contrast, Liu et al. (2020a, b) reported that exogenous application of ABA in rice seedlings inhibited the expression of D10 and D27 that encode for the enzymes involved in strigolactone which eventually lowered the formation of strigolactone in seedlings. In plants exposed to the stressed environment, strigolactone differentially regulates the ABA level in root and shoot of the plants. Min et al. (2019) reported that exogenous application of GR24 (a synthetic strigolactone analog) abrogated the negative impact of drought by enhancing the formation of ABA (by inducing the expression of VvNCED1 and reducing the same of VvHYD1 and VvHYD2) in leaves and shoots of grapevine. Earlier, Liu et al. (2015a, b) reported that GR24 treatment inhibited the expression of LjNCED2 which resulted in reduced formation of ABA in roots of osmotic-stressed Lotus japonicus which contradicted the previous work of Min et al. (2019). However, recent works have proved that there lies an antagonistic relation between ABA and strigolactone. Sun et al. (2021) observed that the exogenous application of ABA upregulated the expression of MdSMXL8.2 which codes for a repressor protein involved in strigolactone signaling pathway. Similarly, ur Rehman et al. (2018) reported that knockdown of GmMAX1a and GmMAX4a (involved in strigolactone biosynthesis) resulted in higher formation of ABA which inhibited root nodulation in Glycine max. Mostofa et al. (2018) reported that strigolactone promotes the process of seed germination by regulating the ABA–gibberellin level during extreme temperature. Thus, it can be stated that the crosstalk between strigolactone and ABA is highly variable and can be either antagonistic or synergistic in nature, depending on the plant type and surrounding environmental stressed conditions.
Conclusion and future scope
Understanding the molecular mechanism of plant responses to abiotic stress such as drought, salinity, and cold is important as it helps in manipulating plants to improve stress tolerance and productivity. From the above discussion, it is clear that plants utilize a complex signaling pathway to confer tolerance against abiotic stress. In addition to other small molecule such as calcium, phytohormones trigger specific signal cascade upon challenged with unfavorable conditions. One such phytohormone is ABA that plays diverse role in plant developmental stages. ABA not only mediates stress tolerance by controlling the expression of various transcription factors (WRKY, bZIP, HDAC, and NAC proteins) but also shares a precise and intrinsic interplay network with other novel phytohormones. A complex synergistic crosstalk of ABA occurs with auxin, jasmonic acid, and ethylene, whereas antagonist crosstalk of ABA occurs with gibberellin and brassinosteroids to promote the dormancy of seedlings. Similarly, during stressed condition, ABA acts antagonistically with auxin and brassinosteroids to maintain root architecture. Furthermore, stomatal closure facilitated by ABA is antagonistically regulated by brassinosteroids and salicylic acid and leaf senescence is promoted by counteracting the action of melatonin and cytokinin. In addition, fruit ripening is positively regulated by ABA which interacts synergistically with ethylene and antagonistically with brassinosteroids, salicylic acid, jasmonic acid, and auxin. Such diverse interactions between ABA and phytohormones help plants to integrate various signal inputs. Existing literature provides a deeper insight of the molecular, biochemical, and physical mechanisms controlled by crosstalk between ABA and other plant hormones that enables plants to cope with the harsh environmental conditions (Table 1).
