Abstract
Key message
This review summarizes the process of thermal acquired tolerance in plants and the knowledge gap compared to systemic acquired resistance that a plant shows after pathogen inoculation.
Abstract
Plants are continuously challenged by several biotic stresses such as pests and pathogens, or abiotic stresses like high light, UV radiation, drought, salt, and very high or low temperature. Interestingly, for most stresses, prior exposure makes plants more tolerant during the subsequent exposures, which is often referred to as acclimatization. Research of the last two decades reveals that the memory of most of the stresses is associated with epigenetic changes. Heat stress causes damage to membrane proteins, denaturation and inactivation of various enzymes, and accumulation of reactive oxygen species leading to cell injury and death. Plants are equipped with thermosensors that can recognize certain specific changes and activate protection machinery. Phytochrome and calcium signaling play critical roles in sensing sudden changes in temperature and activate cascades of signaling, leading to the production of heat shock proteins (HSPs) that keep protein-unfolding under control. Heat shock factors (HSFs) are the transcription factors that read the activation of thermosensors and induce the expression of HSPs. Epigenetic modifications of HSFs are likely to be the key component of thermal acquired tolerance (TAT). Despite the advances in understanding the process of thermomemory generation, it is not known whether plants are equipped with systemic activation thermal protection, as happens in the form of systemic acquired resistance (SAR) upon pathogen infection. This review describes the recent advances in the understanding of thermomemory development in plants and the knowledge gap in comparison with SAR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat stress (HS) can be defined as the temperature higher than the ambient temperature, which causes irreversible loss to plant growth and productivity. The effect of HS on plant productivity depends on the developmental stage of the plant. HS adversely affects anther development, and thus the plants at the reproductive stage are highly sensitive to heat (Li et al. 2015). HS directly impacts on the biochemical, physiological machinery and alter the normal gene expression in the plants (Driedonks et al. 2015). HS mostly damages the leaves and results in curling, necrosis, and senescence of leaves. HS also changes the membrane fluidity that results in electrolytic leakage in the plant cell (Savchenko et al. 2002). Photosynthesis, responsible for almost all biomass production on the earth, is highly susceptible to heat-mediated damage. Though several chloroplast proteins get denatured or become nonfunctional at higher temperatures, the photosystem-II (PS-II) is most vulnerable to get damaged (Tang et al. 2007). HS damages the chloroplast and mitochondrial electron transport system. It generates reactive oxygen species (ROS) such as superoxides, hydrogen peroxide (H2O2), and hydroxyl radicals (•OH) that harm DNA and also cause lipid peroxidation of the cell membrane (Choudhury et al. 2017).
Plants possess several intricate mechanisms through which they protect themselves from excessive heat. Heat shock proteins (HSPs) play the most crucial role in the process (Baniwal et al. 2004; Driedonks et al. 2015; Ul Haq et al. 2019). By functioning as molecular chaperones, HSPs prevent protein denaturation and aggregation. In addition, HSPs interact and modulates functions of heat shock factors (HSFs). HSFs function as transcriptional activators for several genes, including HSPs. Plants activate ROS production upon sensing environmental stresses, including heat. ROS functions as a signaling molecule for activating local response as well as for transmitting to distal parts. Accumulation of ROS in plants activates HSFs, which in turn activate ROS scavenging and detoxifying enzymes like ascorbate peroxidase (APX) and superoxide dismutase (SOD) (Driedonks et al. 2015; Choudhury et al. 2017). A decrease in ROS level occurs due to the enhanced production of antioxidants, osmolytes, and HSPs. The major stress-responsive osmolytes in plants include proline, glycine betaine, and trehalose, which play roles in maintaining cellular ionic homeostasis (Slama et al. 2015).
Prior exposure to elevated temperature helps plants to tolerate a higher temperature, which is otherwise lethal. This thermomemory-mediated enhanced heat tolerance is often referred to as thermal acquired tolerance (TAT). Development of TAT requires the perception of HS, development of stress memory, and priming-mediated altered expression of heat-protective machinery. HSPs and HSFs are critical players for TAT development in plants. These proteins are conserved in evolution and are indispensable for TAT in all the organisms.
A plant that experiences a pathogen attack once becomes resistant to subsequent infections by developing systemic acquired resistance (SAR). In contrast to TAT, which is conserved in evolution across all eukaryotic organisms, SAR is a plant-specific response. Despite that difference, the basic mechanism of development of TAT and SAR is comparable. Besides highlighting the process of TAT development in plants, this review discusses the knowledge gap compared to SAR development.
