Abstract
Key message
The presence of homologous subgenomes inhibited unreduced gamete formation in wheat × Aegilops interspecific hybrids. Unreduced gamete rates were under the control of the wheat nuclear genome.
Abstract
Production of unreduced gametes is common among interspecific hybrids, and may be affected by parental genotypes and genomic similarity. In the present study, five cultivars of Triticum aestivum and two tetraploid Aegilops species (i.e. Ae. triuncialis and Ae. cylindrica) were reciprocally crossed to produce 20 interspecific hybrid combinations. These hybrids comprised two different types: T. aestivum × Aegilops triuncialis; 2n = ABDUtCt (which lack a common subgenome) and T. aestivum × Ae. cylindrica; 2n = ABDDcCc (which share a common subgenome). The frequency of unreduced gametes in F1 hybrids was estimated in sporads from the frequency of dyads, and the frequency of viable pollen, germinated pollen and seed set were recorded. Different meiotic abnormalities recorded in the hybrids included precocious chromosome migration to the poles at metaphase I and II, laggards in anaphase I and II, micronuclei and chromosome stickiness, failure in cell wall formation, premature cytokinesis and microspore fusion. The mean frequency of restitution meiosis was 10.1 %, and the mean frequency of unreduced viable pollen was 4.84 % in T. aestivum × Ae. triuncialis hybrids. By contrast, in T. aestivum × Ae. cylindrica hybrids no meiotic restitution was observed, and a low rate of viable gametes (0.3 %) was recorded. This study present evidence that high levels of homologous pairing between the D and Dc subgenomes may interfere with meiotic restitution and the formation of unreduced gametes. Variation in unreduced gamete production was also observed between T. aestivum × Ae. triuncialis hybrid plants, suggesting genetic control of this trait.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants show a high level of biodiversity and genomic variation. A large part of this genomic variation is due to the capacity for and tolerance of interspecific hybridization, polyploidization and genomic change (de Storme and Mason 2015; Leitch and Leitch 2008). Polyploidy may occur via somatic chromosome doubling or through unreduced or 2n gametes (gametes with the somatic chromosome number) formation. While somatic chromosome doubling is most likely not a common mechanism for transgenerational induction of polyploidy, production of unreduced gametes is now considered to be the main source of polyploidy in nature (Bretagnolle and Thompson 1995; de Storme and Geelen 2013; Harlan 1975; Ramsey and Schemske 1998; Tayalé and Parisod 2013). Genome doubling by unreduced gametes can lead to both autopolyploids (Peloquin et al. 1999; Werner and Peloquin 1991), and allopolyploids (Kihara and Lilienfeld 1949; Ramsey and Schemske 1998).
Although 2n gametes can be produced by premeiotic (Mason et al. 2011) or post-meiotic genome doubling (Bastiaanssen et al. 1998), these mechanisms of unreduced gamete formation are rare (Bretagnolle and Thompson 1995; Mason and Pires 2015). “Meiotic restitution” is considered to be the main mechanism of 2n gamete formation. Meiotic restitution mechanisms convert meiosis into a mitosis-like non-reductional process, generating dyads (and triads) instead of normal tetrads at the end of meiosis II. Two basic pathways by which meiotic restitution takes place have been proposed: first division restitution (FDR) and second division restitution (SDR). In FDR, 2n gametes are genetically equivalent to gametes formed by an omission of meiosis I. Homologous recombination may or may not take place. FDR gametes retain high levels of parental heterozygosity due to retention of both parent homologues, and generate 2n gametes that are genotypically identical to the parent if homologous recombination does not occur. This type of meiotic restitution is often observed in female gametogenesis of apomictic plants and parthenogenically reproducing animals as a reproductive adaptation to generate clonal progeny. In SDR, the 2n gametes are equivalent to those formed by failure of meiosis II. As pairing and recombination occur during meiosis I, the resulting 2n gametes in SDR are mostly homozygous, but retain partial parental heterozygosity (de Storme and Geelen 2013).
Most experiments on production of unreduced gametes have targeted male gametes (Bretagnolle and Thompson 1995), which are more easily assessed than female gametes. In dicotyledonous plant species, a structure known as a sporad is formed after meiosis in microspore mother cells which normally contains four daughter cells within an outer membrane. In monocotyledonous plants and in animals, the products of meiosis are also associated post-meiosis, but without the presence of an outer membrane. For the purposes of this paper, “sporad” will also be used to refer to groups of associated nuclei resulting from a single meiosis. Sporads with four nuclei are referred to as tetrads. Meiotic restitution usually results in sporads that contain unreduced gametes. Such sporads are usually dyads, which contain two unreduced cells (Shamina et al. 1999).