It is apparent that interaction between ABA and other phytohormones is quite common that regulates the growth and developmental processes in plants. ABA controls the action of other hormones by regulating their biosynthesis which modifies their availability by regulating their signaling cascade. In near future, additional crosstalk mechanisms among ABA and other phytohormones will be an important theme in the field of plant research. Furthermore, such complex network due to phytohormone signaling will present valuable new avenues for genetic improvement of crops that can meet the future food demand of growing population in the face of global climate change.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Aleman F, Yazaki J, Lee M, Takahashi Y, Kim AY (2016) An ABA-increased interaction of the PYL6 ABA receptor with MYC2 transcription factor: a putative link of ABA and JA signaling. Sci Rep 6:1–10
Ali F, Qanmber G, Li F, Wang Z (2022) Updated role of ABA in seed maturation, dormancy, and germination. J Adv Res 35:199–214
Arnao MB, Hernández-Ruiz J (2018) Melatonin and its relationship to plant hormones. Ann Bot 121:195–207
Arnao MB, Hernandez-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci 24:38–48
Audenaert K, De Meyer GB, Hofte MM (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 128:491–501
Bandurska H, Stroinski A (2005) The effect of salicylic acid on barley response to water deficit. Acta Physiol Plant 27:379–386
Banerjee A, Roychoudhury A (2019) Melatonin application reduces fluoride uptake and toxicity in rice seedlings by altering abscisic acid, gibberellin, auxin and antioxidant homeostasis. Plant Physiol Biochem 145:164–173
Breitel DA, Chappell-Maor L, Meir S, Panizel I, Puig CP, Hao Y, Yifhar T, Yasuor H, Zouine M, Bouzayen M, Granell Richart A, Rogachev I, Aharoni A (2016a) AUXIN RESPONSE FACTOR 2 intersects hormonal signals in the regulation of tomato fruit ripening. PLOS Genet 12:e1005903
Bulgakov VP, Koren OG (2022) Basic protein modules combining abscisic acid and light signaling in arabidopsis. Front Plant Sci 12:808960
Chandrasekaran U, Luo X, Zhou W, Shu K (2020) Multifaceted signaling networks mediated by abscisic acid insensitive 4. Plant Commun 1:100040
Chang X, Donnelly L, Sun D, Rao J, Reid MS, Jiang C-Z (2014) A petunia homeodomain-leucine zipper protein, PhHD-Zip, plays an important role in flower senescence. PLoS ONE 9:e88320
Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y (2017) Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 29:1425–1439
Cheng W-H, Chiang M-H, Hwang S-G, Lin P-C (2009) Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signaling pathways. Plant Mol Biol 71:61–80
Chini A, Monte I, Zamarreño AM, Hamberg M, Lassueur S, Reymond P, Weiss S, Stintzi A, Schaller A, Porzel A, García-Mina JM, Solano R (2018) An OPR3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis. Nat Chem Biol 14:171–178
Chojak-Kozniewska J, Linkiewicz A, Sowa S, Radzioch MA, Kuzniak E (2017) Interactive effects of salt stress and Pseudomonas syringae pv. lachrymans infection in cucumber: involvement of antioxidant enzymes, abscisic acid and salicylic acid. Environ Exp Bot 136:9–20
Corot A, Roman H, Douillet O, Autret H, Perez-Garcia MD, Citerne S, Bertheloot J, Sakr S, Leduc N, Demotes-Mainard S (2017) Cytokinins and abscisic acid act antagonistically in the regulation of the bud outgrowth pattern by light intensity. Front Plant Sci 8:1724
Dai A (2011) Drought under global warming: a review. Wiley Interdiscip Rev Clim Chang 2:45–65
Ding ZJ, Yan JY, Li CX, Li GX, Wu YR, Zheng SJ (2015) Transcription factor WRKY 46 modulates the development of Arabidopsis lateral roots in osmotic/salt stress conditions via regulation of ABA signaling and auxin homeostasis. The Plant J 84:56–69
Ding Y, Shi Y, Yang S (2019) Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol 222:1690–1704
dos Reis SP, Lima AM, de Souza CRB (2012) Recent molecular advances on downstream plant responses to abiotic stress. Int J Mol Sci 13:8628–8647
