Abstract
Key message
Cadmium sensitivity in sultr1;1 - sultr1;2 double mutant with limiting sulfate supply is attributed to the decreased glutathione content that affected oxidative defense but not phytochelatins’ synthesis.
Abstract
In plants, glutathione (GSH) homeostasis plays pivotal role in cadmium (Cd) detoxification. GSH is synthesized by sulfur (S) assimilation pathway. Many studies have tried to investigate the role of GSH homeostasis on Cd tolerance using mutants; however, most of them have focused on the last few steps of S assimilation. Until now, mutant evidence that explored the relationship between GSH homeostasis on Cd tolerance and S absorption is rare. To further reveal the role of GSH homeostasis on Cd stress, the wild-type and a sultr1;1-sultr1;2 double mutant which had a defect in two distinct high-affinity sulfate transporters were used in this study. Growth parameters, biochemical or zymological indexes and S assimilation-related genes’ expression were compared between the mutant and wild-type Arabidopsis plants. It was found that the mutations of SULTR1;1 and SULTR1;2 did not affect Cd accumulation. Compared to the wild-type, the double mutant was more sensitive to Cd under limited sulfate supply and suffered from stronger oxidative damage. More importantly, under the same condition, lower capacity of S assimilation resulted in decreased GSH content in mutant. Faced to the limited GSH accumulation, mutant seedlings consumed a large majority of GSH in pool for the synthesis of phytochelatins rather than participating in the antioxidative defense. Therefore, homeostasis of GSH, imbalance between antioxidative defense and severe oxidative damage led to hypersensitivity of double mutant to Cd under limited sulfate supply.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) contamination is a widespread serious environmental problem, which has mainly originated from anthropogenic activities (Momodu and Anyakora 2010). In plants, Cd is easily taken up by roots in competition with other divalent ions, then translocated into other organs (Baker et al. 2006). As a non-essential metal, Cd causes various phytotoxic symptoms including growth inhibition, leaf chlorosis, and nutrients deficiency (Ebbs and Uchil 2008).
At cellular level, Cd induces oxidative stress by disturbing the balance between pro-oxidants, for example, reactive oxygen species (ROS) and antioxidants (Xu et al. 2012). ROS cause oxidation of polyunsaturated fatty acids in membranes, depolymerization of nucleic acids, and breakage of peptide bonds. In addition, ROS participate in signaling cascades in defense responses, cell cycle, apoptosis or lignification of cell walls (Foyer and Noctor 2011). Therefore, it is necessary to maintain a certain level of ROS for cell metabolism. This is attained with a complex antioxidant system composed by low molecular weight metabolites like ascorbate (AsA) or glutathione (GSH), and antioxidant enzymes like superoxide dismutase (SOD), ascorbate peroxidases (APX), catalases (CAT) or glutathione reductase (GR) (Hegedüs et al. 2001; Paradiso et al. 2008; Xu et al. 2009).
Sulfur (S) is an essential element and sulfate is the major form of inorganic S utilized by plants (Astolfi et al. 2004). Sulfate uptake depends on the help of sulfate transporters, among which SULTR1;1 and SULTR1;2 are two distinct high-affinity transporters (Takahashi et al. 2001; Vidmar et al. 2000). Absorbed sulfate undergoes S assimilation pathways which direct GSH synthesis (Saito 2000).
GSH homeostasis in plants is closely related to Cd tolerance (Nocito et al. 2006; Sun et al. 2007). Besides the antioxidant role of GSH, this tripeptide is also important in the Cd detoxification through the synthesis of phytochelatins (PCs) (Cobbett 2000). Numerous physiological, biochemical and genetic studies have confirmed that PCs are synthesized from GSH by PCs synthase (Rea 2012). Several studies have provided genetic evidence to elucidate the importance of GSH homeostasis on Cd detoxification. For instance, cad 1–1 exhibited Cd sensitivity due to the defect in PCs synthase (Howden et al. 1995); cad2–1 was another Cd-sensitive mutant with a lower level of PCs that was caused by deficiency in GSH (Cobbett et al. 1998). Recently, overexpression of Lycium chinense GSHS (GSH synthetase) in transgenic Arabidopsis resulted in improved tolerance to Cd stress compared to wild-type (Guan et al. 2015). However, these studies mainly concentrate on the GSH or PCs’ synthesis, which belongs to the last few reactions of S assimilation. Until now, mutant evidence that explored the relationship between GSH homeostasis on Cd tolerance and S absorption process is rare.
In present study, an Arabidopsis double sultr1;1-sultr1;2 null mutant, which had defect in two distinct high-affinity sulfate transporters and displayed low sulfate uptake efficiency (Barberon et al. 2008) was used to investigate the possible role of GSH homeostasis on Cd tolerance under sulfate deficiency. Double mutant and wild-type Arabidopsis seedlings were first exposed to different sulfate supplies with or without Cd for 2 weeks. Then, we compared the root length, biomass, chlorophyll or soluble protein content, oxidative damage, antioxidative defense system, GSH homeostasis and several S assimilation-related genes expression between double mutant and wild-type Arabidopsis seedlings. So far, the potential role of GSH homeostasis on Cd tolerance in a sulfate uptake Arabidopsis has not been established. On one hand, our study reaffirms the importance of high-affinity sulfate transporters or sufficient sulfate supply for Cd tolerance. On the other hand, this research provides a genetic evidence to acknowledge the GSH homeostasis on Cd tolerance under limited sulfate supply. The use of genetic mutant allows us to establish the connections between Cd-induced growth reduction at a whole plant level and its negative effects on biochemical processes at cellular level.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana genotypes used for this study had “Columbia” background. The wild-type accession was obtained from the Nottingham Arabidopsis Stock Centre (N907), while the sultr1;1-sultr1;2 double mutant was obtained from Dr. Françoise Gosti (Institute National de la Recherché Agronomique, Universite Montpellier II, France). According to the methods by Barberon et al. (2008), double mutant was produced after crossing two single mutant (sultr1;1 and sultr1;2), three double mutant lines were selected by PCR, of which one line was chosen for further analysis. This line was then backcrossed three times in the original sel1–8, gl background and retained the homozygous gl mutation. For the sultr1;1 single mutant, it displays a T-DNA insertion in the 10th of the 13 exons of the SULTR1;1 gene (At4g08620) located in chromosome 4 (Barberon et al. 2008). While, the sultr1;2 single mutant (sel1–8 allele) harbors the I551T EMS-induced mutation in the SULTR1;2 gene (At1g78000) (Shibagaki et al. 2002).
Sterilized wild-type and double mutant Arabidopsis seeds were sown on half-strength (1/2) modified MS basal agar medium (Murashige and Skoog 1962) with various treatments in Petri dishes. Sulfate salts of Mg2+, Mn2+, Cu2+, Zn2+ were substituted by equimolar amount of chloride salts (Astolfi et al. 2004). After breaking seed dormancy in dark at 4 °C for 3 days, seeds were geminated and cultivated in a controlled growth chamber with an 8-h light/16-h dark period cycle under a photon flux rate of 200 μmol m−2 s−1 at temperature of 22 °C and relative humidity of 80 %.
Treatments
Pre-experiments were conducted to select the proper limited or sufficient sulfate and Cd concentration. First, seeds of wild-type and double mutant were sown on 1/2 modified MS medium supplemented with 0, 25, 50, 100, 200, 400 or 1500 μM K2SO4, respectively. 1/2 standard MS medium (approximately 860 μM sulfate) was chosen as the control. Primary root length was considered as the basic selection parameter. Then, seeds of wild-type and double mutant were also sown on the medium containing 0, 100, 200, 400, 1/2 standard MS or 1500 μM K2SO4 together with 10, 20, 30, 40 or 50 μM CdCl2. The phenotype and survival situations of both Arabidopsis lines were observed.