Thermosensing by plants
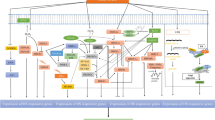
Each component of a cell senses a detrimental temperature. Plants have different thermosensors capable of detecting the heat stimulus (Vu et al. 2019). Plant cellular components like plasma membrane, nucleic acid, proteins mainly receive the heat stimulus and response to HS through reversible changes like protein denaturation, nucleic acid fragmentation, and membrane fluidity, which may function as heat sensors (Vu et al. 2019; Sharma et al. 2020). Red light photoreceptor phytochrome B (PhyB) also act as a thermosensor in addition to the established role in light signaling. The inactive Pr form of phytochrome absorbs red light and converts into the active Pfr form, which reverts back to Pr by absorbing far-red light. The reversion of Pfr to Pr form enhances by high temperature (Rockwell et al. 2006; Vu et al. 2019). The components of PhyB signaling, such as PHYTOCHROME INTERACTING FACTOR 4 (PIF4), also take part in thermosensing (Kumar et al. 2012). The CYCLIC NUCLEOTIDE GATED CALCIUM CHANNELb (CNGCb) of Physcomitrella patens or its orthologue CNGC2 found in Arabidopsis is another possible thermosensor for plants (Finka et al. 2012). CNGCb and CNGC2 are components of plasma membrane-localized cyclic nucleotide-gated Ca++ channel (Fig. 1). This calcium channel acts as primary thermosensors in plants, and their disruptions show hyper-thermosensitive phenotypes (Finka et al. 2012). Mild HS activates heat sensors, which in turn induces possible downstream pathways to activate several transcriptional regulators for altered expression of the stress-related protein to confer tolerance. These downstream signaling also activates RESPIRATORY BURST OXIDASE HOMOLOG D (RBOHD), which expresses in all tissues and acts as a central driving force for ROS signaling in plants (Zhou et al. 2014a). RBOHD is a calcium-dependent NADPH oxidase that generates superoxide in the apoplastic space upon several stresses, including heat and pathogen (Fig. 1).
Thermotolerance in plants. HSFs and HSPs play central roles in thermotolerance. HS (HS) results protein denaturation and activations of HSPs and HSFs. HS also changes the membrane composition and its function. The membrane-localized RBOHD generates apoplastic ROS upon environental stress, which indirectly activates HSFs. RBOHD is regulated by the CDPK, which is activated by the calcium released from the PM and ER via CNGC- and IP3-gated calcium channels that allow an influx of calcium into the cytoplasm. HS induces alternative splicing responsible for production of splice-variants (HsfA2-III) of HsfA2, which is important for thermomemory. Enhanced expression of several HSFs, HSPs and genes for higher levels of osmolytes provide thermotolerant to plants. HS enhances autophgosome formation and expression of autophagy-related genes which protects plants at high temperature. Nucleus-coded small HSP like sHSP21 pay critical role in protecting PS-II and other machineries involved in photosynthesis and significantly contribute towards HS tolerance
Thermomemory and transgenerational protection
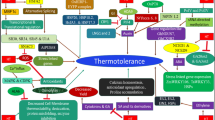
Preexposure to heat changes plants at molecular and biochemical levels that can lead to acquired thermotolerance. A recent study shows that heat priming of wheat at the stem elongation stage significantly inhibits the damage to grain yield, resulting from HS during grain filling (Fan et al. 2018). Enhanced thermotolerance in plants is directly associated with an increased photosynthetic capacity. Genetic and epigenetic regulation, especially of HSPs and HSFs, are associated with stress memory development and stress priming (Fig. 2) (Brzezinka et al. 2016). Plants can retain thermotolerance for several days or even can transfer to the next generation. Progeny of heat-primed wheat plants performs better than non-primed plants under HS conditions (Wang et al. 2016b). Reports suggest that HS causes heritable phenotypic and epigenetic changes in Arabidopsis, wheat, rapeseed, and other plants (Suter and Widmer 2013; Migicovsky et al. 2014; Wang et al. 2016b; Byeon et al. 2019; Liu et al. 2020). The genetic and epigenetic changes associated with thermopriming are discussed in the following sections.
Epigenetic regulation of thermotolerance and thermomemory development HS upregulates the expression of HSFA2. HSFA2 activtaes the H3K27me3 demethylase REF6. REF6 in turn activates HSFA2 and form a positive feedback loop. This loop activates the expression of E3 ligase SGIP1 and indirectly activates HTT5 expression and thermotolerance. The REF6-HSFA2 loop form to activate HTT5 by reducing tasiRNA. HSFA2 also regulated the expression of HSP21, which is responsible for thermomemory development and thermotolerance. HS activates histone acetyl transferase GCN5 that cause H3K9/H314Ac acetylation at the promoter of HSFA3 and UV6 and promotes thermotolerance. HS activates histone deacetylase H2DC, which physically interacts with several member of SWI3/SNF chromatin remodelling complex and negatively regulates thermotolerance
Alternative splicing for thermomemory
Alternative splicing is an important mechanism that increases transcriptome complexity and proteome diversity. Recently it has been reported that thermopriming triggers the splicing memory in Arabidopsis plants (Ling et al. 2018). Splicing is a post-transcriptional process in eukaryotic organisms in which pre-mRNA is processed into mature mRNA by removing introns. Upon heat-stress, non-primed plants show a high-level of intron retention, which is significantly lower in a heat-primed plant, suggesting the splicing of pre-mRNA as a possible mechanism of heat memory in plants (Ling et al. 2018). HSFA2 pre-mRNA undergoes alternative splicing to form a splice variant HSFA2-III (Fig. 1), which encodes a truncated isoform of HSFA2 (S-HsfA2). This truncated isoform binds to the heat shock element (HSE) of HSFA2 itself and other HSP genes to induce their expression (Fig. 1; Liu et al. 2013). Thus, alternative splicing of stress-specific genes is involved in fine-tuning expression of regulatory genes and generation of thermomemory.