Emenecker RJ, Strader LC (2020) Auxin-abscisic acid interactions in plant growth and evelopment. Biomolecules 10:281
Ferrante A, Trivellini A, Scuderi D, Romano D, Vernieri P (2015) Post-production physiology and handling of ornamental potted plants. Posthar Biol Technol 100:99–108
Finkelstein R (2013) Abscisic Acid synthesis and response. Arabidopsis Book 11:e0166
Footitt S, Douterelo-Soler I, Clay H, Finch-Savage WE (2011) Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proc Natl Acad Sci USA 108:20236–20241
Fu J, Wu Y, Miao Y, Xu Y, Zhao E, Wang J, Sun H, Liu Q, Xue Y, Xu Y, Hu T (2017) Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci Rep 7:1–11
Gong T, Shu D, Zhao M, Zhong J, Deng HY, Tan H (2014) Isolation of genes related to abscisic acid production in Botrytis cinerea TB-3-H8 by cDNA-AFLP. J Basic Microbiol 54:204–214
Gui J, Zheng S, Liu C, Shen J, Li J, Li L (2016) OsREM4.1 interacts with OsSERK1 to coordinate the interlinking between abscisic acid and brassinosteroid signaling in rice. Dev Cell 38:201–213
Guilfoyle T, Hagen G, Gazzarrini S, Tsai AYL (2015) Hormone cross-talk during seed germination. Essays Biochem 58:151–164
Ha YM, Shang Y, Yang D, Nam KH (2018) Brassinosteroid reduces ABA accumulation leading to the inhibition of ABA-induced stomatal closure. Biochem Biophys Res Commun 504:143–148
Hewage KAH, Yang JF, Wang D, Hao GF, Yang GF, Zhu JK (2020) Chemical manipulation of abscisic acid signaling: a new approach to abiotic and biotic stress management in agriculture. Adv Sci 7:2001265
Hou Y, Wang Y, Tang L, Tong X, Wang L, Liu L, Huang S, Zhang J (2019) SAPK10-mediated phosphorylation on WRKY72 releases its suppression on jasmonic acid biosynthesis and bacterial blight resistance. iScience 16:499–510
Hu Y, Yu D (2014) BRASSINOSTEROID INSENSITIVE2 interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis. Plant Cell 26:4394–4408
Hu E, Liu M, Zhou R, Jiang F, Sun M, Wen J, Zhu Z, Wu Z (2021) Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci Hortic 285:110176
Huang X, Zhang X, Gong Z, Yang S, Shi Y (2017) ABI4 represses the expression of type-A ARRs to inhibit seed germination in Arabidopsis. Plant J 89:354–365
Huang X, Hou L, Meng J, You H, Li Z, Gong Z, Yang S, Shi Y (2018) The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol Plant 11:970–982
Hyun Y, Richter R, Vincent C, Martinez-Gallegos R, Porri A, Coupland G (2016) Multi-layered regulation of SPL15 and cooperation with SOC1 integrate endogenous flowering pathways at the Arabidopsis shoot meristem. Dev Cell 37:254–266
Izquierdo-Bueno I, González-Rodríguez VE, Simon A, Dalmais B, Pradier J-M, Le Pêcheur P, Mercier A, Walker A-S, Garrido C, Collado IG, Viaud M (2018) Biosynthesis of abscisic acid in fungi: identification of a sesquiterpene cyclase as the key enzyme in Botrytis cinerea. Environ Microbiol. https://doi.org/10.1111/1462-2920.14258
Izydorczyk C, Nguyen T-N, Jo S, Son S, Tuan PA, Ayele BT (2017) Spatiotemporal modulation of abscisic acid and gibberellin metabolism and signaling mediates the effects of suboptimal and supraoptimal temperatures on seed germination in wheat (Triticum aestivum L.). Plant Cell Environ 41:1022–1037
Jakubowicz M, Nowak W, Gałganski Ł, Babula-Skowronska D, Kubiak P (2020) Expression profiling of the genes encoding ABA route components and the ACC oxidase isozymes in the senescing leaves of Populus tremula. J Plant Physiol 248:153143
Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S, Leonhardt N, Ellis BE, Murata Y, Kwak JM (2009) MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci USA 106:20520–20525
Jiang F, Hartung W (2008) Long-distance signaling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. J Exp Bot 59:37–43
Jogawat A (2019) Crosstalk among phytohormone signaling pathways during abiotic stress. In: Roychoudhury A, Tripathi DK (eds) Molecular plant abiotic stress: biology and biotechnology, 1st edn. Wiley, New York, pp 209–220
