Abstract
Key message
Chitinases in Glycine max roots specifically respond to different metal types and reveal a polymorphism that coincides with sensitivity to metal toxicity.
Abstract
Plants evolved various defense mechanisms to cope with metal toxicity. Chitinases (EC 3.2.1.14), belonging to so-called pathogenesis-related proteins, act as possible second line defense compounds in plants exposed to metals. In this work their activity was studied and compared in two selected soybean (Glycine max L.) cultivars, the metal-tolerant cv. Chernyatka and the sensitive cv. Kyivska 98. Roots were exposed to different metal(loid)s such as cadmium, arsenic and aluminum that are expected to cause toxicity in different ways. For comparison, a non-metal, NaCl, was applied as well. The results showed that the sensitivity of roots to different stressors coincides with the responsiveness of chitinases in total protein extracts. Moreover, detailed analyses of acidic and neutral proteins identified one polymorphic chitinase isoform that distinguishes between the two cultivars studied. This isoform was stress responsive and thus could reflect the evolutionary adaptation of soybean to environmental cues. Activities of the individual chitinases were dependent on metal type as well as the cultivar pointing to their more complex role in plant defense during this type of stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution by metals became extensive in the late nineteenth and early twentieth century because of diverse anthropogenic activities. Plants are capable of absorbing metals from soil along with other nutrients. This can have various effects on plants. Some metals like Fe, Mo or Mn are important as micronutrients, while others like Zn, Cr, Cu or Ni are trace elements. On the other hand, Ag, As, Pb or Cd have no known function in plants and their uptake causes different toxicity syndromes. Nevertheless, excess of any type of metal harms plants since it interferes with many essential plant processes such as the carbohydrate metabolism, respiration and photosynthesis, cell division and cell elongation (Lindberg and Greger 2002; Dobroviczká et al. 2013; Sethy and Ghosh 2013). Metal uptake interrupts the nutrient, ion, and water balance in plants, causes oxidative stress and membrane disruption as well as inactivation of important enzymes (Mascher et al. 2002; Rout et al. 2001; Lindberg and Greger 2002; Schützendübel and Polle 2002; Tamás et al. 2004, 2008). General symptoms of metal toxicity include stunted growth, chlorosis, yellowing of leaves, root growth inhibition and necrosis. The levels of metal toxicity are highly different for different species as well as within plants of the same species (Metwally et al. 2005). In addition it depends on several factors including dose, pH and bioavailability (Hall 2002; Drličková et al. 2013).
Plants evolved different defense tactics to cope with metal excess. Different ZIP family transporters regulate the uptake and homeostasis of cations like Fe2+ or Zn2+, unfortunately they also enable transport to cells many other metals including those that are non-essential like Cd2+ (Nakamura et al. 2012). Transport proteins are important also for compartmentation of metal ions into vacuoles (Song et al. 2014). Within the cytosol, metals can be chelated by different ligands to alleviate toxicity. The most important ligands are proteinaceous phytochelatins and metallothioneins, but also low molecular glutathione and different organic acids (e.g. malate, citrate, aspartate) (Clemens 2001; Grennan 2011; Sobrino-Plata et al. 2014). Defense against metals also needs efficient scavenging of reactive oxygen (for example by peroxidases, superoxide dismutases, catalases) (Mittler 2002; Pérez-Chaca et al. 2014), as well as protecting and restoring the native conformation of enzymes attacked by metals (Yu et al. 2006).
Chitinases (EC 3.2.1.14), belonging to so-called pathogenesis-related proteins, have previously been related to metal stress tolerance of plants. Several authors have noted that chitinase activity levels correlate with sensitivity to metals in various species (Metwally et al. 2005; Kieffer et al. 2008). Elevated chitinase enzyme activity and/or transcript levels have been observed in different plant species after exposure to metals, however, contrasting observations have been made as well (reviewed in: Piršelová and Matušíková 2011). Under metal stress the chitinases are believed to act as second line defense components (Mészáros et al. 2013). They possibly modify dynamics and permeability of the cell wall to metals, as well as affect metal binding- and immobilization capability of the cell wall (Corrales et al. 2008). Furthermore they can generate signal molecules triggering further defense responses (Metwally et al. 2005). Though the exact role of these enzymes in defense against metals is still unclear, they appear as stable components of plant defense against metal stress (Piršelová and Matušíková 2011; Békésiová et al. 2008). Indeed, transgenic plants overexpressing chitinases have been shown to confer elevated tolerance to metals (Dana et al. 2006).