According to the root length and phenotype results, 200 or 1500 μM K2SO4 and 20 μM CdCl2 were finally chosen as the limited or sufficient sulfate and Cd treatment concentration. For the subsequent experiments, seedlings were divided into four groups: (1) 1/2 modified MS medium supplemented with 200 μM K2SO4 as the limited sulfate supply (LS); (2) 1/2 modified MS medium supplemented with 200 μM K2SO4 and 20 μM CdCl2 as the combined stress of LS and Cd stress (LS + Cd); (3) 1/2 modified MS medium supplemented with 1500 μM K2SO4 as the control treatment of sufficient sulfate supply (SS); (4) 1/2 modified MS medium supplemented with 1500 μM K2SO4 and 20 μM CdCl2 as the ameliorative effect of SS under Cd stress (SS + Cd). After treatment for 2 weeks, seedlings were harvested, frozen in liquid nitrogen and stored at −80 °C until used, with exception of some fresh seedling materials used for element or chlorophyll content measurement.
Primary root length measurements
After ten days growth, primary root length of seedlings was measured by digitalizing Petri dishes pictures via a scanner (HP ScanJet 7400C; Hewlett-Packard Company) and subsequently analyzing the images by Scion Image software (version 4.02). Forty plants were estimated for each treatment.
Total RNA extraction and gene expression analysis
Total RNA was isolated from wild-type and double mutant seedlings (0.2 g) under different treatments with 1 ml TRIZOL reagent (Invitrogen, Inc., CA, USA) according to manufacturer’s procedure. The RNA integrity was confirmed by 1 % agarose gel electrophoresis and the concentration was determined by an ultraviolet spectrophotometer (Cary 50, Varian, USA). First-strand cDNA was synthesized with M-MLV reverse transcriptase (TaKaRa, Dalian, China) with an oligo d(T)18 primer.
Quantitative real-time PCR (qRT-PCR) was used to analyze genes expression by StepOnePlus™ Real-Time PCR System (Applied Biosystems). A 10 μl real-time PCR system was adopted according to Chen et al. (2011). Primers listed in Supporting Information Table S1 were designed according to the known sequences in NCBI database. Relative quantification values for each target gene were calculated by the 2−ΔΔCt method (Livak and Schmittgen 2001). Gene expression levels were standardized using actin2 as the internal control. The mRNA quantity from SS-treated sample was set as “1” for each gene and other treated samples were expressed relative to the corresponding control. The average expression folds of three biological replicates, including two technical replicates for each biological replicate were calculated. Then, hierarchical clustering of the expression profiles was performed on the log base 2 average expression fold values using Cluster software (version 3.0). Complete linkage algorithm was enabled and the results were plotted employing the Treeview software (version 1.1.3).
Determination of chlorophyll and soluble protein contents
Chlorophyll content was measured by the method of Lichtenthaler (1987) with some modifications. After extraction using 5 ml of 80 % (v/v) aqueous acetone, the total chlorophyll was calculated from the absorbance of leaf chlorophyll extracts at 470, 646, and 663 nm. Soluble protein content was measured according to Bradford (1976) using the Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA, USA) with bovine serum albumin as the standard protein.
Measurement of total S and Cd contents
Fresh Arabidopsis seedlings were washed thoroughly with deionized water, then dried at 70 °C until constant weight. The dried seedlings were ground to a fine power with an agate mortar and pestle. About 10 mg of subsamples were weighted using an ultra-microbalance (Model 4504MP8, Sartorius Crop., Göttingen, Germany) and S elemental content was analyzed by an Carbon–Hydrogen–Nitrogen–Sulfur (CHNS) Elemental Analyzer (Model FlashEA 1112, Thermo Finnigan, San Josa, CA) (Chen et al. 2012b). For Cd elemental measurement, seedling roots and shoots were separated and washed for 10 min in 5 mM ice-cold CaCl2 solution to displace extracellular Cd (Nocito et al. 2002). Then samples were washed twice with deionized water, followed by the digestion with 5 ml concentrated nitric acid in a microwave digestion system (CEM, Inc., Mars-V). The solution was finally diluted to 50 ml with deionized water (Zhang et al. 2010). Cd content was determined by an inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7700 series, Agilent Co. Inc., USA).
Measurement of lipid peroxidation and hydrogen peroxide content
Lipid peroxidation level was determined in terms of malondialdehyde (MDA) content by the thiobarbituric acid reaction (Yan et al. 2010). Hydrogen peroxide (H2O2) was measured spectrophotometrically according to Alexieva et al. (2001).
Analysis of antioxidant enzyme activities
Fresh seedling samples were homogenized on an ice bath with 1 ml of 50 mM phosphate buffer solution (pH 7.0) containing 1 mM ascorbic acid and 1 mM EDTA. The homogenate was centrifuged at 15,000 g for 15 min at 4 °C and the supernatant was used for further assays.
SOD (EC 1.15.1.1) activity was determined by measuring its ability to inhibit photochemical reduction of nitroblue tetrazolium (NBT) (Beauchamp and Fridovich 1971). The 3 ml of reaction mixture consisted of 20 μM riboflavin, 150 mM l-methionine, 600 μM NBT, 400 μl of H2O and 200 μl of enzyme extracts. The reaction was started with the addition of riboflavin and illuminated for 20 min under irradiance of 180 μmol photons m−2 s−1 provided by a white fluorescent lamp. The system without enzyme extracts was conducted as the negative control. One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of the reduction of NBT when measured at 560 nm.
CAT (EC 1.11.1.6) activity was detected at 240 nm in 1.9 ml of 50 mM phosphate buffer solution (pH 7.0) containing 1 ml of 0.1 % H2O2 and 50 μl enzyme extracts, respectively. The enzyme activity was calculated from the initial rate of the reaction using the extinction coefficient of H2O2 at 40 M−1 cm−1 (Dhindsa et al. 1981).
APX (EC 1.11.1.11) activity was conducted by the method of Nakano and Asada (1981). The reaction mixture contained 50 mM phosphate buffer (pH 7.0), 1 mM sodium ascorbate and 150 μl of enzyme extracts. The reaction was started by the addition of 0.5 mM H2O2. The enzyme activity was calculated from the initial rate of the reaction using the extinction coefficient of ascorbate which is 2.8 mM−1 cm−1 at 290 nm.
Peroxidase (POD, EC 1.11.1.7) activity was assayed in 2 ml of 100 mM potassium phosphate buffer (pH 6.5) containing 40 mM guaiacol, 10 mM H2O2 and 100 μl of enzyme extract. Activity of enzyme was evaluated by the rate of tetraguaiacol production using an extinction coefficient of 25.5 mM−1 cm−1 at 436 nm (Chen et al. 2012a).
Analysis of antioxidant compounds
Total glutathione (Total GSH), reduced glutathione (GSH), and disulphide glutathione (GSSG) were estimated using a total GSH/GSSH reagent kit (Jian Cheng Bioengineering Institute, Nanjing, China) according to manufacturer’s instructions. Plant sample (0.3 g) was ground with 1 ml ice-cold freshly made 5 % (w/v) m-phosphoric acid (Semane et al. 2007). GSH was estimated from difference between total GSH and GSSG. GSH/GSSG ratio was calculated by two compound values.
Total ascorbate (Total AsA), reduced ascorbate (AsA), and dehydroascorbate (DHA) were determined spectrophotometrically according to Hodges and Forney (2000). For each sample, DHA was estimated from the difference between total AsA and AsA. AsA/DHA ratio was calculated by two compound values.
Determination of thiols
Cysteine (Cys) in plants was measured according to Gaitonde (1967) with some modifications. Plant materials (0.3 g) were homogenized in 5 % chilled perchloric acid and centrifuged at 10,000 g for 15 min at 4 °C (Mishra et al. 2006). Cys content in supernatant was measured using acid/ninhydrin reagent at 560 nm. Total non-protein thiols (NPTs) content in plants was measured and GSH was used as the standard (Chen et al. 2011). Monobromobimane-labeled PCs content was analyzed by high-performance liquid chromatography (HPLC) (Li et al. 2014; Sneller et al. 2000). Detailed PCs measurement procedures were described in Supporting Information Methods: Determination of PCs. PCs content was expressed as GSH equivalent concentration.
Statistical analysis
For root length measurement, forty seedlings were used. For physiological and biochemical measurements, three replicates were used. Statistical treatment of the data was carried out by one-way analysis of variance (One-way ANOVA) with SPSS 19.0 package (SPSS, Chicago, Illionis USA). Duncan’s post-test (P < 0.05) was used for multiple comparisons and Student’s t-test (two tailed) was used for pairwise comparisons. Data are mean ± SE. In all of figures and tables, different capital letters indicate significant differences between different treatments, while the lowercase letters indicate significant differences between two kinds of plant materials under the same treatment.