Kang J, Hwang JU, Lee M, Kim YY, Assmann SM, Martinoia E, Lee Y (2010) PDR-type ABC transporter mediates cellular uptake of the Phytohormone abscisic acid. Proc Natl Acad Sci USA 107:2355–2360
Kanno Y, Hanada A, Chiba Y, Ichikawa T, Nakazawa M, Matsui M, Koshiba T, Kamiya Y, Seo M (2012) Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci USA 109:9653–9658
Kim SY, Warpeha KM, Huber SC (2019) The brassinosteroid receptor kinase, BRI1, plays a role in seed germination and the release of dormancy by cold stratification. J Plant Physiol 241:153031
Kozaki A, Aoyanagi T (2022) Molecular aspects of seed development controlled by gibberellins and abscisic acids. Int J Mol Sci 23:1876
Kumar D, Hazra S, Datta R, Chattopadhyay S (2016) Transcriptome analysis of Arabidopsis mutants suggests a crosstalk between ABA, ethylene and GSH against combined cold and osmotic stress. Sci Rep 6:1–13
Kumar M, Kesawat MS, Ali A, Lee SC, Gill SS, Kim U (2019) Integration of abscisic acid signaling with other signaling pathways in plant stress responses and development. Plants 8:592
Kundu S, Gantait S (2017) Abscisic acid signal crosstalk during abiotic stress response. Plant Gene 11:61–69
Kuromori T, Miyaji T, Yabuuchi H, Shimizu H, Suqimoto E, Kamiya A, Moriyama Y, Shinozaki K (2010) ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc Natl Acad Sci USA 107:2361–2366
Kusajima M, Okumura Y, Fujita M, Nakashita H (2017) Abscisic acid modulates salicylic acid biosynthesis for systemic acquired resistance in tomato. Biosci Biotechnol Biochem 81:1850–1853
Lee HY, Yoon GM (2018) Regulation of ethylene biosynthesis by phytohormones in etiolated rice (Oryza sativa L.) seedlings. Mol Cells 41:311–319
Lee HY, Chen YC, Kieber JJ, Yoon GM (2017) Regulation of the turnover of ACC synthases by phytohormones and heterodimerization in Arabidopsis. Plant J 91:491–504
Li C, Tan D-X, Liang D, Chang C, Jia D, Ma F (2015a) Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J Exp Bot 66:669–680
Li H, Mo Y, Cui Q (2019) Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and-susceptible watermelon genotypes. Plant Sci 278:32–43
Li H, Guo Y, Lan Z (2020a) Melatonin antagonizes ABA action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci 303:110761
Li K, Wang S, Wu H, Wang H (2020b) Protein levels of several Arabidopsis auxin response factors are regulated by multiple factors and ABA promotes ARF6 protein ubiquitination. Int J Mol Sci 21:9437
Liu J, He H, Vitali M, Visentin I, Charnikhova T, Haider I, Schubert A, Ruyter-Spira C, Bouwmeester HJ, Lovisolo C, Cardinale F (2015a) Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: exploring the interaction between strigolactones and ABA under abiotic stress. Planta 241:1435–1451
Liu W, Li RJ, Han TT, Cai W, Fu ZW, Lu YT (2015b) Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol 168:343–356
Liu S, Lv Z, Liu Y, Li L, Zhang L (2018) Network analysis of ABA-dependent and ABA-independent drought responsive genes in Arabidopsis thaliana. Genet Mol Biol 41:624–637
Liu S, Huang H, Huber DJ, Pan Y, Shi X, Zhang Z (2020a) Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol Technol 163:111136
Liu X, Hu Q, Yan J, Sun K, Liang Y, Jia M, Meng X, Fang S, Wang Y, Jing Y, Liu G, Wu D, Chu C, Smith SM, Chu J, Wang Y, Li J, Wang B (2020b) ζ-Carotene isomerase suppresses tillering in rice through the coordinated biosynthesis of strigolactone and abscisic acid. Mol Plant 3:1784–1801
Lopez-Orenes A, Alba JM, Kant MR, Calderon AA, Ferrer MA (2020) OPDA and ABA accumulation in Pb-stressed Zygophyllum fabago can be primed by salicylic acid and coincides with organ-specific differences in accumulation of phenolics. Plant Physiol Biochem 154:612–621
Lopez-Raez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK, Sergeant MJ, Verstappen F, Bugg TDH, Thompson AJ, Ruyter-Spira C, Bouwmeester H (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytol 187:343–354
Lopez-Ruiz BA, Zluhan-Martínez E, Sanchez MDLP, Alvarez-Buylla ER, Garay-Arroyo A (2020) Interplay between hormones and several abiotic stress conditions on Arabidopsis thaliana primary root development. Cells 9:2576