The plasma membrane, glycerolipids, and glucose in thermomemory development
HS affects plasma membrane composition and fluidity of the lipid bilayer. Plants have generated several complex mechanisms through which they can stabilize their membrane in response to HS. A sudden increase in temperature activates two membrane proteins, PHOSPHATIDYLINOSITOL PHOSPHATE KINASE (PIPK) and PHOSPHOLIPASE D (PLD) (Mishkind et al. 2009). PIPK and PLD generate phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidic acid (PA), respectively, from membrane lipids (Mishkind et al. 2009). PIP2 acts as a precursor of inositol 1,4,5-triphosphate (IP3), which further activates IP3-gated calcium channels and increases the calcium level in the cytoplasm (Fig. 1). The spike of calcium is required for stress-induced ROS production and the activation of heat shock responsive genes (Fig. 1; Liu et al. 2006). Under HS, plants release polyunsaturated fatty acids like alpha-linolenic acid (18:3) from chloroplast membrane glycerolipids, such as monogalactosyldiacylglycerol (MGDG), with the help of a lipase enzyme HIKESHI-LIKE PROTEIN1 (HLP1) (Higashi et al. 2018). Glucose also plays an indispensable role in the generation of thermomemory by regulating HLP1 in Arabidopsis (Sharma et al. 2019).
Chloroplasts for thermomemory
Chloroplast being the powerhouse of green tissues, plants have evolved elaborate mechanisms to protect them from heat damage. Transcription of plastidic genome alters upon HS (Danilova et al. 2018). A group of smaller heat shock proteins (sHSP) protect photosynthetic machinery from denaturation (Fig. 1). sHSP21 plays an essential role in the development of thermomemory. HSP21 binds with the thylakoid membrane, and this binding increases upon HS (Bernfur et al. 2017). The duration of memory depends on the level of sHSP21 produced after heat priming (Sedaghatmehr et al. 2016). sHSP21 is a nuclear-encoded protein (monomer size 21 kDa), and localized in the plastid in Arabidopsis (Härndahl et al. 1999). Under unfavorable conditions, sHSPs rapidly bind to selective unfolded protein independent of ATP and facilitate the refolding of the protein. It contains conserved methionine residues in the N-terminal region that recognizes and attaches to the hydrophobic regions of the unfolded protein (Sundby et al. 2005). It is reported that, in the tomato, these HSP21 mostly involve in the protection of photosystem II (PSII) under high-temperature stress (Neta-Sharir et al. 2005). Transgenic Arabidopsis plants overexpressing HSP21 show high tolerance to HS. The expression of HSP21 is regulated by HSFA2, the transcriptional regulator of thermomemory, while hsfA2 mutants show defective in thermomemory development (Charng et al. 2007).
Autophagy in thermopriming
Autophagy plays a crucial role in nutrient cycling and tolerance to various biotic and abiotic stress. HS in tomato and Arabidopsis leads to enhanced accumulation of autophagosomes (Fig. 1) with concomitant induced expression of AUTOPHAGY-RELATED GENE (ATG5), ATG7, and autophagy cargo receptor NEIGHBOUR OF BREAST CANCER 1 (NBR1) (Zhou et al. 2013, 2014b). Arabidopsis atg5, atg7, and nbr1 mutants show reduced heat tolerance compared to wild type. Transgenic apple (Malus domestica) plants overexpressing MdATG18a gene show enhanced autophagic activity, photosynthetic component protection, ROS scavenging, expression of HSPs, and thermotolerance (Huo et al. 2020). The MDATG18a overexpressing lines show the accumulation of autophagosomes in the leaves of apple upon exposure to HS (Huo et al. 2020). Arabidopsis ATG8-INTERACTING PROTEIN 3 (ATI3) is essential for thermopriming (Zhou et al. 2018). ATI3 interacts with the protein UBIQUITIN-ASSOCIATED PROTEIN 2 (UBAC2), which is required for ER-associated degradation as well as heat tolerance (Zhou et al. 2018), suggesting the critical role of autophagy in thermopriming (Fig. 1).