Results
Effects of Cd on genes expression of sulfate transporter in wild-type Arabidopsis
To investigate the effect of Cd on sulfate uptake in wild-type Arabidopsis seedlings, gene expression levels of SULTR1;1 and SULTR1;2 were analyzed by qRT-PCR (Fig. 1). Limited sulfate treatment (LS) caused seven- or three-fold higher genes expression than control treatment (SS). Meanwhile, 20 μM Cd up-regulated the SULTR1;1 and SULTR1;2 genes expression. SULTR1;1 expression level increased approximately 44-fold under LS + Cd treatment than SS treatment, and it was about four times higher under SS + Cd treatment (Fig. 1a). Similarly, the expression level of SULTR1;2 showed 5- or 1.5-fold increase under LS + Cd or SS + Cd treatment compared to control (Fig. 1b), which is much lower than SULTR1;1. Genes expression results also showed that these two genes were suppressed completely in sultr1;1-sultr1;2 double mutant in each condition analyzed (Fig. 1a, b).
Effects of Cd on SULTR1;1 (a) and SULTR1;2 (b) transcript level in 2-week-old wild-type and double sultr1;1-sultr1;2 null mutant Arabidopsis seedlings under different treatment conditions. Data are mean ± SE of three independent replicates. The SULTR1;1 and SULTR1;2 mRNA transcriptional levels were suppressed completely in mutant. ND represents not detectable. LS limited sulfate supply treatment: 200 μM K2SO4; LS + Cd, limited sulfate supply with Cd treatment: 200 μM K2SO4 + 20 μM CdCl2; SS sufficient sulfate supply treatment: 1500 μM K2SO4; SS + Cd, sufficient sulfate supply with Cd treatment: 1500 μM K2SO4 + 20 μM CdCl2
Effects of Cd on biomass, root length, total chlorophyll, soluble protein, total S content and Cd accumulation in double mutant and wild-type Arabidopsis under LS and SS treatments
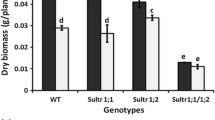
Compared to LS treatment, the seedling biomass of both lines was higher under SS treatment and there was no significant difference between wild-type and double mutant. Meanwhile, the biomass was obviously reduced by Cd in both Arabidopsis lines. Under LS treatment, Cd caused a 41 or 50 % decrease in wild-type and mutant seedlings (Table 1).
In both Arabidopsis lines, LS treatment inhibited the root elongation compared to SS treatment. Similarly, Cd exposure also inhibited the root elongation significantly. Under LS treatment, decrease percentage induced by Cd reached 14.6 % in wild-type seedlings and 22 % in double mutant seedlings (Table 1).
In both Arabidopsis lines, the total leaf chlorophyll content reached the maximum under SS treatment and decreased under both LS and Cd treatments. Interestingly, under LS condition, the total chlorophyll content in double mutant seemed to be more affected by Cd compared to wild-type (Table 1).
In wild-type Arabidopsis, we found that the soluble protein content was almost not affected by different sulfate supplies. Furthermore, the protein content for LS + Cd-treated seedlings decreased by 24 % in comparison to LS-treated seedlings, while no difference was observed between SS and SS + Cd treatment. However, in double mutant, an obvious decrease of soluble protein content was found under LS treatment. The decrease percentage of soluble protein content induced by Cd reached 69 % under LS treatment (Table 1).
To examine the effects of Cd on S content in two lines of Arabidopsis seedlings under different sulfate supplies, we measured the total S content (Fig. 2a). Double mutant accumulated less S than wild-type. In wild-type Arabidopsis, it was more than two-fold higher under SS than LS treatment. In addition, Cd exposure enhanced S accumulation regardless of sulfate supply. Changes in double mutant were consistent with those in wild-type.
Cd accumulation was also measured as shown in Fig. 2b, c. In both Arabidopsis lines, SS treatment enhanced Cd accumulation in roots and shoots. Moreover, there was no noticeable difference between double mutant and wild-type seedlings grown under LS + Cd or SS + Cd treatment.
Oxidative damage and quantification of enzymatic antioxidants in double mutant and wild-type Arabidopsis under LS and SS treatments with or without Cd
To investigate the oxidative damage induced by Cd, lipid peroxidation was estimated by measuring MDA content (Fig. 3a). In wild-type Arabidopsis, Cd exposure had no effect on MDA content under LS or SS treatment. However, for double mutant seedlings, MDA content was 76 % higher under LS + Cd combined stress compared to LS treatment, while only 23 % increase of MDA was measured at SS + Cd stress compared to SS treatment.
Effects of Cd on MDA content (a), H2O2 content (b) and enzymatic antioxidants activities of superoxide dismutase (SOD) (c), catalase (CAT) (d), ascorbate peroxidase (APX) (e) and peroxidase (POD) (f) in wild-type and double sultr1;1-sultr1;2 null mutant Arabidopsis seedlings under limited or sufficient sulfate conditions for 2 weeks. Data are mean ± SE of three independent replicates
Cd exposure induced the accumulation of H2O2 (Fig. 3b). In wild-type Arabidopsis, H2O2 content in seedlings grown under LS + Cd treatment increased by 11 % compared to LS treatment, while it was 32 % in double mutant. Interestingly, double mutant seedlings accumulated more H2O2 content than wild-type in each treatment group.
Compared to LS treatment, LS + Cd treatment increased the activity of SOD by 14 % in wild-type seedlings. However, in double mutant seedlings, SOD activity showed no marked change between LS and LS + Cd treatment (Fig. 3c). Unlike the changes pattern of SOD under LS and LS + Cd treatments, Cd caused 49 and 34 % decrease of SOD activity under SS treatment for wild-type and double mutant, respectively. The activity of CAT decreased by 58 % under LS + Cd combined stress compared to LS treatment in double mutant, while no significant difference was observed in wild-type (Fig. 3d). Furthermore, we also found that the CAT activity in double mutant was lower than those in wild-type under LS + Cd combined stress. No significant change in APX activity was measured in wild-type between LS and LS + Cd treatment, while Cd exposure caused an obvious increase at SS treatment (Fig. 3e). However, in double mutant, Cd exposure resulted in 27 % decrease or 52 % increase of APX activity under LS or SS treatment, respectively. In addition, we also examined the activity of POD as shown in Fig. 3f. Results suggested that Cd did not alter POD activity under LS treatment in both Arabidopsis lines, while Cd increased POD activity under SS treatment.
Non-enzymatic antioxidant defense in double mutant and wild-type Arabidopsis under LS and SS treatments with or without Cd
We further measured the changes of GSH and AsA which play an important role in ROS detoxification. For total GSH content, Cd exposure caused marked increase under LS or SS treatment in both Arabidopsis lines. Furthermore, double mutant kept lower total GSH than wild-type among all treatments except for SS + Cd (Fig. 4a). Similarly, double mutant also kept lower level of reduced GSH than wild-type under all treatments except for SS + Cd (Fig. 4c). Besides, Cd caused approximately 30- or 58-fold elevation in double mutant seedlings under LS or SS treatment, respectively, while the increase was only 18- or 10-fold higher in wild-type. Compared to LS treatment, Cd decreased GSSG content, the oxidized form of GSH, under LS + Cd combined stress in wild-type (Fig. 4e). However, in double mutant, there was no significant difference between LS + Cd and LS treatment (Fig. 4e). Finally, in wild-type seedlings, GSH/GSSG ratio was 0.83 in control, but it turned out to 0.42, 8.66 and 7.99 under LS, LS + Cd and SS + Cd treatment, respectively. Consistent with the results from wild-type, Cd exposure also increased the ratio under LS or SS treatment in double mutant. Moreover, it was noteworthy that double mutant seedlings kept 26 % lower GSH/GSSG ratio under LS + Cd treatment compared to wild-type (Fig. 4g).