Magome H, Kamiya Y (2018) Inactivation processes. Annu Plant Rev Online 73–93
Manohar M, Wang D, Manosalva PM, Choi HW, Kombrink E, Klessig DF (2017) Members of the abscisic acid co-receptor PP2C protein family mediate salicylic acid–abscisic acid crosstalk. Plant Direct 1:e00020
Mantri N, Patade V, Penna S, Ford R, Pang E (2012) Abiotic stress responses in plants: present and future. In: Ahmad P, Prasad M (eds) Abiotic stress responses in plants. Springer, New York, pp 1–19
Min Z, Li R, Chen L, Zhang Y, Li Z, Liu M, Ju Y, Fang Y (2019) Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol Biochem 135:99–110
Mostofa MG, Li W, Nguyen KH, Fujita M, Tran LSP (2018) Strigolactones in plant adaptation to abiotic stresses: an emerging avenue of plant research. Plant, Cell Environ 41:2227–2243
Muller M, Munne-Bosch S (2021) Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol 185:1500–1522
Munoz-Espinoza VA, Lopez-Climent MF, Casaretto JA, Gomez-Cadenas A (2015) Water stress responses of tomato mutants impaired in hormone biosynthesis reveal abscisic acid, jasmonic acid and salicylic acid interactions. Front Plant Sci 6:997
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Nguyen KH, Ha CV, Nishiyama R, Watanabe Y, Leyva-Gonzalez MA, Fujita Y, Tran UT, Li W, Tanaka M, Seki M, Schaller GE, Herrera-Estrella L, Tran LS (2016) Arabidopsis type B cytokinin response regulators ARR1, ARR10, and ARR12 negatively regulate plant responses to drought. Proc Natl Acad Sci USA 113:3090–3095
North HM, De Almeida A, Boutin JP, Frey A, To A, Botran Sotta B, Marion-Poll A (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50:810–824
Novikova GV, Stepanchenko NS, Zorina AA, Nosov AV, Rakitin VY, Moshkov IE, Los DA (2020) Coupling of cell division and differentiation in Arabidopsis thaliana cultured cells with interaction of ethylene and ABA signaling pathways. Life 10:15
Peian Z, Haifeng J, Peijie G, Sadeghnezhad E, Qianqian P, Tianyu D, Teng L, Huanchun J, Jinggui F (2020) Chitosan induces jasmonic acid production leading to resistance of ripened fruit against Botrytis cinerea infection. Food Chem 337:127772
Per TS, Khan MIR, Anjum NA, Masood A, Hussain SJ, Khan NA (2018) Jasmonates in plants under abiotic stresses: crosstalk with other phytohormones matters. Environ Exp Bot 145:104–120
Perez-Alonso MM, Ortiz-García P, Moya-Cuevas J, Lehmann T, Sánchez-Parra B, Björk RG, Karim S, Amirjani MR, Aronsson H, Wilkinson MD, Pollmann S (2021) Endogenous indole-3-acetamide levels contribute to the crosstalk between auxin and abscisic acid, and trigger plant stress responses in Arabidopsis. J Exp Bot 72:459–475
Prodhan MY, Munemasa S, Nahar MNEN, Nakamura Y, Murata Y (2018) Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol 178:441–450
Prodhan Y, Issak M, Munemasa S, Nakamura Y, Murata Y (2020) Salicylic acid receptor NPR1 is involved in guard cell chitosan signaling. Biosci Biotechnol Biochem 84:963–969
Promchuea S, Zhu Y, Chen Z, Zhang J, Gong Z (2017) ARF2 coordinates with PLETHORAs and PINs to orchestrate ABA-mediated root meristem activity in Arabidopsis. J Integr Plant Biol 59:30–43
Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Drechsel P, Noble AD (2014) Economics of salt-induced land degradation and restoration. In: Natural Resources Forum (United Nations: Wiley Online Library), pp 282–295
Raftery AE, Zimmer A, Frierson DMW, Startz R, Liu P (2017) Less than 2°C warming by 2100 unlikely. Nat Clim Chang 7:637
Rehman K, Fatima F, Waheed I, Akash MSH (2018) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119:157–184
Riboni M, Galbiati M, Tonelli C, Conti L (2013) GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and suppressor of overexpression of constans. Plant Physiol 162:1706–1719
Ronen G, Karchi H, Diber A, Vinocur BJ, Ayal S, Emmanuel E, Gang M, Dimet D (2019) Polynucleotides, polypeptides encoded thereby, and methods of using same for increasing abiotic stress tolerance and/or biomass and/or yield in plants expressing same. U.S. Pat Appl 16: 833.