Genetic, epigenetic and environmental factors contribute to the frequent observation of unreduced gametes (de Storme and Geelen 2013; Islam and Shepherd 1980; Ramsey and Schemske 1998; Xu and Joppa 1995; Kobel 1996; Schmidt et al. 2015; Mason et al. 2011; Younis et al. 2014). Unreduced gametes are commonly produced in interspecific or intergeneric hybrids at higher frequencies than in the progenitor species (Ramsey and Schemske 1998; Silkova et al. 2011b), with additional genotype-specific effects (Zhang et al. 2010; Xu and Joppa 2000; Zhang et al. 2007). Various amphidiploids have arisen spontaneously in the tribe Triticeae by formation and union of unreduced gametes (Blanco et al. 1983; Islam and Shepherd 1980; Loureiro et al. 2009; Maan and Sasakuma 1977; Tiwari et al. 2008; Xu and Dong 1992). In the case of intercrossing between two closely related species, resulting F1 hybrids may also exhibit events of meiotic restitution and 2n gamete formation, albeit at significantly lower rates compared to hybrids with more divergent genomes (Tayalé and Parisod 2013). In interspecific hybrids resulting from parents with partial genome homology, a combination of both bivalents and univalents may be formed in which bivalents attach properly to the bipolar spindle in anaphase I, and univalents segregate randomly. Consequently, meiotic restitution normally involves a reductional division of bivalents (separation of homologous chromosomes) together with an equational segregation of univalents (separation of sister chromatids), giving rise to unreduced gametes that are equivalent to a so-called indeterminate (IMR)-type of meiotic restitution (Lim et al. 2001). Furthermore, in interspecific hybrids with homologous subgenomes, formation of unreduced gametes might be impeded by homologous chromosome pairing. This phenomenon has been shown in tetraploid Triticum turgidum × tetraploid Aegilops tauschii hybrids (Wang et al. 2010). However, whether the presence of homologous subgenomes in interspecific Aegilops hybrids impedes meiotic restitution has not been clearly investigated. In the present study, we evaluate the effect of shared parental subgenomes on unreduced gamete formation in two types of interspecific hybrids from crosses between tetraploid Aegilops species and five different bread wheat cultivars. Interspecific hybrids lacking homologous subgenomes were produced by crossing Ae. triuncialis L. (2n = 4x = 28; UtUtCtCt) and bread wheat cultivars (T. aestivum L., 2n = 6x = 42, AABBDD), while for the production of hybrids containing homologous subgenomes, Ae. cylindrica L. (2n = 4x = 28; CcCcDcDc) was crossed with the bread wheat cultivars.
Materials and methods
Plant material
Wheat cultivars and Aegilops accessions used in the present study were provided by the Seeds and Plant Improvement Institute and the Research Institute of Forests and Rangelands of Iran. Crosses were made between five cultivars of bread wheat (‘Mv17’, ‘Navid’, ‘Omid’, ‘Pishgam’ and ‘Zarin’) and two Aegilops species (Ae. triuncialis; accession S101 and Ae. cylindrica; accession S376). Crosses were made between the months of May and Jun in 2013 and 2014 under field conditions on the University of Kurdistan main campus. Temperature during the crossing period ranged from 19–38 °C day to 5–18 °C night, with low humidity and precipitation. The parents were reciprocally crossed in both hybrid types (i.e. wheat-Ae. triuncialis and wheat-Ae. cylindrica), and 20 hybrid combinations were produced in total (Table S1). Only the two outermost florets of spikelets were pollinated. No embryo rescue or hormone treatment was applied. For each hybrid combination at least 15 spikes (in total 340 spikes) were pollinated in each cross combination. Hybrids from the crosses of ‘Mv17’, ‘Navid’, ‘Omid’, ‘Pishgam’ and ‘Zarin’ with Ae. triuncialis were named MT, NT, OT, PT and ZT (for direct crosses) and TM, TN, TO, TP and TZ (for reverse crosses), respectively. In the case of wheat × Ae. cylindrica hybrids, ‘C’ was used for Ae. cylindrica and the hybrids were named MC, NC, OC, PC and ZC for direct crosses and CM, CN, CO, CP and CZ for reverse crosses.
Seed set of hybrid plants
At least 15 F1 seeds from each cross combination were germinated in Petri dishes in the autumn and transplanted to the field at the University of Kurdistan. In the spring of the following year, non-hybrid plants (wheat or Aegilops plants) were deleted at the flowering stage and only the true hybrid plants—which were morphologically distinguishable—were retained in the field. The F1 spikes were bagged to enforce self-pollination and the percentage of plump seeds over total selfed florets was calculated for each cross combination.
Meiotic observations, sporad analysis and unreduced gamete rate measurement
For meiotic studies young spikes of hybrid plants at the appropriate booting stage were fixed in Carnoy’s fixative I for 24 h. Slides were made by squashing the immature anthers in a drop of acetocarmine. Some slides were prepared using 4′,6-diamidino-2-phenylindole (DAPI): these slides were made by squashing the immature anthers in a drop of 45 % acetic acid. Coverslips were removed using liquid nitrogen. Slides were then dried in ethanol series and stained by adding 20 µl of DAPI at 1 µg ml−1 in Vectashield antifade mounting medium (Vector H-1200) and mounted with a coverslip. Male unreduced gamete production was estimated by assessment of meiotic products at the sporad stage. It was assumed that each dyad produced two unreduced nuclei and that each tetrad produced four reduced nuclei at the end of meiosis. Micronuclei (which generally result from laggard chromosomes) were discounted for the purposes of quantification of dyad vs. tetrad frequency. Male unreduced gamete frequencies in each evaluated F1 hybrid anther were estimated using the formula:
In each cross combination, five spikes were randomly selected and an anther from the outer flower of the middle spikelet was evaluated in each spike.
Pollen viability and diameter
Pollen viability was assessed as the percentage of pollen grains stained with Alexander’s solution as described by Peterson et al. (2010), or with 1 % acetocarmine. Mature, swollen pollen grains strongly staining red were assumed to be viable. Mature anthers were randomly selected from seven spikelets of different spikes in each hybrid combination and more than 2000 pollen grains were analysed, and minimum pollen diameter of at least 40 viable pollen grains was measured.
Pollen germination
Sequential pollen germination and staining were done based on Cheng and McComb (1992) and Bartek et al. (2012) with some modifications. The pollen germination medium was made up of 0.7 % agar, 100 mg l−1 H3BO3, 300 mg l−1 CaCl2·2H2O and 0.75 M raffinose in distilled water. A few drops of the medium were added to the surface of a slide. After spreading fresh mature pollen grains, slides were placed on moist filter paper in petri dishes and incubated at 25 °C for 1 h. The cultured pollens were cleared overnight by adding a few drops of 0.8 M NaOH to each slide, which was then replaced with a solution of 0.025 % (w/v) aniline blue in 0.1 M K2PO4 for 2 h followed by adding a few drops of Vectashield antifade mounting medium containing DAPI. No coverslip was applied. Slides were then observed with an Olympus fluorescent microscope using 4× or 10× objectives, under the UV filter. A pollen grain was considered germinated when pollen tube length was at least equal to or greater than the grain diameter. Pollen grains from three anthers (each from a randomly chosen spike) were scored in each of the 20 cross combinations, and only the anther with the highest germination rate was considered.