Role of phytohormones in thermotolerance
In addition to growth and development, phytohormones also regulate all types of stress responses. Functions of all plant hormones are implicated in thermotolerance (Sharma et al. 2020). HS leads to increased transcription of auxin biosynthetic YUCCA genes (Blakeslee et al. 2019). High temperature results in hypocotyl elongation in an auxin-dependent manner (Gray et al. 1998). HSP90 and the co-chaperon SUPPRESSOR OF G-TWO ALLELE OF SKP1 (SGT1) interact with auxin receptor protein TRANSPORT INHIBITOR RESPONSE1 (TIR1), and the module integrates growth and development under HS condition (Fig. 2; Wang et al. 2016a). Jasmonic acid (JA) signaling is also like to have a direct impact on thermotolerance. CORONATINE INSENSITIVE 1 (COI1) and JASMONATE-ZIM DOMAIN (JAZ) are co-receptors of JA-Ile, the bioactive form of JA. HS also results in elevated accumulation of 12-oxo-phytodienoic acid (OPDA) and dinor-OPDA (dn-OPDA) in plants (Monte et al. 2020). Exogenous application OPDA and dn-OPDA protects Arabidopsis and Marchantia polymorpha from HS in a COI1-independent manner (Monte et al. 2020). Application of these oxylipin precursors of JA or heat treatment enhance the accumulation of HSP101 HSP26.5, DREB2A, and HSF2A (Muench et al. 2016). Among the plant hormones, ABA plays the most significant roles in abiotic stress responses, largely through regulating stomatal opening. Exogenous ABA application leads to the accumulation of ROS and several smaller HSPs like sHSP17.2, sHSP17.4, and sHSP26 (Hu et al. 2010). Inhibition of cytokinin degradation using inhibitor of cytokinin oxidase/dehydrogenase modestly improves HS tolerance and improve recovery after HS in heat-acclimatized plants (Prerostova et al. 2020).
Role of epigenetics in HS response
Epigenetic regulation holds the key to the stress memory generation in plants (Fig. 2). The process of the epigenetic regulation of gene expression is highly conserved among the eukaryotic organisms. Alteration of histone proteins modulates chromatin composition and thereby influences gene expression (Ueda and Seki 2020). Epigenetic modifications of stress memory genes are associated with an altered expression between first exposure and subsequent exposures (Bäurle and Trindade 2020). Two types of enzymes are mainly involved in histone modifications, writers and erasers. Writers such as HATs (histone acetyltransferase), HMTs (histone methyltransferase), kinases, and ubiquitinase are responsible for the addition of methyl or acetyl group to the tail or core domain of histones. Erasers are HDACs (histone deacetylase), HDMs (histone demethylase), phosphatase, and deubiquitinase, which undo the modifications done by writers (Lohmann et al. 2004; Xu et al. 2017; Ueda and Seki 2020).
Histone acetyltransferases (HATs) in thermotolerance
Arabidopsis genome encodes several HAT genes that play a role in HS response. HATs transfer the acetyl group to amino acids (mostly lysine residues) in histone that weakens the interaction between DNA and histone protein and results in the transcriptional activation of genes. One of the HAT family genes is GENERAL CONTROL NONDEREPRESSIBLE5 (GCN5). Studies on GCN5 described their functional role in both salinity and HS. GCN5 induces the cell wall-related genes ZmEXPANSIN B2 and ZmXYLOGLUCAN ENDOTRANSGLUCOSYLASE/ HYDROLASE1 by H3K9 acetylation (Li et al. 2014). The gcn5 mutants show sensitivity to salinity stress due to defects in the integrity of the cell wall. Interestingly, GCN5 also plays an essential role in providing thermotolerance (Hu et al. 2015). GCN5 enhances H3K9/K14Ac acetylation in the promoter region of the HSFA3 and ULTRAVIOLET HYPERSENSITIVITY6 (UVH6) genes, which is associated with their enhanced expression and thermotolerance in Arabidopsis (Hu et al. 2015).