Roychoudhury A, Basu S (2012) Ascorbate-glutathione and plant tolerance to various abiotic stresses. In: Anjum NA, Umar S, Ahmad A (eds) Oxidative stress in plants causes, consequences and tolerance. IK International Publishing House Pvt. Ltd., New Delhi, pp 177–258
Roychoudhury A, Paul S, Basu S (2013) Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep 32:985–1006
Ruan J, Zhou Y, Zhou M, Yan J, Khurshid M, Weng W, Cheng J, Zhang K (2019a) Jasmonic acid signaling pathway in plants. Int J Mol Sci 20:2479
Sah SK, Reddy KR, Li J (2016) Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci 7:571
Saika H, Okamoto M, Miyoshi K, Kushiro T, Shinoda S, Jikumaru Y, Fujimoto M, Arikawa T, Takahashi H, Ando M, Arimura S, Miyao A, Hirochika H, Kamiya Y, Tsutsumi N, Nambara E, Nakazono M (2006) Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8’-hydroxylase in rice. Plant Cell Physiol 48:287–298
Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M (2004) Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol 134:1439–1449
Samanta S, Banerjee A, Roychoudhury A (2021) Exogenous melatonin regulates endogenous phytohormone homeostasis and thiol-mediated detoxification in two indica rice cultivars under arsenic stress. Plant Cell Rep 40:1585–1602
Seo M, Koshiba T (2011) Transport of ABA from the site of biosynthesis to the site of action. J Plant Res 124:501–507
Seo M, Aoki H, Koiwai H, Kamiya Y, Nambara E, Koshiba T (2004) Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds. Plant Cell Physiol 45:1694–1703
Setsungnern A, Munoz P, Perez-Llorca M, Müller M, Thiravetyan P, Munne-Bosch S (2020) A defect in BRI1-EMS-SUPPRESSOR 1 (BES1)-mediated brassinosteroid signaling increases photoinhibition and photo-oxidative stress during heat stress acclimation in Arabidopsis. Plant Sci 296:110470
Shakirova FM, Bezrukova MV, Allagulova CR, Maslennikova DR, Lubyanova AR (2017) Wheat germ agglutinin and dehydrins as ABA-regulated components of SA-induced cadmium resistance in wheat plants. In: Khan NA, Iqbal N, Nazar R (eds) Salicylic acid: a multifaceted hormone. Springer, Singapore, pp 77–96
Sharp RE (2002) LeNoble ME (2002) ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot 53(366):33–37
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress tolerance and response. J Exp Bot 58:221–227
Shu K, Chen Q, Wu Y, Liu R, Zhang H, Wang P, Li Y, Wang S, Tang S, Liu C, Yang W, Cao X, Serino G, Xie Q (2016a) ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels. Plant J 85:348–361
Shu K, Liu XD, Xie Q, He ZH (2016b) Two faces of one seed: hormonal regulation of dormancy and germination. Mol Plant 9:34–45
Shu K, Zhou W, Chen F, Luo X, Yang W (2018) Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Front Plant Sci 9:416
Singh A, Banerjee A, Roychoudhury A (2022) Fluoride tolerance in rice is negatively regulated by the “stress-phytohormone” abscisic acid (ABA), but promoted by ABA-antagonist growth regulators, melatonin, and gibberellic acid. Protoplasma 259:1331–1350
Sirko A, Wawrzynska A, Brzywczy J, Sienko M (2021) Control of ABA signaling and crosstalk with other hormones by the selective degradation of pathway components. Int J Mol Sci 22:4638
Skalak J, Nicolas KL, Vankova R, Hejatko J (2021) Signal integration in plant abiotic stress responses via multistep phosphorelay signaling. Front Plant Sci 12:12
Skubacz A, Daszkowska-Golec A, Szarejko I (2016) The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front Plant Sci 7:1884
Sun LR, Wang YB, He SB, Hao FS (2018a) Mechanisms for abscisic acid inhibition of primary root growth. Plant Signal Behav 13:e1500069
Sun LR, Wang YB, He SB, Hao FS (2018b) Mechanisms for abscisic acid inhibition of primary root growth. Plant Signal Behav 13:e1500069
Sun M, Tuan PA, Izydorczyk MS, Ayele BT (2020) Ethylene regulates post-germination seedling growth in wheat through spatial and temporal modulation of ABA/GA balance. J Exp Bot 71:1985–2004
Sun W, Ji X, Song L, Wang X, You C, Hao Y (2021) Functional identification of MdSMXL8. 2, the homologous gene of strigolactones pathway repressor protein gene in Malus × domestica. Hortic Plant J 7:275–285