Statistical tests
Statistical analyses were carried out using Minitab software version 15.2 (Minitab 2010). Standard errors for unreduced gamete and viable pollen rates were computed in each cross combination. Graphs were generated in Excel 2010 (Microsoft Corporation) or Minitab. Student’s paired t test was used to compare mean plump seed set rates between reciprocal genotype crosses of T. aestivum × Ae. triuncialis. Furthermore, Pearson’s correlation coefficient (Pearson’s r) was used to demonstrate whether plump seed set rate is correlated with viable gamete rate or unreduced gamete rate.
Results
Crossing and F1 seed production
F1 seeds were obtained from 20 different cross combinations involving five bread wheat cultivars (‘Mv17’, ‘Navid’, ‘Omid’, ‘Pishgam’ and ‘Zarin’) and two Aegilops spp. (Ae. triuncialis; accession S101 and Ae. cylindrica; accession S376). The parents were readily crossable and no embryo rescue or hormone treatment was needed. In total, 702 F1 seeds from T. aestivum (♀) × Ae. triuncialis (♂) crosses, 283 F1 seeds from Ae. triuncialis (♀) × T. aestivum (♂) crosses, 243 F1 seeds from T. aestivum (♀) × Ae. cylindrica (♂) crosses and 499 F1 seeds from Ae. cylindrica (♀) × T. aestivum (♂) crosses were obtained. The dry F1 seeds were generally healthy with normal size. F1 seeds of wheat × Ae. triuncialis were shrivelled whereas the F1 seeds of wheat × Ae. cylindrica were smooth. The germination rate of F1 seeds was 100 % in both cross types. Vigorous F1 plants were obtained from all cross combinations; all had tough tenacious glumes, obviously inherited from the Aegilops parent. Mitotic chromosome counts confirmed that the F1 plants were pentaploid with 2n = 35 chromosomes (data not shown). Moreover, spike morphology and high sterility rate confirmed that the F1 plants were true hybrids.
Meiosis in the T. aestivum × Ae. cylindrica hybrids
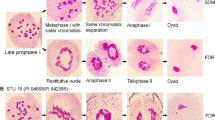
Male sporogenesis was studied in the F1 plants from different cross combinations. Generally, meiotic restitution was not observed in T. aestivum × Ae. cylindrica hybrids (2n = 5x = 35; ABDDcCc). In this hybrid type, meiosis was non-reductional, i.e. neither FDR nor SDR was observed, and the progression of cytokinesis was almost normal (Figs. 1, 2; pathway A). Chromosomes remained unconnected or formed ring and rod bivalents at metaphase I. Subsequent events, including random segregation of the univalents at anaphase I and separation of chromatids toward the poles in anaphase II, led to the production of tetrads. Metaphase-anaphase I chromosome configurations in pollen mother cells of T. aestivum × Ae. cylindrica hybrids (in both directions) were predominantly 5–7 ring bivalents, 6–7 rod bivalents and 0–2 trivalents (Fig. 3). The bivalents presumably comprised chromosomes from the D and Dc genomes. Two daughter cells were usually produced at the end of meiosis I. The chromosomes then aligned on the equatorial plates at metaphase II, and lagging chromosomes and chromosome bridges were common at anaphase II. The meiotic products were usually tetrads. Many tetrads contained one or more micronuclei and dyads were not observed.
Telophase II of meiosis in pollen mother cells (PMCs) of Triticum aestivum-Aegilops hybrids. a Meiotic restitution leading to symmetric dyad and finally unreduced gamete production in T. aestivum cv. ‘Omid’ × Ae. triuncialis F1 plants. Small and big arrow heads point to the partially unreduced and the complete unreduced meiotic products, respectively. b Only reductional meiosis was observed in all T. aestivum × Ae. cylindrica hybrids. Bar 100 µm
Meiotic pathways observed in Triticum aestivum-Aegilops hybrids. Both reductional (A) and non-reductional (B) pathways were observed in T. aestivum × Ae. triuncialis while only the reductional pathway was observed in T. aestivum × Ae. cylindrica hybrids; different stages for each pathway have been depicted and the representative meiotic products produced for each pathway are shown to the right side of the picture: a tetrad; b tetrad with decondensed lagging chromosomes inside a microspore; c micronucleus; d unbalanced chromosome distribution at metaphase I producing polyads; e a triad with two reduced and one unreduced microspores; f a dyad resulting from FDR; g, h, j, k Unipolar and unequal chromosome segregation during the first meiotic division leading to complete and partial 2n gametes and micronuclei; i a tetrad and a monad produced by an F1 plant. In the restitution meiocytes, premature cytokinesis occurred earlier in meiocyte development (during meiosis I), generating asymmetrical dyads with one cell containing a small decondensed chromatin segment and one containing a diploid cell nucleus
Typical chromosome arrangements of Triticum aestivum-Aegilops hybrids at metaphase. a Metaphase-anaphase I in a pollen mother cell (PMC) of T. aestivum-Ae. cylindrica hybrid showing 5–7 ring bivalents and 6–7 rod bivalents and trivalents. b Metaphase I in PMCs of T. aestivum × Ae. triuncialis hybrids predominantly showing 7 rod bivalents. No ring bivalents were observed in this hybrid type. c A T. aestivum-Ae. triuncialis hybrid PMC at metaphase I, in which univalents were arranged near the equational plate at anaphase I with a circular pattern from a polar view. Both ‘b’ and ‘c’ forms frequently occurred in T. aestivum-Ae. triuncialis hybrids. Bar 10 µm
Non-reductional meiosis and unreduced gametes in the T. aestivum × Ae. triuncialis hybrids
In the T. aestivum–Ae. triuncialis hybrids, unreduced gametes—estimated through sporad observations—were produced with a frequency of 0.1 on average (Fig. 4). In this type of hybrid, metaphase I chromosome configurations in PMCs with reductional meiosis were predominantly 21 univalents and seven rod bivalents (Fig. 3b). The non-reductional meiosis was mitosis-like: univalent chromosomes were arranged near the equational plate at metaphase I with a clear circular pattern from a polar view, and a ring-shaped restitution nucleus was formed without cytokinesis (Fig. 3c). At late metaphase, the univalents split into sister chromatids, but failed to move to the poles and formed a restitution nucleus. Chromosomes of the PMCs underwent equational division at anaphase and started to decondense at telophase. Symmetric dyad daughter cells were the final products of the process (Fig. 1) which transformed into unreduced microspores. The average frequencies of unreduced gametes in different T. aestivum–Ae. triuncialis hybrid combinations varied from 2.0 % (in ZT hybrids) to 24.8 % (in TO hybrids) with an overall average of 10.1 %.