Influence of Cd on glutathione–ascorbic acid metabolism in wild-type and sultr1;1-sultr1;2 double mutant Arabidopsis seedlings under limited or sufficient sulfate conditions for 2 weeks. a total glutathione (total GSH) content, c reduced glutathione (GSH) content, e oxidized glutathione (GSSG) content, g the GSH/GSSG ratio, b total ascorbic acid (total AsA) content, d reduced ascorbic acid (AsA) content and f dehydroascorbic acid content (DHA), h the AsA/DHA ratio. Data are mean ± SE of three independent replicates
As other non-enzymatic antioxidants, total AsA content showed no significant difference among all treatments in this study (Fig. 4b). In both Arabidopsis lines, Cd exposure increased the content of reduced AsA under LS treatment. Furthermore, AsA content in double mutant was much higher than that in wild-type under LS + Cd combined treatment (Fig. 4d). For the oxidized form, DHA content decreased under LS + Cd combined stress compared to LS treatment in both Arabidopsis lines; however, it was much lower in double mutant than that in wild-type under LS + Cd combined stress (Fig. 4f). Besides, the AsA/DHA ratio increased after Cd exposure under LS treatment in both plants, especially in double mutant (Fig. 4h). No evident changes were observed between SS and SS + Cd treatment.
Thiol compound changes in double mutant and wild-type Arabidopsis under LS and SS treatments with or without Cd
In wild-type, LS treatment increased Cys content compared to control, while Cd addition further promoted Cys synthesis, with increment of 1.3- or 1-fold, respectively, when seedlings were treated by LS or SS. The changes in mutant were similar as described above in wild-type. However, under LS + Cd combined stress, double mutant seedlings accumulated 53 % more Cys than wild-type (Fig. 5a).
Effects of Cd on three major thiol compounds content in wild-type and double sultr1;1-sultr1;2 null mutant Arabidopsis seedlings under limited or sufficient sulfate conditions for 2 weeks. a cysteine (Cys) content, b total non-protein thiols (NPTs) content, c phytochelatin 2 (PC2) content and d phytochelatin 3 (PC3) content. Data are mean ± SE of three independent replicates. ND represents not detectable
Then we evaluated total content of NPTs. In both plants, different sulfate concentrations did not affect the NPTs content (Fig. 5b). However, after Cd exposure, NPTs content in wild-type or double mutant seedling was 3.45- or 2.78-fold higher than that under LS treatment, while which was approximately 7.25- or 2.57-fold higher than that under SS treatment.
Finally, the content of phytochelatin 2 (PC2), phytochelatin 3 (PC3) or phytochelatin 4 (PC4) was analyzed by HPLC, but only PC2 and PC3 were detected in our study (Fig. 5c, d). In both Arabidopsis lines, Cd stress induced production of PC2 and PC3, especially under SS treatment. Interestingly, under LS + Cd combined stress, double mutant seedlings could accumulate equivalent PCs compared to wild-type.
S assimilation-related genes expression
Cluster analysis of transcriptional fold changes for S assimilation-related genes is shown in Fig. 6. Original genes transcriptional changes are listed in Supporting Information Table S2. Genes expression of five important sulfate transporters was analyzed first. In both Arabidopsis lines, LS treatment down-regulated SULTR2;1, SULTR 1;3, SULTR 3;4 and SULTR 3;5 expression levels. Compared to LS treatment, Cd did not affect the transcript levels of these four genes in wild-type but caused up-regulation in double mutant. Interestingly, SULTR 4;2 expression level was up-regulated in both Arabidopsis lines under LS and LS + Cd treatments.
Cluster analyses of S metabolism-related genes expression in wild-type and double sultr1;1-sultr1;2 null mutant Arabidopsis seedlings under limited or sufficient sulfate conditions for 2 weeks. Each horizontal line displays the abundance of one gene. Red and green indicate up- and down-regulation in treated plants compared to control, respectively. Intensity of the colors is proportional to the absolute value of the fold difference. The SS-treated sample mRNA quantity was set at “1” for each gene and other treated samples were expressed relative to the corresponding control. Sulfate transporters (SULTR 2;1, At5g10180; SULRE1;3, At1g22150; SULTR3;4 At3g15990; SULTR3;5, At5g19600; SULTR4;2, At3g12520), ATP sulfurylase (ATPS, At3g22890), adenosine 5′-phosphosulfate reductase (APR2, At1g62180), sulfide reductase (SIR, At5g04590) and O-acetylserine(thiol)lyase (OASTL), γ-glutamylcysteine synthase (ECS1, At5g23100), GSH synthase (GSH2, At5g27380) phytochelatin synthase (PCS, At5g44070), GSH reductase (GR, At3g24170), GSH S-transferase (GST, class phi, At1g02930) (color figure online)
Cd led to significant increase of ATP sulfurylase (ATPS), adenosine 5′-phosphosulfate reductase (APR2), sulfide reductase (SIR) and O-acetylserine(thiol)lyase (OASTL) mRNA levels under LS treatment in both Arabidopsis lines (Fig. 6). Generally, the transcriptional levels of these four genes were much lower in double mutant than those in wild-type (Fig. 6).
For the GSH biosynthesis-related genes, Cd did not affect the genes expression level of γ-glutamylcysteine synthase (ECS1) and glutathione synthetase 2 (GSH2) in wild-type. On the contrary, Cd led to increment of GSH2 gene expression level in double mutant seedlings under LS or SS treatment. Meanwhile, no significant difference was observed for expression of ECS1 between LS and LS + Cd treatment in double mutant.
For the GSH metabolism-related genes expression, we detected that Cd up-regulated the expression levels of encoding glutathione reductase (GR1), encoding monodehydroascorbate reductase (DHAR1) and encoding glutathione-S-transferase (GST1) in wild-type. Differentially, GST1 was down-regulated by Cd in double mutant under LS + Cd combined stress. Overall, the transcriptional levels of these three genes were much lower in double mutant than those in wild-type. We also analyzed the genes encoding PCs synthase (PCS1). In both Arabidopsis lines, PCS1 was slightly up-regulated after Cd exposure. Interestingly, no significant difference was observed between the wild-type and double mutant seedlings under LS + Cd stress.
Discussion
Cd up-regulates genes’ expression of sulfate transporter in wild-type Arabidopsis seedlings
The increased expression of sulfate transporter genes in response to sulfate deficiency has been considered as one of the most important mechanisms to improve sulfate utilization by plants (Hawkesford 2000). SULTR1;1 and SULTR1;2 are two important high-affinity transporters and their transcriptions are responsive to sulfate deprivation in Arabidopsis (Takahashi et al. 2001). Our results showed that LS treatment enhanced SULTR1;1 and SULTR1;2 transcript abundance (Fig. 1). Similar results have been certified by Hubberten et al. (2012). Based on their results, the expression of SULTR1;1 and SULTR1;2 was up-regulated ten- and three-fold in LS-treated Arabidopsis roots. Besides, in our study, Cd exposure also up-regulated expression of these two genes regardless of sulfate concentration (Fig. 1). In accordance with the results by Nocito et al. (2006), Cd exposure increased ZmST1;1 expression in maize roots, which was confirmed as a high-affinity sulfate transporter. It was noteworthy that Cd-induced genes’ expression increased significantly in seedlings under LS treatment (Fig. 1). These findings were consistent with recent studies in Brassica juncea using semi-quantitative RT-PCR (Lancilli et al. 2014). Their results indicated that BjSultr1;1 and BjSultr1;2 genes’ expression could be elevated by Cd exposure and sulfate limitation. Moreover, the transcript level of SULTR1;1 was higher than SULTR1;2 for seedlings treated by LS or Cd toxicity, suggesting that SULTR1;1 was easily induced by sulfate deficiency or Cd toxicity. Reports by Yoshimoto et al. (2002, 2007) have proved that these two sulfate transporters exhibited slight differences in mRNA inducibility. Under LS condition, SULTR1;1 mRNA was predominantly detected, whereas SULTR1;2 was found abundantly even when plants were supplied with adequate sulfate and was less responsive to the fluctuation of S status compared to SULTR1;1.
The double sultr1;1-sultr1;2 null mutant shows Cd hypersensitivity under LS treatment
Since SULTR1;1 and SULTR1;2 are the main genes involved in sulfate uptake in Arabidopsis, double mutation results in an even much lower sulfate uptake. Poor sulfate status in sultr1;1-sultr1;2 double mutant led to reduced growth, but mutant did flower and produced viable seeds. It is noteworthy that the mutant did not always show reduced growth, as revealed by the progressive recovery observed after increasing the sulfate concentration (Barberon et al. 2008). In recent years, this mutant has been utilized to investigate the relationship between sulfate transporters and metal tolerance. For example, Barberon et al. (2008) reports that double mutant plants possess selenate tolerance; El-Zohri et al. (2015) also reports that poor sulfate status could influence arsenate tolerance in mutant. Nevertheless, our study focused on the correlation between sulfate transporter and Cd tolerance using the double sultr1;1-sultr1;2 null mutant.