Swamy PM, Smith B (1999) Role of abscisic acid in plant stress tolerance. Curr Sci 76:1220–1227
Szalai G, Pál M, Janda T (2011) Abscisic acid may alter the salicylic acid-related abiotic stress response in maize. Acta Biol Szeged 55:155–157
Szepesi Á, Csiszár J, Gémes K, Horváth E, Horváth F, Simon ML, Tari I (2009) Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation, and increases Na+ content in leaves without toxicity symptoms in Solanum lycopersicum L. J Plant Physiol 166:914–925
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56
Tan X, Fan Z, Kuang J, Lu W, Reiter RJ, Prakash L, Su X-G, Zhou J, Chen J-Y, Shan W (2019) Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J Pineal Res 67:e12570
Tigchelaar M, Battisti DS, Naylor RL, Ray DK (2018) Future warming increases probability of globally synchronized maize production shocks. Proc Natl Acad Sci 115:6644–6649
Tosetti R, Waters A, Chope GA, Cools K, Alamar MC, McWilliam S, Thompson AJ, Terry LA (2021) New insights into the effects of ethylene on ABA catabolism, sweetening and dormancy in stored potato tubers. Postharvest Biol Technol 173:111420
Tran LSP, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K (2007) Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA 104:20623–20628
Tuan PA, Kumar R, Rehal PK, Toora PK, Ayele BT (2018) Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front Plant Sci 9:668
Ueno Y, Yoshida R, Kishi-Kaboshi M, Matsushita A, Jiang CJ, Goto S, Takahashi A, Hirochika H, Takatsuji H (2015) Abiotic stresses antagonize the rice defence pathway through the tyrosine-dephosphorylation of OsMPK6. PLoS Pathog 11:e1005231
ur Rehman N, Ali M, Ahmad MZ, Liang G, Zhao J (2018) Strigolactones promote rhizobia interaction and increase nodulation in soybean (Glycine max). Microb Pathog 114:420–430
Urano K, Maruyama K, Jikumaru Y, Kamiya Y, Yamaguchi-Shinozaki K, Shinozaki K (2017) Analysis of plant hormone profiles in response to moderate dehydration stress. Plant J 90:17–36
Vaistij FE, Barros-Galvao T, Cole AF, Gilday AD, He Z, Li Y, Harvey D, Larson TR, Graham IA (2018) MOTHER-OF-FT-AND-TFL1 represses seed germination under far-red light by modulating phytohormone responses in Arabidopsis thaliana. Proc Natl Acad Sci USA 115:8442–8447
Verma V, Ravindran P, Kumar PP (2016) Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16:86
Vishal B, Kumar PP (2018) Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front Plant Sci 9:838
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S (2017) Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8:161
Wang Y, Li L, Ye T, Lu Y, Chen X, Wu Y (2013) The inhibitory effect of ABA on floral transition is mediated by ABI5 in Arabidopsis. J Exp Bot 64:675–684
Wang S, Takahashi H, Saito T (2015) Jasmonate application influences endogenous abscisic acid, jasmonic acid and aroma volatiles in grapes infected by a pathogen (Glomerella cingulata). Sci Hortic 192:166–172
Wang F, Guo Z, Li H, Wang M, Onac E, Zhou J, Xia X, Shi K, Yu J, Zhou Y (2016a) Phytochrome A and B function antagonistically to regulate cold tolerance via abscisic acid-dependent jasmonate signaling. Plant Physiol 170:459–471
Wang S, Saito T, Ohkawa K, Ohara H, Shishido M, Ikeura H, Takagi K, Ogawa S, Yokoyama M, Kondo S (2016b) α-Ketol linolenic acid (KODA) application affects endogenous abscisic acid, jasmonic acid and aromatic volatiles in grapes infected by a pathogen (Glomerella cingulata). J Plant Physiol 192:90–97
Wang B, Wei H, Xue Z, Zhang WH (2017) Gibberellins regulate iron deficiency-response by influencing iron transport and translocation in rice seedlings (Oryza sativa). Ann Bot 119:945–956
Wang W, Wang X, Huang M, Cai J, Zhou Q, Dai T, Cao W, Jiang D (2018) Hydrogen peroxide and abscisic acid mediate salicylic acid-induced freezing tolerance in wheat. Front Plant Sci 9:1137
Wang X, Zeng W, Ding Y, Wang Y, Niu L, Yao JL, Pan L, Lu Z, Cui G, Li G, Wang Z (2019a) Peach ethylene response factor PpeERF2 represses the expression of ABA biosynthesis and cell wall degradation genes during fruit ripening. Plant Sci 283:116–126