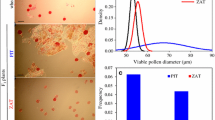
Rates of unreduced gamete production (±standard error), pollen viability (±standard error), pollen germination and seed set (a) and viable pollen size distribution estimates (b) for F1 plants derived from Triticum aestivum × Aegilops triuncialis and T. aestivum × Ae. cylindrica hybrids. Hybrids from the crosses of bread wheat cultivars ‘Mv17’, ‘Navid’, ‘Omid’, ‘Pishgam’ and ‘Zarin’ with Ae. triuncialis were named MT, NT, OT, PT and ZT (for direct crosses) and TM, TN, TO, TP and TZ (for reverse crosses) respectively. The letter ‘T’ has been used instead of ‘C’ for Ae. cylindrica in wheat-Ae. cylindrica hybrids
Genotype-specific and maternal effects
Although T. aestivum × Ae. triuncialis hybrids underwent primarily reductional meiosis, this trait was affected by the T. aestivum genotypes involved. ‘Pishgam’ × Ae. triuncialis hybrids in both directions (PT and TP) produced significantly higher rates of unreduced gametes (20.6 and 24.8 %, respectively on average), while ‘Zarin’ × Ae. triuncialis hybrids in both directions (ZT and TZ), produced the lowest rates of unreduced gamete (2 and 4.5 %, respectively on average; Fig. 4). No dyad (or unreduced gamete) was observed in T. aestivum × Ae. cylindrica hybrids.
Types of meiotic abnormalities
Different meiotic abnormalities were observed during both reductional and non-reductional meiosis and in both hybrid types. These are depicted in Fig. 2 and examples provided in the right-hand column of the figure. These abnormalities included irregular cytokinesis, lagging chromosomes, unequal segregation of chromosomes to one pole during the first division, asymmetric diakinesis and chromosome bridges. Abnormal sporads (other than dyads) were also observed, including monads, triads and pentads. These were assumed to contain abnormal chromosome numbers. The frequency of triads was very low, ranging from 0 to 5 in each evaluated anther. Many tetrads contained one or more micronuclei in T. aestivum × Ae. cylindrica hybrids, evidence of isolated lagging chromosomes in anaphase I or II. Cell fusion was detected in meiosis in the “Pishgam” × Ae. triuncialis hybrids, including cells connected by a thin or a thick cytoplasmic channel (Fig. 5a, b) showing chromatin transfer between meiocytes. Completely fused cells were also observed in which the meiotic products were present in the same cytoplasm (Fig. 5c).
Pollen viability and diameter
Hybrid combinations varied significantly in pollen viability (as revealed by acetocarmine or Alexander’s staining), pollen germination rate and plump seed set. All T. aestivum × Ae. triuncialis (ABDUtCt) hybrids produced viable pollen grains at a frequency ranging from 0.84 to 7.33 % on average by genotype. However, the mean frequencies of viable pollen grains in the T. aestivum–Ae. cylindrica hybrids were significantly lower than those of the T. aestivum × Ae. triuncialis hybrids, varying from 0.25 % (for CM hybrids) to 0.43 % (for MC hybrids) depending on the T. aestivum parent (Fig. 4a).
The mean pollen diameter was significantly higher in T. aestivum × Ae. triuncialis hybrids than that of T. aestivum × Ae. cylindrica hybrids, ranging from 47.68 to 67.30 µm (58.72 µm on average) for the first hybrids type and from 37.52 to 48.48 µm (43.02 µm on average) for the second hybrids type (Fig. 4b). Significant differences in average pollen diameter were observed within hybrid combinations in each hybrid type as well. Pollen diameter distributions were generally positively skewed and showed more variable diameter in T. aestivum × Ae. triuncialis hybrids compared with that of T. aestivum × Ae. cylindrica hybrids as shown in Fig. 4b. In T. aestivum × Ae. triuncialis hybrids, viable gamete rate was correlated with the rate of plump seed set (r = 0.707, P = 0.022).