Histone deacetylase (HDACs) in thermotolerance
Histone deacetylases are the group of enzymes involved in removing the acetyl group from N-acetyl lysine amino acid residues in histone proteins. They allow the wrapping of DNA on histone more tightly and negatively regulates the transcription of genes. The action plays by HDACs is directly opposite to the effect of HATs. HDACs are divided into four classes. Class I, II, and IV HDACs are Zinc-dependent, require Zn++ as a cofactor, while type III is NAD+ dependent (Imai et al. 2000). These classes of HDACs are both positive and negative regulators of the drought, salinity, and HS-responsive genes (Fig. 2) (Ueda and Seki 2020). HDACs are part of the multiprotein chromatin remodeling complex. They interact with chromatin remodeler protein and negatively regulate the expression of genes. One example of HDAC that negatively regulates the thermotolerance is HD2C of Arabidopsis (Buszewicz et al. 2016). Heat-induced expression level of HSFA3, HSFC, HSP101 and APX genes are significantly higher in hd2c mutant. The HD2C deacetylase interacts with SW13B, a protein of BRAHMA (BRM)-containing SWI/SNF (SWITCH/SUC NONFERMENTING) chromatin remodeling complex (Fig. 2) (Buszewicz et al. 2016). BRM, HD2C, and SWI13B, all control thermotolerance in plants (Brzezinka et al. 2016; Buszewicz et al. 2016). Interestingly, HS induces HD2C transcript level upon heat treatment, (Buszewicz et al. 2016) suggesting the involvement of this gene in fine-tuning the stress reponse output.
Demethylation in thermotolerance
Demethylation of histones results in both activation and suppression of expression, depending on the specific positions of amino acids. Whereas methylation at H3K4 activates gene expression, methylation at H3K9 or H3K27 suppresses it (Ueda and Seki 2020). HS induces the expression of HSFA2, which directly activates the H3K27Me3 demethylase enzyme coding gene RELATIVE OF EARLY FLOWERING 6 (REF6) (Liu et al. 2019). This REF6 is also directly involved in the derepressing of HSFA2. Thus, HSFA2 and H3K27Me3 demethylase create a positive feedback loop, which maintains the epigenetic heat shock memory for a longer time by stabilizing HSFA2 in “on-state.” This loop also activates an E3 ubiquitin ligase SUPPRESSOR OF GENE SILENCING 3 (SGS3)-INTERACTING PROTEIN 1 (SGIP1), which eventually upregulates the expression of HEAT-INDUCED TAS1 TARGET 5 (HTT5) and positively regulates thermotolerance (Fig. 2) (Liu et al. 2019). Epigenetic changes are heritable and thus may contribute to long-term and transgenerational thermomemory.
The knowledge gap
An encounter with pathogen results in systemic acquired resistance in plants (SAR), which provides protection against a broad range of pathogens during subsequent infections. A local pathogen infection results in the production of mobile signals that travel through the vasculature and provide systemic immunity (Dempsey and Klessig 2012). Plants can retain infection memory from days to months. Repeated exposures to pathogens can result in inheritable protection (Luna et al. 2012; Slaughter et al. 2012). Thus, the physiological responses towards heat and pathogens are comparable for plants. When TAT is compared with SAR, one finds significant differences in the process of initiation, with substantial similarity in the mechanism of infection memory development and retention. The fundamental difference in the process of perception of these two stresses lies in the virtue of the signal's composition. In contrast to the perception of HS as described above, pathogens are perceived by classical receptor-like kinases (Tang et al. 2017). However, the process of infection-memory development is quite similar, which relies on the epigenetic modifications of the key transcription factors (Luna et al. 2012; Singh et al. 2013; Banday and Nandi 2015). Despite this similarity, it is not known whether HS generates any vascular mobile signal for activating systemic tolerance of HS. The systemic spread of infection immunity makes sense as the pathogens are likely to invade certain parts of a plant and not the whole plant simultaneously. However, one may assume the HS is expected to affect the entire plant equally with a changing environment and thus may not evolve a mechanism of systemic alertness. But how about the plants that grow beneath the canopy? Ray of sunlight falling directly in a part of the plant for an hour in the morning could be a possible trigger of thermotolerance development for the entire plant to protect from the afternoon heat in a tropical forest. Thus, positive selection pressure for the evolution of systemic thermo-tolerance is not unimaginable. However, a systematic study addressing to unravel such a signaling mechanism is lacking. Experiments may be performed by applying heat to a few leaves and testing the systemic immunity a few hours later. Experiments show that SAR mobile signals can be collected from a pathogen inoculated leaf as petiole exudates. Pathogen-free petiole exudates further can induce SAR in a naïve plant (Chaturvedi et al. 2008; Singh et al. 2013). However, it is not known whether there are any such vascular mobile signals for TAT.
Author contribution statement
The concept was given by AKN. AN made the draft manuscript, which was further modified by AKN and approved by both the authors.