Aiming to select a proper limited or sufficient sulfate concentration, the primary root length under a set of K2SO4 concentration was measured in two Arabidopsis lines. In mutant, root length was shorter than that in wild-type except when 1500 µM sulfate was added into medium (Supporting Information Fig. S1). Based on this result, 1500 µM K2SO4 was chosen as the SS treatment. Then, different Cd concentrations were added in the medium containing a gradient of K2SO4 concentration, and we mainly observed the survival situations (data were not shown). We found mutant seedlings could not grow normally in presence of Cd when sulfate added in medium was equal or lower to 100 µM. Similarly, mutant seedlings also could not survive in 30, 40 and 50 µM Cd when supplied with 200, 400 and 1/2 standard MS medium sulfate. To avoid the early death of double mutant seedlings under LS + Cd combined stress and ensure the samples needed for subsequent experiments, 200 μM K2SO4 was used as the LS treatment in this study, while 20 μM CdCl2 was selected as the Cd stress.
Sanita di Toppi and Gabbrielli (1999) report that the main visualized symptoms induced by Cd are root growth inhibition, biomass reduction and leaf chlorosis. Soluble protein content in organisms, an important indicator of reversible and irreversible changes in metabolism, is known to respond to a wide variety of stressors such as natural and xenobiotic (Singh and Tewari 2003). Our data showed that 20 μM CdCl2 decreased the biomass, root length, chlorophyll and soluble protein content in both wild-type and double mutant plants under LS treatment (Table 1). Similarly, in maize seedlings, these growth parameters were inhibited by 100 μM Cd (Astolfi et al. 2004). Besides, Cd also resulted in a significant decrease of protein level in duckweed (Hou et al. 2007).
More importantly, under LS + Cd treatment, Cd-induced growth inhibition was much greater in mutant than that in wild-type (Table 1 and Supporting Information Fig. S2b, f), suggesting the mutant is more sensitive to Cd stress under LS condition. However, all reductions caused by Cd could be alleviated under SS treatment (Table 1 and Supporting Information Fig. S2f, h). Based on these results, we conclude that double mutant is significantly more impaired in presence of Cd under LS. Moreover, the sensitivity could be recovered by SS treatment.
Cd in the growth medium is easily taken up by plant tissues (Gill et al. 2012). Compared to LS treatment, SS promoted roots and shoots Cd accumulation in both Arabidopsis lines, suggesting that SS could help plant to take up more Cd from cultural medium. In addition, it was worth noticing that Cd content had no significant difference between double mutant and wild-type seedlings under LS + Cd or SS + Cd treatment (Fig. 2c, d), indicating the mutations of SULTR1;1 and SULTR1;2 did not affect the capacity of Cd accumulation.
Functions of antioxidative enzymes in Cd sensitivity for sultr1;1-sultr1;2 double mutant under LS + Cd combined stress
Being a non-transition metal, Cd leads to oxidative stress characterized by increased H2O2 level and lipid peroxidation (Hsu and Kao 2007; Paradiso et al. 2008). In Arabidopsis, oxidative stress after exposure to Cd is due to H2O2 accumulation (Cho and Seo 2005). In present study, compared with wild-type, higher MDA and H2O2 accumulation was found in double mutant under LS + Cd combined stress (Fig. 3a, b), implying that Cd hypersensitivity in double mutant can be attributed to severe oxidative damage under LS + Cd combined stress. Cd-induced increase of thiobarbituric acid and H2O2 content was also detected in leaves of Lepidium sativum (Gill et al. 2012).
Enzymatic antioxidant system, such as SOD, CAT, APX and POD, is capable to scavenge excess ROS and prevent plants from lipid peroxidation (Hegedüs et al. 2001; Salin 2007). SOD is a key enzyme against oxidative stress as it catalyzes the dismutation of O ·−2 to H2O2 and O2, which is essential for quenching ROS (Chu et al. 2005). In our study, Cd elevated SOD activity under LS condition in wild-type seedlings but did not alter SOD activity in mutant at the same treatment. Compared to wild-type, mutant even held lower SOD activity under LS + Cd treatment (Fig. 3c). These results indicate that double mutant fails to increase SOD activity under LS + Cd. The decrease of SOD activity might be caused by the inactivation of enzyme by overproduced H2O2, low availability of Cys residue and failure or limitation in iron (Fe)–S cluster synthesis (Sandalio et al. 2001; Ryan et al. 2010). CAT, APX and POD, other three antioxidative enzymes, play an important role in removal of H2O2 (Gratão et al. 2005). In wild-type seedlings, Cd did not alter CAT activity under LS treatment, while in double mutant, CAT activity was decreased. Under LS + Cd combined stress, CAT activity was much lower in mutant than wild-type (Fig. 3d). Consistent with the results of Bashir et al. (2013), Cd failed to stimulate SOD and CAT activities in Arabidopsis under S deficiency. The decline of CAT activity reflects reduced H2O2 scavenging capacity (Azpilicueta et al. 2007), which results in H2O2 accumulation. Besides, in wild-type, the activity of APX was not influenced by Cd under LS condition, but it showed a slight decrease in double mutant (Fig. 3e). Decreased APX activity induced by Cd also led to H2O2 accumulation in double mutant under LS treatment. Surprisingly, the POD activity in wild-type seedlings was much lower than that in double mutant under LS + Cd (Fig. 3f), suggesting mutant still needs to retain enough POD to encounter H2O2. The contradictory responses of POD and APX to Cd stress indicate that different mechanisms may be involved in their operations against oxidative stress (Ali et al. 2002).
Overall, H2O2 scavenging ability was challenged by LS + Cd combined stress in double mutant, which directly caused excess H2O2 accumulation.
Role of glutathione homeostasis in Cd hypersensitivity for sultr1;1-sultr1;2 double mutant under LS + Cd combined stress
The functions of GSH due to its unique structural properties, abundance, broad redox potential, and wide distribution in most living organisms have been drawn (May et al. 1998). Being a non-protein S-containing tripeptide, GSH is related to the sequestration of heavy metals and is also an essential component of the cellular antioxidative defense system which keeps ROS under control (Sobrino-Plata et al. 2014). In past few years, the power of mutant analysis in elucidation role of GSH homeostasis on heavy metal tolerance has been demonstrated in various species. In Arabidopsis, cad2-1 (Cd sensitive 2–1) with a reduced capacity to produce GSH was found to be hypersensitive to both Cd and copper (Cu) (Cobbett et al. 1998); The phytoalexin-deficient 2–1 (pad2–1) and ascorbate-deficient 2–1 (vtc2–1) mutants showed enhanced susceptibility to Cd, which was also related to lower subcellular GSH content (Koffler et al. 2014); In roots of mercury (Hg)-treated regulator of APX2 1–1 (rax1–1) Arabidopsis mutant, there was a strong accumulation of GSH (Sobrino-Plata et al. 2014). Similar studies have been addressed in cultivated plants of economic importance. For example, a GSH-deficient mutant of grass pea, gshL-1, also exhibited Cd sensitivity (Talukdar 2012); Liu et al. (2015) indicated that overexpression of StGCS-GS (encoding γ-glutamylcysteine synthetase-glutathione synthetase in Streptococcus thermophilus) was an efficient mean of enhancing heavy metal tolerance in transgenic sugar beet. Until now, mutant evidence that explored the relationship between GSH homeostasis on Cd tolerance and S absorption process is rare. In present study, we assessed the possible role of GSH homeostasis on Cd tolerance in double sultr1;1-sultr1;2 null mutant, especially under LS treatment.
Regulation of GSH biosynthesis
We measured the content of total S, which is necessary for the biosynthesis of thiol compounds through S assimilation pathway (Saito 2000). In both Arabidopsis lines, Cd increased sulfate uptake independently (Fig. 2a). This result is consistent with previous work reported in Brassica napus (Sun et al. 2007). Although the expression of SULTR1;1 and SULTR1;2 was suppressed, Cd stress could still enhance sulfate absorption in double mutant at low efficiency. Possible explanations are that plants might accumulate sulfate via a nonspecific anion uptake mechanism (Frachisse et al. 1999) or by activation of other sulfate transporters.