Seed set
The T. aestivum × Ae. triuncialis hybrids produced plump seeds ranging from 0.1 to 4.52 % per pollinated floret with a mean value of 0.96 %. Hybrids from ‘Pishgam’ and ‘Omid’ × Ae. triuncialis (namely PT and OT) produced the highest viable gamete ratios (6.26 and 8.92 %, respectively), but ‘Pishgam’ × Ae. triuncialis hybrids in both directions (PT and TP) produced the most plump self-pollinated seed (1.71 and 4.52 %, respectively; Fig. 6a). The rate of seed set in T. aestivum × Ae. triuncialis hybrids was not significantly different between the two cross directions (Student’s pared t test, P = 0.737) Although all T. aestivum–Ae. cylindrica hybrids produced viable gametes, fertilities were extremely low with seed sets ranging from 0 (for MC, NC, OC, CM, CP and CZ) to 0.22 % (for CO) with an average of 0.044 % per pollinated floret (Fig. 4a). Only plump seed was produced in this hybrid type. Germination rates of the F2 seed ranged from 61 to 81 %. In T. aestivum × Ae. triuncialis hybrids, the plump seed set rate was significantly correlated with the rate of unreduced gamete formation (r = 0.874, P = 0.001).
Pollen viability in Triticum aestivum × Aegilops triuncialis hybrids: a differential staining of aborted and non-aborted pollen grains of F1 plants T. aestivum cv. ‘Pishgam’ × Ae. triuncialis (accession S101) visualized using Alexander’s stain. Swollen red pollen grains were considered to be viable. b Germinated pollen grains of wheat cultivar ‘Pishgam’ on pollen culture medium. Bar 50 µm
Pollen germination
As the germination rate of pollen is highly dependent on the medium, we used the pollen germination rate of wheat cv. ‘Roushan’ as control (Fig. 6). The mean pollen germination rate in ‘Pishgam’ on raffinose medium was 68.6 %. Pollen from wheat × Ae. cylindrica hybrids did not germinate, except for OC and CP hybrids which showed 0.07 and 0.03 % germination, respectively (Fig. 4a). As expected, the other hybrid type (wheat-Ae. triuncialis), showed higher germination rates, from 0 (for ZT, TN and TZ) to 2.66 % on average (Fig. 4a).
Discussion
We hypothesized that unreduced gametes would be produced at higher frequencies in interspecific T. aestivum × Ae. triuncialis hybrids, which do not contain homologous chromosome pairs, than in interspecific T. aestivum × Ae. cylindrica hybrids, whose parents share a subgenome in common. This hypothesis was supported by the results from the meiotic studies of reciprocal crosses. The two types of hybrids differed in meiotic behaviour, production of unreduced gametes and seed set. While meiosis was generally reductional in hybrids involving Ae. cylindrica, an FDR-type of non-reductional meiosis was frequent in the Ae. triuncialis hybrid type, leading to viable 2n gametes and consequently F2 seed production. The final product of meiosis in T. aestivum–Ae. cylindrica hybrids was usually tetrads maturing to unbalanced gametes with reduced viability, and hence F2 seeds were rarely produced. On average, 4.84 % viable gametes were produced in the first hybrid type but only 0.30 % viable gametes in the second hybrid type. Most of the viable gametes in T. aestivum × Ae. triuncialis hybrids were assumed to be unreduced gametes resulting from non-reductional meiosis (Fig. 2). Our results provide quantifiable evidence that the presence of homologous subgenomes inhibits unreduced gamete formation in interspecific hybrids, with implications for the subsequent fertility and viability of interspecific hybrid lines (Fig. 7).
A model for the rate of n or 2n gamete formation in interspecific F1 plants. In the ‘U-shaped’ curve, production of n (green colour) or 2n (blue colour) gametes increase depending on the extent of genome similarity between the parents. Hybrids from divergent parents usually undergo non-reductional meiosis and produce unreduced gametes. As in this case, none or a small number of (rod) bivalents are formed at metaphase I: non-reductional meiosis is believed to be univalent dependent. However, hybrids between closely related parents that share some level of genome similarity undergo reductional meiosis, as a higher number of (rod and ring) bivalents are formed at metaphase I, preventing the occurrence of non-reductional meiosis. However, to what extent the resulting gametes would be viable depends on the parental divergence, such that more closely related parents produce more viable gametes. Indeterminate meiotic restitution (IMR) may hypothetically occur if only some chromosomes form bivalents; however, to date few observations of this phenomenon have been recorded
Non-reductional meiosis has been frequently observed in interspecific hybrids in the Triticeae tribe (Matsuoka et al. 2013; Silkova et al. 2011b; Zhang et al. 2010). Cai et al. (2010) reported that in T. turgidum × Ae. tauschii hybrids, chromosomes appeared as univalents: sister kinetochores of the univalents oriented amphitelically, and kinetochores bipolarly attached to microtubules in one-third of meiocytes. Both bipolar and monopolar attachments were observed in other meiocytes. These observations led to the conclusion that the amphitelic orientation of sister kinetochores and persistence of centromeric cohesion between sister chromatids at meiosis I leads to the onset of first division restitution (Cai et al. 2010). Similar conclusions were drawn by FISH using rye centromere probes on PMCs of F1 wheat–rye amphihaploid hybrids (Zeng et al. 2014). These results generally support the hypothesis that hybrids between more distinct parents are more likely to trigger univalent dependent non-reductional meiosis and produce unreduced gametes. However, the rate of unreduced gamete formation is also affected by genotype (e.g. parental allele composition and chromosome substitution). Other factors such as genotype × environment interaction have been reported to be effective on the rate of unreduced gamete (Xu and Joppa 2000; Mason et al. 2011).