Wang YT, Chen ZY, Jiang Y, Duan BB, Xi ZM (2019b) Involvement of ABA and antioxidant system in brassinosteroid-induced water stress tolerance of grapevine (Vitis vinifera L.). Sci Hortic 256:108596
Wang X, Li Q, Xie J (2020) Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J 9:120–132
Wang D, Chen Q, Chen W, Guo Q, Xia Y, Wang S, Jing D, Liang G (2021) Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resistance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ Exp Bot 181: 104291
Wilmowicz E, Kaaaesy J, Kopcewicz J (2008a) Ethylene and ABA interactions in the regulation of flower induction in Pharbitis nil. J Plant Physiol 165:1917–1928
Wilmowicz E, Kęsy J, Kopcewicz J (2008b) Ethylene and ABA interactions in the regulation of flower induction in Pharbitis nil. J Plant Physiol 165:1917–1928
Wilmowicz E, Frankowski K, Kućko A, Kaaaesy J, Kopcewicz J (2014) Involvement of the iaa-regulated acc oxidase gene PNACO3 in Pharbitis nil flower inhibition. Acta Biol Crac Ser Bot 56:90–96
Xu ZY, Lee KH, Dong T, Jeong JC, Jin JB, Kanno Y, Kim DH, Kim SY, Seo M, Bressan RA, Yun DJ, Hwang I (2012) A vacuolar b-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell 24:2184–2199
Xu P, Chen H, Cai W (2020) Transcription factor CDF4 promotes leaf senescence and floral organ abscission by regulating abscisic acid and reactive oxygen species pathways in Arabidopsis. EMBO Rep 21:e48967
Xue LW, Du JB, Yang H, Xu F, Yuan S, Lin HH (2009) Brassinosteroids counteract abscisic acid in germination and growth of Arabidopsis. Z Für Naturforsch C 64:225–230
Yaish MW, El-Kereamy A, Zhu T, Beatty PH, Good AG, Bi YM, Rothstein SJ (2010) The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet 6:e1001098
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yang L, Zhang J, He J, Qin Y, Hua D, Duan Y, Chen Z, Gong Z (2014) ABA mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet 10:e1004791
Yang X, Bai Y, Shang J, Xin R, Tang W (2016) The antagonistic regulation of abscisic acid-inhibited root growth by brassinosteroids is partially mediated via direct suppression of abscisic acid insensitive 5 expression by brassinazole resistant 1. Plant Cell Environ 39:1994–2003
Yoneyama K, Brewer PB (2021) Strigolactones, how are they synthesized to regulate plant growth and development? Curr Opin Plant Biol 63:102072
Yu Y, Wang J, Li S, Kakan X, Zhou Y, Miao Y, Wang F, Qin H, Huang R (2019) Ascorbic acid integrates the antagonistic modulation of ethylene and abscisic acid in the accumulation of reactive oxygen species. Plant Physiol 179:1861–1875
Zaharah SS, Singh Z, Symons GM, Reid JB (2013) Mode of action of abscisic acid in triggering ethylene biosynthesis and softening during ripening in mango fruit. Postharvest Biol Technol 75:37–44
Zahedi SM, Hosseini MS, Abadía J, Marjani M (2020) Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Plant Physiol Biochem 149:313–323
Zhang J, Shi Y, Zhang X, Du H, Xu B, Huang B (2017) Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ Exp Bot 138:36–45
Acknowledgements
Financial assistance from Science and Engineering Research Board, Government of India through the grant [EMR/2016/004799] and Department of Higher Education, Science and Technology and Biotechnology, Government of West Bengal, through the grant [264(Sanc.)/ST/P/S&T/1G-80/2017] to Prof. Aryadeep Roychoudhury is gratefully acknowledged.
Funding
This study was funded by Science and Engineering Research Board, Government of India, and Department of Higher Education, Science and Technology and Biotechnology, Government of West Bengal.
Author information
Authors and Affiliations
Contributions
AS drafted the entire manuscript. AR critically analyzed the manuscript, incorporated necessary modifications, and supervised the whole work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in publishing the manuscript.
Additional information
Communicated by Wusheng Liu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, A., Roychoudhury, A. Abscisic acid in plants under abiotic stress: crosstalk with major phytohormones. Plant Cell Rep 42, 961–974 (2023). https://doi.org/10.1007/s00299-023-03013-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-023-03013-w