The first step of S assimilation pathway is sulfate uptake by roots and transport to other different organs (Kopriva et al. 2009). To study the roles of sulfate transporters, transcriptional levels of five genes were analyzed (Fig. 6). In wild-type seedlings, Cd down-regulated the expression levels of SULTR2;1, SULTR1;3, SULTR3;4 and SULTR3;5 under LS treatment. Conversely, Cd increased the expression levels of these four genes in mutant seedlings under LS treatment, indicating the mutations of SULTR1;1 and SULTR1;2 did not restrict sulfate transport completely. Cd-induced increase expression of other sulfate transporters was highly important for double mutant. This viewpoint has already been proved by El-Zohri et al. (2015) recently. In their research, sultr1;1-sultr1;2 double mutant also had drastically very low S contents in its root and shoot tissues. The low amounts of S in the double mutant could be explained by the expression of other sulfate transporters, such as SULTR 1;3, SULTR2;1, and SULTR 2;2. In double mutant, compensation process is responsible for adequate S supply for Cd tolerance.
The second step in the S assimilation pathway is the sulfate activation followed by its reduction to sulfide, catalyzed by ATPS and APR; while, the rate-limiting step for GSH biosynthesis is considered to be the availability of reduced S for needed Cys synthesis that occurs at the last step of the S reduction pathway (Cobbett 2000). Regardless of sulfate supply, Cd increased the expression levels of ATPS, APR, SIR and OASTL in wild-type seedlings (Fig. 6). Similar results were observed in both Arabidopsis (Harada et al. 2001) and other plants such as Brassica juncea (Heiss et al. 1999; Lee and Leustek 1999). Even though up-regulation induced by Cd was also observed in double mutant under LS treatment, the transcriptional levels of these four genes were much lower in double mutant than wild-type (Figs. 6, 8). Lower capacity of S assimilation in mutant after loss of functions of SULTR1;1 and SULTR1;2 could not meet enough GSH needs to tolerate Cd. Increment of GSH biosynthesis was closely related with Cd response (Semane et al. 2007). Biosynthesis of GSH occurs in two steps: first, synthesis of γ-glutamylcysteine (γ-EC) catalyzed by γ-EC synthetase (ECS); then, synthesis of GSH catalyzed by GSH synthetase. In our study, mutations of SULTR1;1 and SULTR1;2 did not affect gene expression of ECS1 under LS + Cd stress, but it was worth noticing that the GSH2 transcriptional level was increased (Fig. 6). This result suggests that GSH2 might be a potential gene responsible for maintaining GSH content when mutant plants were faced to lower S assimilation capacity.
Regulatory role of AsA–GSH pathway
Thiol groups, such as in GSH, possess the particularity of high affinity to Cd. In presence of Cd, an imbalance of the available GSH pool can lead to the disturbance of the AsA–GSH cycle (Di Cagno et al. 2001; Noctor and Foyer 1998; Jozefczak et al. 2012).
In the present study, enhanced GSH content was related to Cd tolerance in Arabidopsis (Fig. 4a), and the change in total GSH mainly resulted from the increase of the reduced form (GSH) rather than the oxidized form (GSSG) (Fig. 4a, c, e). In cells, GSH is supplemented by regeneration from GSSG reduction catalyzed by GR (Semane et al. 2007). Since Cd induced GR1 expression (Fig. 6), we suppose up-regulated expression of GR1 may promote this reaction. Although the GSH content was increased obviously in presence of Cd, double mutant seedlings accumulated less GSH than wild-type (Fig. 4c). Considering GSH functions directly as a free radical scavenger by reacting with ROS, GSH deficiency also contributes to the severe oxidative damage to double mutant. GSH/GSSG ratio is often used to indicate the redox state in organisms. Lower GSH/GSSG ratio implies more severe oxidative stress (Smeets et al. 2005). In present study, double mutant maintained lower GSH/GSSG ratio in comparison with wild-type under LS + Cd stress (Fig. 4g). This result was relevant to the excess H2O2 accumulation observed in Fig. 3b. Decreased GSH generation in mutant under LS + Cd was also related to the down-regulated GR1 transcriptional abundance (Fig. 6). Lower expression of GR1 transcript suggested that the function of GR was challenged by Cd in double mutant under S-deficiency condition. In other words, normal conversion between GSH and GSSG is impaired by combined stress. Additionally, GST, a group of dimeric, multifunctional enzymes, catalyzes conjugation of GSH with xenobiotic compounds for detoxification (Marrs 1996). As an important cellular detoxifier of metabolites, it is also involved in alleviating Cd-induced oxidative stress (Aravind and Prasad 2005). In our study, the expression of GST1 was down-regulated by Cd in double mutant under LS + Cd stress (Fig. 6), which might result in severe oxidative damage in double mutant.
AsA takes part in growth process, electron transport and removal of H2O2 through APX (Semane et al. 2007). In plants, GSH can be used as electron donor by dehydroascorbate reductase (DHAR) to recycle DHA to AsA (Semane et al. 2007). In general, reduced conversion procedure from GSSG to GSH was accompanied with low DHAR activity, which directly resulted in low AsA and high DHA accumulation. In our study, under LS + Cd, DHAR1 mRNA transcript level was lower in mutant than wild-type (Fig. 6). DHAR1 transcript level has been correlated to plants’ resistance to stress (Chen and Gallie 2004; Ushimaru et al. 2006). Reduced DHAR1 reflects the poor resistance of double mutant to Cd under LS. Surprisingly, compared to wild-type, AsA content was slightly higher in double mutant, while its oxidized form, DHA content exhibited lower content when seedlings were all exposed to LS + Cd (Fig. 4d, f). We suppose DHAR may undergo post-transcriptional regulation (Chen and Gallie 2004). Increase of AsA and decrease of DHA resulted in the highest AsA/DHA ratio in double mutant under LS + Cd stress (Fig. 4h). One hypothesis to explain the highest ratio is the increased accumulation of H2O2. H2O2 is reduced to H2O by APX using AsA as the specific electron donor (Semane et al. 2007). The above-mentioned Cd-reduced APX activity in double mutant under LS treatment led to AsA accumulation. Moreover, it is possible that regeneration of DHA from AsA by DHAR using GSH as an electron donor could not be maintained at a sufficient level due to decreased GSH content.
Relationship between PCs and GSH in Cd hypersensitivity
Binding mechanism of thiol compounds is crucial to heavy metal detoxification. One of the most important compounds for binding metal ions is non-protein thiol (NPTs). Many studies have indicated that Cd enhances NPTs formation (Cho and Seo 2006; Vögeli-Lange and Wagner 1996). Similarly, in our study, Cd also enhanced NPTs formation and SS treatment could promote this biosynthesis process in both Arabidopsis lines (Fig. 5b). Double mutant seedlings accumulated less NPTs than wild-type. These results suggest that S content and/or loss function of two high-affinity sulfate transporters are closely related to NPTs accumulation.
Cys, as predominant NPTs and the precursor molecule for GSH synthesis, plays an important role in plant stress responses (Sobrino-Plata et al. 2014). Since Cys is a potent chelator of heavy metals ions, Cd exposure increases the Cys demands (Heiss et al. 1999). In our study, LS and LS + Cd treatments triggered Cys accumulation, while double mutant accumulated much more Cys under LS + Cd combined stress (Fig. 5a). It has been reported that unstressed cells contain low Cys concentrations and free Cys levels would be increased in response to various abiotic stresses (Ruiz and Blumwald 2002). However, accumulation of free Cys is not always a benefit for plant. Cys–metal ion complex can trigger the Fenton reaction, thereby producing the highly toxic •OH radical (Zagorchev et al. 2013). From these viewpoints, overaccumulation of Cys could be another important reason why mutant seedlings suffered from severe oxidative stress under LS + Cd.