In our previous study, immunolabeling demonstrated that an FDR mechanism is the main mechanism involved in non-reductional meiosis, and in unreduced gamete formation in T. aestivum × Ae. triuncialis hybrids (Mirzaghaderi and Fathi 2015). FDR was also the main mechanism of unreduced gamete formation in the present study (Fig. 2, pathway B). In some PMCs, unipolar or unequal segregation of chromosomes were the cause of non-reductional meiosis, where chromosomes unequally segregated to one pole at metaphase I followed by asymmetric diakinesis, a normal second division and formation of dyads (Fig. 2f–h). Similar mechanisms have been observed in hybrids of T. durum × Haynaldia villosa (Stefani 1986), T. aestivum × S. cereale (Silkova et al. 2011a) and T. persicum × Ae. squarrosa (Xu and Dong 1992). Unreduced gamete formation in interspecific hybrids of Triticeae is believed to be univalent dependent (Cai et al. 2010; Jauhar 2007). In haploid wheat, orientation of sister kinetochores was found to be responsible for unreduced gamete formation (Cai et al. 2010), while in Triticum × Ae. tauschii hybrids univalent alignment was also implicated (Matsuoka et al. 2013). Although FDR is the predominant mechanism observed in interspecific crosses between wheat and different Aegilops species, SDR has also frequently been observed (Silkova et al. 2003, 2011b; Maan and Sasakuma 1977; Matsuoka and Nasuda 2004). However, in the present study neither FDR nor SDR was observed in hybrids of T. aestivum and Ae. cylindrica accession S376 (Fig. 2; pathway A). The presence of more bivalents in interspecific hybrids with homologous subgenomes may inhibit first meiotic restitution by induction of syntelic orientation of kinetochores at metaphase I, leading to the reductional meiotic cell division.
A circular arrangement of chromosomes at metaphase I was the dominant configuration in T. aestivum × Ae. triuncialis hybrids, although this observation depended on wheat genotype. ‘Pishgam’ and ‘Omid’ showed the highest frequencies of dyads relative to tetrads, probably as a product of circular meiotic configurations. These configurations appeared to force the meiotic process to convert to a mitotic-like pathway that ensured 2n gamete formation. Such a mechanism of meiotic restitution has been described in other hybrids, such as wheat × Ae. tauschii (Fukuda and Sakamoto 1992; Matsuoka et al. 2013; Zhang et al. 2007) and wheat × Secale cereale (Silkova et al. 2011b). However, in ‘Zarin’ × Ae. triuncialis hybrids, the chromosomes remained unconnected or formed rod bivalents at metaphase I. Random segregation of the univalents at anaphase I and subsequent events, including separation of chromatids toward the poles in anaphase II, led to the production of tetrads. Most of these microspores matured into non-viable pollen grains.
Figure 4 shows that the type of meiosis and frequency of unreduced gametes were not affected by cross direction, indicating that non-reductional meiosis and the ratio of unreduced gamete formation is controlled by nuclear genes. These findings are in agreement with those of previous studies which reported that the occurrence of hybrid genome doubling through unreduced gamete production is controlled by nuclear genes (Matsuoka and Nasuda 2004; Zhang et al. 2007, 2010; Xu and Joppa 1995; Xu and Dong 1992). Genes for high frequencies of FDR or normal second-division of FDR cells were mapped onto chromosome 4A or to chromosomes 3A and 6A of durum wheat cultivar ‘Langdon’, respectively (Xu and Joppa 2000). Moreover, six QTLs in the Ae. tauschii genome were found to be responsible for the FDR type of unreduced gamete formation in F1 hybrids between Triticum turgidum and Aegilops tauschii (Matsuoka et al. 2013). Although the underlying molecular mechanisms behind the different FDR unreduced gamete production pathways are not well characterized in Triticum and Aegilops, recent evidence suggests that paralogous genes (e.g. tam) linked to unreduced gamete formation in Arabidopsis (d’Erfurth et al. 2010) are also implicated in FDR in wheat × Aegilops hybrids (Hao et al. 2014).
The presence of 2n pollen is often associated with the occurrence of each of monads, dyads and triads during microsporogenesis (Mason et al. 2011), except when 2n gamete formation is the result of pre- or postmeiotic restitution. However, very few triads (0–5 per anther) were observed in our study. Different mechanisms of meiotic non-reduction were likely operating in our study relative to previous observations in other genera. For instance, Nelson et al. (2009) observed parallel spindles at anaphase II to be a common mechanism of FDR unreduced gamete production, but this mechanism is unlikely in monocotyledonous plants, where cytokinesis separates daughter nuclei after meiosis I. By contrast, both distribution of chromosomes across the meiotic plate and cytomixis were implicated as mechanisms of gametic non-reduction in our study. Cell fusion was detected in meiosis in the “Pishgam” × Ae. triuncialis hybrids, including cells connected by a thin or a thick cytoplasmic channel (Fig. 5a, b) typical of those recorded during cytomixis, showing chromatin transfer between meiocytes. If the meiotic process evolves normally in the fused cells, restitution nuclei will give rise to 2n gametes. Similar cytoplasmic channels promoted cytomixis in tetraploid accessions of Brachiaria dictyoneura (Risso-Pascotto et al. 2006) and B. nigropedata (Utsunomiya et al. 2004), as well as in hybrids between Aegilops tauschii and T. turgidum (Peng et al. 2003).
The seed set rate on the T. aestivum × Ae. triuncialis hybrids in the present study was correlated with the rate of unreduced gamete formation (r = 0.874, P = 0.001). However, there was a moderately high correlation between seed set rate and viable gametes (r = 0.707, P = 0.022) indicating that seed set could be a good indicator of unreduced gamete formation in wheat × Aegilops interspecific hybrids. Supporting this result, Zhang et al. (Zhang et al. 2010) observed an average seed set of 4.93 and 7.97 in the hybrids of T. turgidum × Ae. tauschii ssp. strangulate and T. turgidum × Ae. tauschii ssp. tauschii respectively, and concluded that self-pollinated seed set is a good indicator of unreduced gamete formation. In another study, Zhang et al. (2007) observed an average seed set of 25 % in T. turgidum–Ae. tauschii interspecific hybrids and stated that meiotic restitution results in higher self-pollinated seed set in the hybrids between T. turgidum–Ae. tauschii amphidiploids and Ae. variabilis (2n = 28, UUSlSl). The greater viability advantage of unreduced gametes produced by meiotic restitution is putatively conferred by the presence of a full, balanced complement of chromosomes and hence genetic information from one parent species in interspecific hybridization events.