Abbreviations
- APx:
-
Ascorbate peroxidase
- BRM:
-
Brahma
- CDPK:
-
Calcium-dependent protein kinases
- CNGC:
-
Cyclic nucleotide gated calcium channel
- GCN5:
-
General control nonderepressible5
- HATs:
-
Histone acetyl transferase
- HD2C:
-
Histone deacetylases 2C
- HDACs:
-
Histone deacetylases
- HDMs:
-
Histone demethylase
- HS:
-
Heat stress
- HLP1:
-
Hikeshi-like proteins 1
- HMTs:
-
Histone methyl transferases
- HSE:
-
Heat shock element
- HSF:
-
Heat shock transcription factor
- HSP:
-
Heat shock proteins
- HTT5:
-
Heat inducible Tas1 target 5
- IP3:
-
Inositol-1,4,5-triphosphate
- MGDG:
-
Monogalactosyldiacylglycerol
- NAD+ :
-
Nicotinamide adenine diphosphate
- PhyB:
-
Phytochrome B
- PIF4:
-
Phytochrome interacting factor 4
- PIP2:
-
Phophatidyl-4,5-inositol bisphosphate
- PIPK:
-
Phosphatidylinositol phosphate kinase
- PLD:
-
Phospholipase D
- PS-II:
-
Photosystem-II
- RBOHD:
-
Respiratory burst oxidase homolog
- REF6:
-
Relative of early flowering 6
- ROS:
-
Reactive oxygen species
- SAR:
-
Systemic acquired resistance
- sHSP:
-
Smaller heat shock proteins
- SGIP1:
-
SGS3 interacting proteins
- SGS3:
-
Suppressor of gene silencing 3
- SWI/SNF:
-
Switch/Suc non-fermenting
- TAT:
-
Thermal acquired tolerance
- UVH6:
-
Ultraviolet hypersensitivity6
References
Banday ZZ, Nandi AK (2015) Interconnection between flowering time control and activation of systemic acquired resistance. Front Plant Sci 6:174
Baniwal SK, Bharti K, Chan KY, Fauth M, Ganguli A, Kotak S, Mishra SK, Nover L, Port M, Scharf KD, Tripp J, Weber C, Zielinski D, von Koskull-Döring P (2004) Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Biosci 29:471–487
Bäurle I, Trindade I (2020) Chromatin regulation of somatic abiotic stress memory. J Exp Bot 71(17):5269–5279. https://doi.org/10.1093/jxb/eraa098
Bernfur K, Rutsdottir G, Emanuelsson C (2017) The chloroplast-localized small heat shock protein Hsp21 associates with the thylakoid membranes in heat-stressed plants. Protein Sci 26:1773–1784
Blakeslee JJ, Spatola Rossi T, Kriechbaumer V (2019) Auxin biosynthesis: spatial regulation and adaptation to stress. J Exp Bot 70:5041–5049
Brzezinka K, Altmann S, Czesnick H, Nicolas P, Gorka M, Benke E, Kabelitz T, Jähne F, Graf A, Kappel C, Bäurle I (2016) Arabidopsis FORGETTER1 mediates stress-induced chromatin memory through nucleosome remodeling. Elife 5:e17061. https://doi.org/10.7554/eLife.17061
Buszewicz D, Archacki R, Palusiński A, Kotliński M, Fogtman A, Iwanicka-Nowicka R, Sosnowska K, Kuciński J, Pupel P, Olędzki J, Dadlez M, Misicka A, Jerzmanowski A, Koblowska MK (2016) HD2C histone deacetylase and a SWI/SNF chromatin remodelling complex interact and both are involved in mediating the heat stress response in Arabidopsis. Plant Cell Environ 39:2108–2122
Byeon B, Bilichak A, Kovalchuk I (2019) Transgenerational response to heat stress in the form of differential expression of noncoding RNA fragments in Brassica rapa plants. Plant Genome. https://doi.org/10.3835/plantgenome2018.04.0022
Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT (2007) A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol 143:251–262
Chaturvedi R, Krothapalli K, Makandar R, Nandi A, Sparks AA, Roth MR, Welti R, Shah J (2008) Plastid omega3-fatty acid desaturase-dependent accumulation of a systemic acquired resistance inducing activity in petiole exudates of Arabidopsis thaliana is independent of jasmonic acid. Plant J 54:106–117
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Danilova MN, Kudryakova NV, Andreeva AA, Doroshenko AS, Pojidaeva ES, Kusnetsov VV (2018) Differential impact of heat stress on the expression of chloroplast-encoded genes. Plant Physiol Biochem 129:90–100
Dempsey DA, Klessig DF (2012) SOS - too many signals for systemic acquired resistance? Trends Plant Sci 17:538–545
Driedonks N, Xu J, Peters JL, Park S, Rieu I (2015) Multi-level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front Plant Sci 6:999
Fan Y, Ma C, Huang Z, Abid M, Jiang S, Dai T, Zhang W, Ma S, Jiang D, Han X (2018) Heat priming during early reproductive stages enhances thermo-tolerance to post-anthesis heat stress via improving photosynthesis and plant productivity in winter wheat (Triticum aestivum L.). Front Plant Sci 9:805