Chelation by PCs is considered as another efficient mechanism against Cd toxicity. PCs bind Cd by thiol groups of Cys. After chelation, Cd–PCs complexes are transported into vacuole through some ATP binding cassette transporters for sequestration of Cd, thereby reducing Cd toxicity (Park et al. 2012). We detected the PCS1 was up-regulated after Cd exposure. In previous studies, it has been reported that PCS1 expression was constitutively expressed and post-transcriptionally regulated by activation of the enzyme in presence of metal (Cobbett 2000). Interestingly, we observed that double mutant seedlings suffered from LS + Cd combined stress maintained comparable PC2 and PC3 content as wild-type seedlings (Fig. 5c). Both Cd accumulation and PCs chelation were not influenced by lower GSH content induced by mutations of SULTR1;1 and SULTR1;2. Correspondingly, regarding the expression level of PCS1, there was no difference between double mutant and wild-type under LS + Cd stress (Fig. 6). These results suggest that the chelation of PCs to Cd is not affected in double mutant, even under LS + Cd combined stress.
To know the amount of GSH involved in PCs’ synthesis, we referred to the data processing method according to Sobrino-Plata et al. (2014) and designed an additional pie chart (Fig. 7). New figure exhibited the total GSH pool (black boxes) and proportion (%) of GSH, GSSG, PC2, PC3 in wild-type and double mutant. We calculated the total GSH pool by adding up the content of reduced GSH, two-fold GSSG, PC2 and PC3. It was obvious that compared to wild-type under LS + Cd, total GSH pool in mutant was lower. More importantly, having faced the GSH pool deficiency condition, 31 and 27 % of GSH was used to synthesize PC2 and PC3, respectively, while the percentage was 26 and 19 % in wild-type. Thus, it can be seen that a majority of GSH in pool is consumed for the synthesis of PCs.
Total GSH pool concentration (black boxes) and proportion (%) of reduced GSH (Blue part), GSSG (light blue part), PC2 (red part) and PC3 (green part) in wild-type and sultr1;1-sultr1;2 double mutant Arabidopsis seedlings under limited or sufficient sulfate with Cd conditions for 2 weeks (color figure online)
In summary, to further acknowledge the role of GSH homeostasis on Cd stress, a double sultr1;1-sultr1;2 mutant was used in our study. Compared to wild-type, double mutant was more sensitive to Cd under limited sulfate supply. More importantly, the sensitive phenotype was mainly attributed to the decreased GSH content that affected oxidative defense but not PCs’ synthesis (Fig. 8).
A model of differential processes involved in Cd tolerance in mutant and wild-type Arabidopsis under LS + Cd combined stress. Mutations of SULTR1;1 and SULTR1;2 affected sulfate uptake, then sulfur (S) deficiency reduced S assimilation genes expression level, which ultimately resulted in decrease of GSH content. Having faced the deficiency of GSH, a large proportion of GSH was used for the PCs’ synthesis to chelate Cd rather than participating in antioxidative defense. Finally, imbalance between antioxidative defense and severe oxidative damage led to Cd hypersensitivity in mutant. Combined stress-induced Cd hypersensitivity can be turned over by additional sufficient sulfate in medium. In small GSH pie chart, white section means percentage of GSH involved in antioxidative reaction, the dark gray section means the percentage involved in PCs’ synthesis (color figure online)
References
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Ali M, Chun H, Kim B, Lee C (2002) Cadmium-induced changes in antioxidant enzyme activities in rice (Oryza sativa L. cv. Dongjin). J Plant Biol 45:134–140
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Astolfi S, Zuchi S, Passera C (2004) Role of sulphur availability on cadmium-induced changes of nitrogen and sulphur metabolism in maize (Zea mays L.) leaves. J Plant Physiol 161:795–802
Azpilicueta CE, Benavides MP, Tomaro ML, Gallego SM (2007) Mechanism of CATA3 induction by cadmium in sunflower leaves. Plant Physiol Biochem 45:589–595
Baker A, Reeves R, Hajar A (2006) Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. and C. Presl (Brassicaceae). New Phytol 127:61–68
Barberon M, Berthomieu P, Clairotte M, Shibagaki N, Davidian JC, Gosti F (2008) Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2. New Phytol 180:608–619
Bashir H, Ahmad J, Bagheri R, Nauman M, Qureshi MI (2013) Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ Exp Bot 94:19–32
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16:1143–1162
Chen J, Wu FH, Wang WH, Zheng CJ, Lin GH, Dong XJ, He JX, Pei ZM, Zheng HL (2011) Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot 62:4481–4493
Chen J, Wang WH, Wu FH, You CY, Liu TW, Dong XJ, He JX, Zheng HL (2012a) Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362:301–318
Chen J, Wu FH, Liu TW, Chen L, Xiao Q, Dong XJ, He JX, Pei ZM, Zheng HL (2012b) Emissions of nitric oxide from 79 plant species in response to simulated nitrogen deposition. Environ Pollut 160:192–200
Cho UH, Seo NH (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168:113–120
Cho UH, Seo NH (2006) Changes of thiols and oxidative stress in tomato seedlings exposed to cadmium. J Ecol Field Biol 29:61–67
Chu CC, Lee WC, Guo WY, Pan SM, Chen LJ, Li HM, Jinn TL (2005) A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant Physiol 139:425–436
Cobbett CS (2000) Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr Opin Plant Biol 3:211–216
Cobbett CS, May MJ, Howden R, Rolls B (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J 16:73–78
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Di Cagno R, Guidi L, De Gara L, Soldatini GF (2001) Combined cadmium and ozone treatments affect photosynthesis and ascorbate-dependent defences in sunflower. New Phytol 151:627–636
Ebbs S, Uchil S (2008) Cadmium and zinc induced chlorosis in Indian mustard [Brassica juncea (L.) Czern] involves preferential loss of chlorophyll b. Photosynthetica 46:49–55
El-Zohri M, Odjegba V, Ma L, Rathinasabapathi B (2015) Sulfate influx transporters in Arabidopsis thaliana are not involved in arsenate uptake but critical for tissue nutrient status and arsenate tolerance. Planta 241:1109–1118
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Frachisse JM, Thomine S, Colcombet J, Guern J, Barbier-Brygoo H (1999) Sulfate is both a substrate and an activator of the voltage-dependent anion channel of Arabidopsis hypocotyl cells. Plant Physiol 121:253–262
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Gill SS, Khan NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Guan C, Ji J, Jia C, Guan W, Li X, Jin C, Wang G (2015) A GSHS-like gene from Lycium chinense maybe regulated by cadmium-induced endogenous salicylic acid and overexpression of this gene enhances tolerance to cadmium stress in Arabidopsis. Plant Cell Rep 34:871–884
Harada E, Choi YE, Tsuchisaka A, Obata H, Sano H (2001) Transgenic tobacco plants expressing a rice cysteine synthase gene are tolerant to toxic levels of cadmium. J Plant Physiol 158:655–661
Hawkesford MJ (2000) Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J Exp Bot 51:131–138
Hegedüs A, Erdei S, Horváth G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci 160:1085–1093
Heiss S, Schäfer H, Haag-Kerwer A, Rausch T (1999) Cloning sulfur assimilation genes of Brassica juncea L.: cadmium differentially affects the expression of a putative low-affinity sulfate transporter and isoforms of ATP sulfurylase and APS reductase. Plant Mol Biol 39:847–857
Hodges DM, Forney CF (2000) The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J Exp Bot 51:645–655
Hou W, Chen X, Song G, Wang Q, Chi Chang C (2007) Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol Biochem 45:62–69
Howden R, Andersen CR, Goldsbrough PB, Cobbett CS (1995) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107:1067–1073
Hsu YT, Kao CH (2007) Toxicity in leaves of rice exposed to cadmium is due to hydrogen peroxide accumulation. Plant Soil 298:231–241
Hubberten HM, Drozd A, Tran BV, Hesse H, Hoefgen R (2012) Local and systemic regulation of sulfur homeostasis in roots of Arabidopsis thaliana. Plant J 72:625–635
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175
Koffler BE, Polanschütz L, Zechmann B (2014) Higher sensitivity of pad2-1 and vtc2-1 mutants to cadmium is related to lower subcellular glutathione rather than ascorbate contents. Protoplasma 251:755–769