Conclusions
Hybrids without a common subgenome (i.e. T. aestivum × Ae. triuncialis hybrids) produced unreduced gametes and thereby putatively polyploid seeds. By contrast, hybrids with a common subgenome (i.e. T. aestivum × Ae. cylindrica hybrids) failed to produce unreduced gametes. Unreduced gametes were produced at higher frequencies on average in some interspecific hybrids depending on the T. aestivum parent genotype, but no maternal parent effects were observed, implying nuclear genetic control of this trait. Unreduced gametes were also more viable than reduced gametes in interspecific hybrids. However, what level of genome homology (or number of bivalents formed) turns reductional meiosis to non-reductional to produce unreduced gametes needs more evaluation to be understood. The results of the present study have potential applications in breeding programmes aimed at creating new cultivars at higher ploidy levels and creating bridges to transfer desirable genes from wild diploid species into cultivated polyploid gene pools.
Author contribution statement
ZF and SA: Laboratory work; GM: Conception and design of the study; providing of genetic material, analysis and interpretation of data; manuscript writing; ASM analysis and interpretation of data; critical discussion and manuscript corrections. All authors read and approved the final manuscript.
References
Bartek M, Hodnett G, Burson B, Stelly D, Rooney W (2012) Pollen tube growth after intergeneric pollinations of iap-homozygous Sorghum. Crop Sci 52:1553–1560
Bastiaanssen HJM, van den Berg PMMM, Lindhout P, Jacobsen E, Ramanna MS (1998) Postmeiotic restitution in 2n egg formation of diploid potato. Heredity 81:20–27
Blanco A, Simeone R, Tanzarella OA, Greco B (1983) Morphology and chromosome pairing of a hybrid between Triticum durum Desf. and Haynaldia villosa (L.) Schur. Theor Appl Genet 64:333–337
Bretagnolle F, Thompson J (1995) Tansley review no. 78. Gametes with the stomatic chromosome number: mechanisms of their formation and role in the evolution of autopolypoid plants. New Phytol 129:1–22
Cai X, Xu SS, Zhu X (2010) Mechanism of haploidy-dependent unreductional meiotic cell division in polyploid wheat. Chromosoma 119:275–285
Cheng C, McComb J (1992) In vitro germination of wheat pollen on raffinose medium. New Phytol 120:459–462
de Storme N, Geelen D (2013) Sexual polyploidization in plants—cytological mechanisms and molecular regulation. New Phytol 198:670–684
de Storme N, Mason A (2015) Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr Plant Biol 1:10–33
d’Erfurth I, Cromer L, Jolivet S, Girard C, Horlow C, Sun Y, To JP, Berchowitz LE, Copenhaver GP, Mercier R (2010) The cyclin-A CYCA1; 2/TAM is required for the meiosis I to meiosis II transition and cooperates with OSD1 for the prophase to first meiotic division transition. PLoS Genet 6:e1000989
Fukuda K, Sakamoto S (1992) Studies on unreduced gamete formation in hybrids between tetraploid wheats and Aegilops squarrosa L. Hereditas 116:253–255
Hao M, Luo J, Zeng D, Zhang L, Ning S, Yuan Z, Yan Z, Zhang H, Zheng Y, Feuillet C, Choulet F, Yen Y, Zhang L, Liu D (2014) QTug.sau-3B is a major quantitative trait locus for wheat hexaploidization. G3 (Bethesda) 4:1943–1953
Harlan JR (1975) On Ö. Winge and a prayer: the origins of polyploidy. Bot Rev 41:361–390
Islam AKMR, Shepherd KW (1980) Meiotic restitution in wheat barley hybrids. Chromosoma 68:252–261
Jauhar PP (2007) Meiotic restitution in wheat polyhaploids (amphihaploids): a potent evolutionary force. J Hered 98:188–193
Kihara H, Lilienfeld F (1949) A new synthesized 6x-wheat. Hereditas 35(S1):307–319
Kobel HR (1996) Allopolyploid speciation. In: Tinsley RC, Kobel HR (eds) The biology of Xenopus. Clarendon Press, Oxford, pp 391–401
Leitch A, Leitch I (2008) Genomic plasticity and the diversity of polyploid plants. Science 320:481–483
Lim KB, Ramanna MS, de Jong JH, Jacobsen E, van Tuyl JM (2001) Indeterminate meiotic restitution (IMR): a novel type of meiotic nuclear restitution mechanism detected in interspecific lily hybrids by GISH. Theor Appl Genet 103:219–230
Loureiro I, Escorial C, Garcıa-Baudin JM, Chueca MC (2009) Spontaneous wheat-Aegilops biuncialis, Ae. geniculata and Ae. triuncialis amphiploid production, a potential way of gene transference. Span J Agric Res 7:614–620
Maan SS, Sasakuma T (1977) Fertility of amphihaploids in Triticinae. J Hered 57:76–83
Mason AS, Pires JC (2015) Unreduced gametes: meiotic mishap or evolutionary mechanism? Trends Genet 31:5–10
Mason A, Nelson M, Yan G, Cowling W (2011) Production of viable male unreduced gametes in Brassica interspecific hybrids is genotype specific and stimulated by cold temperatures. BMC Plant Biol 11:103
Matsuoka Y, Nasuda S (2004) Durum wheat as candidate for the unknown female progenitor of bread wheat: an empirical study with a highly fertile F1 hybrid with Aegilops tauschii Coss. Theor Appl Genet 109:1710–1717