Finka A, Cuendet AF, Maathuis FJ, Saidi Y, Goloubinoff P (2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24:3333–3348
Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95:7197–7202
Härndahl U, Hall RB, Osteryoung KW, Vierling E, Bornman JF, Sundby C (1999) The chloroplast small heat shock protein undergoes oxidation-dependent conformational changes and may protect plants from oxidative stress. Cell Stress Chaperones 4:129–138
Higashi Y, Okazaki Y, Takano K, Myouga F, Shinozaki K, Knoch E, Fukushima A, Saito K (2018) HEAT INDUCIBLE LIPASE1 remodels chloroplastic monogalactosyldiacylglycerol by liberating α-linolenic acid in Arabidopsis leaves under heat stress. Plant Cell 30:1887–1905
Hu X, Li Y, Li C, Yang H, Wang W, Lu M (2010) Characterization of small heat shock proteins associated with maize tolerance to combined drought and heat stress. J Plant Growth Regul 29:455–464
Hu Z, Song N, Zheng M, Liu X, Liu Z, Xing J, Ma J, Guo W, Yao Y, Peng H, Xin M, Zhou DX, Ni Z, Sun Q (2015) Histone acetyltransferase GCN5 is essential for heat stress-responsive gene activation and thermotolerance in Arabidopsis. Plant J 84:1178–1191
Huo L, Sun X, Guo Z, Jia X, Che R, Sun Y, Zhu Y, Wang P, Gong X, Ma F (2020) MdATG18a overexpression improves basal thermotolerance in transgenic apple by decreasing damage to chloroplasts. Hortic Res 7:21
Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795–800
Kumar SV, Lucyshyn D, Jaeger KE, Alos E, Alvey E, Harberd NP, Wigge PA (2012) Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484:242–245
Li H, Yan S, Zhao L, Tan J, Zhang Q, Gao F, Wang P, Hou H, Li L (2014) Histone acetylation associated up-regulation of the cell wall related genes is involved in salt stress induced maize root swelling. BMC Plant Biol 14:105
Li X, Lawas LM, Malo R, Glaubitz U, Erban A, Mauleon R, Heuer S, Zuther E, Kopka J, Hincha DK, Jagadish KS (2015) Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant Cell Environ 38:2171–2192
Ling Y, Serrano N, Gao G, Atia M, Mokhtar M, Woo YH, Bazin J, Veluchamy A, Benhamed M, Crespi M, Gehring C, Reddy ASN, Mahfouz MM (2018) Thermopriming triggers splicing memory in Arabidopsis. J Exp Bot 69:2659–2675
Liu HT, Gao F, Cui SJ, Han JL, Sun DY, Zhou RG (2006) Primary evidence for involvement of IP3 in heat-shock signal transduction in Arabidopsis. Cell Res 16:394–400
Liu J, Sun N, Liu M, Liu J, Du B, Wang X, Qi X (2013) An autoregulatory loop controlling Arabidopsis HsfA2 expression: role of heat shock-induced alternative splicing. Plant Physiol 162:512–521
Liu J, Feng L, Gu X, Deng X, Qiu Q, Li Q, Zhang Y, Wang M, Deng Y, Wang E, He Y, Bäurle I, Li J, Cao X, He Z (2019) An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res 29:379–390
Liu H, Able AJ, Able JA (2020) Transgenerational effects of water-deficit and heat stress on germination and seedling vigour-new insights from Durum wheat microRNAs. Plants (Basel) 9:189. https://doi.org/10.3390/plants9020189
Lohmann C, Eggers-Schumacher G, Wunderlich M, Schöffl F (2004) Two different heat shock transcription factors regulate immediate early expression of stress genes in Arabidopsis. Mol Genet Genom 271:11–21
Luna E, Bruce TJ, Roberts MR, Flors V, Ton J (2012) Next-generation systemic acquired resistance. Plant Physiol 158:844–853
Migicovsky Z, Yao Y, Kovalchuk I (2014) Transgenerational phenotypic and epigenetic changes in response to heat stress in Arabidopsis thaliana. Plant Signal Behav 9:e27971
Mishkind M, Vermeer JE, Darwish E, Munnik T (2009) Heat stress activates phospholipase D and triggers PIP accumulation at the plasma membrane and nucleus. Plant J 60:10–21
Monte I, Kneeshaw S, Franco-Zorrilla JM, Chini A, Zamarreno AM, Garcia-Mina JM, Solano R (2020) An ancient COI1-independent function for reactive electrophilic oxylipins in thermotolerance. Curr Biol 30(962–971):e963
Muench M, Hsin CH, Ferber E, Berger S, Mueller MJ (2016) Reactive electrophilic oxylipins trigger a heat stress-like response through HSFA1 transcription factors. J Exp Bot 67:6139–6148
Neta-Sharir I, Isaacson T, Lurie S, Weiss D (2005) Dual role for tomato heat shock protein 21: protecting photosystem II from oxidative stress and promoting color changes during fruit maturation. Plant Cell 17:1829–1838