Kopriva S, Mugford S, Matthewman C, Koprivova A (2009) Plant sulfate assimilation genes: redundancy versus specialization. Plant Cell Rep 28:1769–1780
Lancilli C, Giacomini B, Lucchini G, Davidian JC, Cocucci M, Sacchi GA, Nocito FF (2014) Cadmium exposure and sulfate limitation reveal differences in the transcriptional control of three sulfate transporter (Sultr1; 2) genes in Brassica juncea. BMC Plant Biol 14:132
Lee S, Leustek T (1999) The affect of cadmium on sulfate assimilation enzymes in Brassica juncea. Plant Sci 141:201–207
Li JX, Gao LJ, Zheng L, Chen JH, Wang JT, Wang XR (2014) Determination of monobromobimane-labeled phytochelatins by high-performance liquid chromatography. Anal Lett 47:2148–2155
Lichtenthaler H (1987) Chlorophylls and carotenoids-pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Liu D, An Z, Mao Z, Ma L, Lu Z (2015) Enhanced heavy metal tolerance and accumulation by transgenic sugar beets expressing Streptococcus thermophilus STGCS-GS in the presence of Cd, Zn and Cu alone or in combination. PLoS One 10:e0128824
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47:127–158
May MJ, Vernoux T, Leaver C, Montagu MV, Inzé D (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49:649–667
Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MN (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44:25–37
Momodu M, Anyakora C (2010) Heavy metal contamination of ground water: the surulere case study. Res J Environ Earth Sci 2:39–43
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol 22:867–880
Nocito FF, Pirovano L, Cocucci M, Sacchi GA (2002) Cadmium-induced sulfate uptake in maize roots. Plant Physiol 129:1872–1879
Nocito FF, Lancilli C, Crema B, Fourcroy P, Davidian JC, Sacchi GA (2006) Heavy metal stress and sulfate uptake in maize roots. Plant Physiol 141:1138–1148
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49:249–279
Paradiso A, Berardino R, de Pinto MC, Sanita di Toppi L, Storelli MM, Tommasi F, De Gara L (2008) Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol 49:362–374
Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y (2012) The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J 69:278–288
Rea PA (2012) Phytochelatin synthase: of a protease a peptide polymerase made. Physiol Plant 145:154–164
Ruiz J, Blumwald E (2002) Salinity-induced glutathione synthesis in Brassica napus. Planta 214:965–969
Ryan KC, Johnson OE, Cabelli DE, Brunold TC, Maroney MJ (2010) Nickel superoxide dismutase: structural and functional roles of Cys2 and Cys6. J Biol Inorg Chem 15:795–807
Saito K (2000) Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol 3:188–195
Salin ML (2007) Toxic oxygen species and protective systems of the chloroplast. Physiol Plantarum 72:681–689
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Semane B, Cuypers A, Smeets K, Van Belleghem F, Horemans N, Schat H, Vangronsveld J (2007) Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiol Plant 129:519–528
Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1; 2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29:475–486
Singh P, Tewari R (2003) Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L. plants. J Environ Biol 24:107–112
Smeets K, Cuypers A, Lambrechts A, Semane B, Hoet P, Van Laere A, Vangronsveld J (2005) Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol Biochem 43:437–444
Sneller FEC, van Heerwaarden LM, Koevoets PL, Vooijs R, Schat H, Verkleij JA (2000) Derivatization of phytochelatins from Silene vulgaris, induced upon exposure to arsenate and cadmium: comparison of derivatization with Ellman’s reagent and monobromobimane. J Agr Food Chem 48:4014–4019
Sobrino-Plata J, Meyssen D, Cuypers A, Escobar C, Hernández LE (2014) Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil 377:369–381
Sun X, Lu B, Huang S, Mehta S, Xu L, Yang Z (2007) Coordinated expression of sulfate transporters and its relation with sulfur metabolites in Brassica napus exposed to cadmium. Bot Stud 48:43–54
Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K (2001) The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J 23:171–182
Talukdar D (2012) An induced glutathione-deficient mutant in grass pea (Lathyrus sativus L.): modifications in plant morphology, alteration in antioxidant activities and increased sensitivity to cadmium. Biorem Biodivers Bioavailab 6:75–86
Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, Yamauchi Y, Nonaka H, Amako K, Yamawaki K, Murata N (2006) Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol 163:1179–1184
Vidmar JJ, Tagmount A, Cathala N, Touraine B, Davidian JCE (2000) Cloning and characterization of a root specific high-affinity sulfate transporter from Arabidopsis thaliana. FEBS Lett 475:65–69
Vögeli-Lange R, Wagner GJ (1996) Relationship between cadmium, glutathione and cadmium-binding peptides (phytochelatins) in leaves of intact tobacco seedlings. Plant Sci 114:11–18
Xu J, Yin H, Li X (2009) Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep 28:325–333
Xu J, Zhu Y, Ge Q, Li Y, Sun J, Zhang Y, Liu X (2012) Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol 196:125–138
Yan K, Chen W, Zhang G, Xu S, Liu Z, He X, Wang L (2010) Elevated CO2 ameliorated oxidative stress induced by elevated O3 in Quercus mongolica. Acta Physiol Plant 32:375–385
Yoshimoto N, Takahashi H, Smith FW, Yamaya T, Saito K (2002) Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J 29:465–473
Yoshimoto N, Inoue E, Watanabe-Takahashi A, Saito K, Takahashi H (2007) Posttranscriptional regulation of high-affinity sulfate transporters in Arabidopsis by sulfur nutrition. Plant Physiol 145:378–388
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432
Zhang ZC, Chen BX, Qiu BS (2010) Phytochelatin synthesis plays a similar role in shoots of the cadmium hyperaccumulator Sedum alfredii as in non-resistant plants. Plant Cell Environ 33:1248–1255
Acknowledgments
We are grateful to Dr. Françoise Gosti from France for kindly providing sultr1;1-sultr1;2 double mutant seeds. This study was financially supported by the Natural Science Foundation of China (NSFC Nos. 30930076, 31260057, 31300505 and 31570586), Research Fund of State Key Laboratory of Soil and Sustainable Agriculture, Nanjing Institute of Soil Science, Chinese Academy of Science (Y412201449), China Postdoctoral Science Foundation (2012M521278).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contribution statement
X.L., F.H.W. and H.L.Z. designed the experiments and wrote the manuscript. X.L., F.H.W., J.X.L., J.C., G.H.W., W.H.W., W.J.H., L.J.G., Z.L.W. and J.H.C. conducted experiments. X.L. and M.S. analyzed data. F.H.W. and W.H.W. provided technical assistance. All authors read and approved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. Feher.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1
Supplementary Table S1. Lists of primers used in quantitative real-time PCR. Supplementary Table S2. Sulfate uptake or metabolism related genes expression in wild-type and double sultr1;1-sultr1;2 null mutant Arabidopsis seedlings under limited or sufficient sulfate conditions for two weeks. Supplementary Methods: Determination of phytochelatins (PCs). Supplementary Figure S1. The primary root length of ten-day-old wild-type and sultr1;1-sultr1;2 double mutant seedlings grown in 1/2 modified MS medium supplemented with 0, 25, 50, 100, 200, 400 and 1500 μM K2SO4 concentrations. 1/2 standard MS medium was chosen as control. Forty seedlings were used for each treatment. Data are mean ± SE. Supplementary Figure S2. The phenotype of wild-type and double sultr1;1-sultr1;2 null mutant of Arabidopsis seedlings under four treatment conditions for two weeks. (a) LS: 200 μM K2SO4, wild-type; (b) LS+Cd: 200 μM K2SO4 + 20 μM CdCl2, wild-type; (c) SS: 1500 μM K2SO4, wild-type; (d) SS+Cd: 1500 μM K2SO4 + 20 μM CdCl2, wild-type; (e) LS: 200 μM K2SO4 , double sultr1;1-sultr1;2 null mutant; (f) LS+Cd: 200 μM K2SO4 + 20 μM CdCl2, double mutant; (g) SS: 1500 μM K2SO4, double mutant; (h) SS+Cd: 1500 μM K2SO4 + 20 μM CdCl2, double mutant. (DOCX 2048 kb)
Rights and permissions
About this article
Cite this article
Liu, X., Wu, FH., Li, JX. et al. Glutathione homeostasis and Cd tolerance in the Arabidopsis sultr1;1-sultr1;2 double mutant with limiting sulfate supply. Plant Cell Rep 35, 397–413 (2016). https://doi.org/10.1007/s00299-015-1892-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1892-8