Matsuoka Y, Nasuda S, Ashida Y, Nitta M, Tsujimoto H, Takumi S, Kawahara T (2013) Genetic basis for spontaneous hybrid genome doubling during allopolyploid speciation of common wheat shown by natural variation analyses of the paternal species. PLoS One 8:e68310
Minitab (2010) Minitab 16 statistical software. Minitab Inc., State College
Mirzaghaderi G, Fathi N (2015) Unreduced gamete formation in wheat: Aegilops triuncialis interspecific hybrids leads to spontaneous complete and partial amphiploids. Euphytica 206:67–75
Nelson MN, Mason AS, Castello M-C, Thomson L, Yan G, Cowling WA (2009) Microspore culture preferentially selects unreduced (2n) gametes from an interspecific hybrid of Brassica napus L.× Brassica carinata Braun. Theor Appl Genet 119:497–505
Peloquin SJ, Boiteux LS, Carputo D (1999) Meiotic mutants in potato: valuable variants. Genetics 153:1493–1499
Peng Z-S, Yang J, Zheng G-C (2003) Cytomixis in pollen mother cells of new synthetic hexaploid amphidiploid (Aegilops tauschii × Triticum turgidum). Cytologia 68:335–340
Peterson R, Slovin JP, Chen C (2010) A simplified method for differential staining of aborted and non-aborted pollen grains. Int J Plant Biol 1:e13
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29:467–501
Risso-Pascotto C, Pagliarini MS, Valle CB (2006) Microsporogenesis in Brachiaria dictyoneura (Fig. & Fe Not.) Stapf (Poaceae: Paniceae). Genet Mol Res 5:837–845
Schmidt A, Schmid MW, Grossniklaus U (2015) Plant germline formation: common concepts and developmental flexibility in sexual and asexual reproduction. Development 142:229–241
Shamina N, Dorogova N, Goncharov N, Orlova A, Trunova S (1999) Abnormalities of spindle and cytokine behavior leading to the formation of meiotic restitution nuclei in intergeneric cereal hybrids. Cell Biol Int 23:863–870
Silkova OG, Shchapova AI, Kravtsova LA (2003) Mechanisms of meiotic restitution and their genetic regulation in wheat–rye polyhaploids. Russ J Genet 39:1271–1280
Silkova O, Shchapova A, Shumny V (2011a) Meiotic restitution in amphihaploids in the tribe Triticeae. Russ J Genet 47:383–393
Silkova OG, Shchapova AI, Shumny VK (2011b) Patterns of meiosis in ABDR amphihaploids depend on the specific type of univalent chromosome division. Euphytica 178:415–426
Stefani A (1986) Unreduced gametes in the F1 hybrid of Triticum durum Desf. × Haynaldia villosa Schur. Zeitschrift für Pflanzenzüchtung 96:8–14
Tayalé A, Parisod C (2013) Natural pathways to polyploidy in plants and consequences for genome reorganization. Cytogenet Genome Res 140:79–96
Tiwari VK, Rawat N, Neelam K, Randhawa GS, Singh K, Chhuneja P, Dhaliwal HS (2008) Development of Triticum turgidum subsp. durum–Aegilops longissima amphiploids with high iron and zinc content through unreduced gamete formation in F1 hybrids. Genome 51:757–766
Utsunomiya KS, Pagliarini MS, Valle CBd (2004) Chromosome transfer among meiocytes in Brachiaria nigropedata (Ficalho & Hiern) Stapf (Gramineae). Cytologia 69:395–398
Wang C-J, Zhang L-Q, Dai S-F, Zheng Y-L, Zhang H-G, Liu D-C (2010) Formation of unreduced gametes is impeded by homologous chromosome pairing in tetraploid Triticum turgidum × Aegilops tauschii hybrids. Euphytica 175:323–329
Werner JE, Peloquin SJ (1991) Occurrence and mechanisms of 2n egg formation in 2x potato. Genome 34:975–982
Xu S, Dong Y (1992) Fertility and meiotic mechanisms of hybrids between chromosome autoduplication tetraploid wheats and Aegilops species. Genome 35:379–384
Xu S, Joppa L (1995) Mechanisms and inheritance of first division restitution in hybrids of wheat, rye, and Aegilops squarrosa. Genome 38:607–615
Xu S, Joppa L (2000) First-division restitution in hybrids of Langdon durum disomic substitution lines with rye and Aegilops squarrosa. Plant Breeding 119:233–241
Younis A, Hwang Y-J, Lim K-B (2014) Exploitation of induced 2n-gametes for plant breeding. Plant Cell Rep 33:215–223
Zeng D-Y, Hao M, Luo J-T, Zhang L-Q, Yuan Z-W, Ning S-Z, Zheng Y-L, Liu D-C (2014) Amphitelic orientation of centromeres at metaphase I is an important feature for univalent-dependent meiotic nonreduction. J Genet 93:531–534
Zhang L-Q, Yen Y, Zheng Y-L, Liu D-C (2007) Meiotic restriction in emmer wheat is controlled by one or more nuclear genes that continue to function in derived lines. Sex Plant Reprod 20:159–166
Zhang L-Q, Liu D-C, Zheng Y-L, Yan Z-H, Dai S-F, Li Y-F, Jiang Q, Ye Y-Q, Yen Y (2010) Frequent occurrence of unreduced gametes in Triticum turgidum–Aegilops tauschii hybrids. Euphytica 172:285–294
Acknowledgments
This research was financially supported by the University of Kurdistan, Sanandaj, Iran. ASM is funded by an Emmy Noether DFG award (MA 6473/1-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by I. Hwang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fakhri, Z., Mirzaghaderi, G., Ahmadian, S. et al. Unreduced gamete formation in wheat × Aegilops spp. hybrids is genotype specific and prevented by shared homologous subgenomes. Plant Cell Rep 35, 1143–1154 (2016). https://doi.org/10.1007/s00299-016-1951-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1951-9