Prerostova S, Dobrev PI, Kramna B, Gaudinova A, Knirsch V, Spichal L, Zatloukal M, Vankova R (2020) Heat acclimation and inhibition of cytokinin degradation positively affect heat stress tolerance of arabidopsis. Front Plant Sci 11:87. https://doi.org/10.3389/fpls.2020.00087
Rockwell NC, Su YS, Lagarias JC (2006) Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol 57:837–858
Savchenko G, Klyuchareva E, Abramchik L, Serdyuchenko E (2002) Effect of periodic heat shock on the inner membrane system of etioplasts. Russ J Plant Physiol 49:349–359
Sedaghatmehr M, Mueller-Roeber B, Balazadeh S (2016) The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat Commun 7:12439
Sharma M, Banday ZZ, Shukla BN, Laxmi A (2019) Glucose-regulated HLP1 acts as a key molecule in governing thermomemory. Plant Physiol 180:1081–1100
Sharma L, Priya M, Kaushal N, Bhandhari K, Chaudhary S, Dhankher OP, Prasad PVV, Siddique KHM, Nayyar H (2020) Plant growth-regulating molecules as thermoprotectants: functional relevance and prospects for improving heat tolerance in food crops. J Exp Bot 71:569–594
Singh V, Roy S, Giri MK, Chaturvedi R, Chowdhury Z, Shah J, Nandi AK (2013) Arabidopsis thaliana FLOWERING LOCUS D is required for systemic acquired resistance. Mol Plant Microbe Interact 26:1079–1088
Slama I, Abdelly C, Bouchereau A, Flowers T, Savoure A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot 115:433–447
Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B (2012) Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol 158:835–843
Sundby C, Härndahl U, Gustavsson N, Ahrman E, Murphy DJ (2005) Conserved methionines in chloroplasts. Biochim Biophys Acta 1703:191–202
Suter L, Widmer A (2013) Environmental heat and salt stress induce transgenerational phenotypic changes in Arabidopsis thaliana. PLoS ONE 8:e60364
Tang Y, Wen X, Lu Q, Yang Z, Cheng Z, Lu C (2007) Heat stress induces an aggregation of the light-harvesting complex of photosystem II in spinach plants. Plant Physiol 143:629–638
Tang D, Wang G, Zhou JM (2017) Receptor kinases in plant–pathogen interactions: more than pattern recognition. Plant Cell 29:618–637
Ueda M, Seki M (2020) Histone modifications form epigenetic regulatory networks to regulate abiotic stress response. Plant Physiol 182:15–26
Ul Haq S, Khan A, Ali M, Khattak AM, Gai WX, Zhang HX, Wei AM, Gong ZH (2019) Heat shock proteins: dynamic biomolecules to counter plant biotic and abiotic stresses. Int J Mol Sci 20:5321. https://doi.org/10.3390/ijms20215321
Vu LD, Gevaert K, De Smet I (2019) Feeling the heat: searching for plant thermosensors. Trends Plant Sci 24:210–219
Wang R, Zhang Y, Kieffer M, Yu H, Kepinski S, Estelle M (2016a) HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nat Commun 7:10269
Wang X, Xin C, Cai J, Zhou Q, Dai T, Cao W, Jiang D (2016b) Heat priming induces trans-generational tolerance to high temperature stress in wheat. Front Plant Sci 7:501
Xu Y, Zhang S, Lin S, Guo Y, Deng W, Zhang Y, Xue Y (2017) WERAM: a database of writers, erasers and readers of histone acetylation and methylation in eukaryotes. Nucleic Acids Res 45:D264–d270
Zhou J, Wang J, Cheng Y, Chi YJ, Fan B, Yu JQ, Chen Z (2013) NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. PLoS Genet 9:e1003196
Zhou J, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ (2014a) RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J Exp Bot 65:595–607
Zhou J, Zhang Y, Qi J, Chi Y, Fan B, Yu JQ, Chen Z (2014b) E3 ubiquitin ligase CHIP and NBR1-mediated selective autophagy protect additively against proteotoxicity in plant stress responses. PLoS Genet 10:e1004116
Zhou J, Wang Z, Wang X, Li X, Zhang Z, Fan B, Zhu C, Chen Z (2018) Dicot-specific ATG8-interacting ATI3 proteins interact with conserved UBAC2 proteins and play critical roles in plant stress responses. Autophagy 14:487–504
Acknowledgements
Authors acknowledge the UGC Grant (F.No. 6-8/2017(IC)). Anand Nishad is a recipient of the UGC non-NET fellowship.
Funding
This study was funded by UGC grant (F.No. 6-8/2017(IC)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Neal Stewart.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishad, A., Nandi, A.K. Recent advances in plant thermomemory. Plant Cell Rep 40, 19–27 (2021). https://doi.org/10.1007/s00299-020-02604-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-020-02604-1