Previously the metal specificity of chitinases has been proposed (Békésiová et al. 2008). This work studies the profile and behavior of chitinase enzymes in soybean roots (Glycine max L.) after application of metals with different mechanisms of action in plants. To estimate if the noted responses are specific to metals or not, a stress by NaCl was included into the experimental setup since it causes tissue dehydration as occurs with metals (Singh and Tewari 2003). Cadmium (Cd) was chosen as a typical heavy metal (transition element) (Appenroth 2010). Arsenic (As) (p-group element) and aluminum (Al) (lightweight, lead-group element) were applied as metalloids with chemical properties between metals and non-metals (Appenroth 2010). (For clarity of the text, all three are mentioned as “metal(s)” henceforth). The Cd2+ and Al3+ ions enter the plant cells through cation channels and their toxicity mainly results from replacing cofactors of enzymes (Krupa and Baszynski 1995) or direct attacking of membrane proteins and lipids (Lindberg and Griffiths 1993). In contrast, arsenic replaces phosphorus in the phosphate groups of molecules and interferes with the physiology and biochemistry in several ways (Patra et al. 2004). The analyses were performed from two soybean cultivars selected from 10 cultivars grown in Middle Europe.
Materials and methods
Plant material and growth conditions
Seeds of ten soybean (Glycine max L.) cultivars were used: Boróka, Bólyi 56, BS 31, Evans (provided by the Bóly Agricultural Production and Trade Ltd., Hungary); Cardiff, Color, Essor, Merlin (SAATBAU LINZ Slovakia Ltd., Slovakia) and Chernyatka, Kyivska 98 (Institute of Agriculture of Ukrainian Agrarian Academy of Science, Ukraine). The biological material was surface-sterilized with 0.5 % (w/v) sodium hypochlorite for 10 min, then rinsed three times in distilled water. The seeds were germinated in Petri dishes lined with two layers of water-moistened filter paper (Whatman No. 1) in the dark at 25 °C, and grown until the roots reached 3–8 mm in length. Uniformly germinated seeds were collected and transferred to fresh filter paper moistened with distilled water (control) or different metal solutions. We applied 44.4 or 444 µmol dm−3 (5 or 50 mg dm−3, indicated in text) of Cd2+ as for a typically heavy metal, 66.7 µmol dm−3 (5 mg dm−3) solution of metalloid arsenic As3+ and 80 µmol dm−3 Al3+ as a light metal, respectively. As a stressor different from metals we applied 50 mmol dm−3 NaCl. Solutions were prepared from Cd(NO3)2 × 4H2O (Centralchem); As2O3 (Merck) according to Tamari et al. (1988) and AlCl3 (Merck). Roots were analyzed after 48 h of exposure. The signs of ongoing stress were monitored in tissues by measuring root growth, cell viability and also by quantification of generation of some typical stress molecules. As indicators of activation of defense the chitinase enzymes were analyzed more in detail.
Measurement of root weights
Germinating seeds were exposed to the tested stressors as described above. Fresh (FW) and dry weights (DW) of the roots of 10–15 seedlings per cultivar and replicate were measured. For sensitivity screening of the soybean genotypes, tolerance indexes (TIs) were expressed in percentage: TI = (root weight of treated plants/root weight of untreated plants) × 100 %. For analyses the transferred plants were cultivated under the same conditions as for the germination, and sampled after 48 h.
Determination of cell viability
The cell viability in root tips (~1 cm long, a total of 100 mg) was determined after staining with Evans blue according to (Baker and Mock 1994).
Quantitative detection of H2O2 based on staining with xylenol orange
The level of H2O2 molecules was measured in the homogenates of root tips (~1 cm) using the Pierce® Quantitative Peroxide Assay Kit (Thermo Scientific). Values are expressed as nmol g−1 of FW.
Determination of lipid peroxidation
The levels of malondialdehyde (MDA) as product of lipid peroxidation were measured according to Karabal et al. (2003).
Analyses on chitinase enzymes
Total proteins were extracted from roots according to (Hurkman and Tanaka 1986), and their concentration in the samples was determined (Bradford 1976). Aliquots of proteins (20 µg) were separated on 1.5 mm thick minigels on the Mini-PROTEAN Tetra Cell apparatus (Bio-Rad Laboratories) according to (Laemmli 1970). The 12.5 % polyacrylamide gels contained 0.01 % (w/v) glycol chitin that was obtained by acetylation of glycol chitosan (Sigma G-7753) (Trudel and Asselin 1989). Molecular weights of proteins were estimated by co-electrophoresis with a protein ladder (Mark 12 Unstained Standard, Invitrogen). Electrophoresis was run under a constant current of 18 mA for the stacking gel and 24 mA for the separation gel for 3–4 h. After electrophoresis, proteins were re-natured by shaking the gels in 50 mM sodium acetate buffer (pH 5.0), 1 % (v/v) Triton X-100 overnight. The chitinase activities of separated proteins were detected according to Pan et al. (1991) upon staining with 0.01 % (w/v) Fluorescent Brightener 28 (Sigma) in 250 mM Tris–HCl (pH 8.9) for 15 min, and subsequent illumination under UV light.
The active chitinases appeared as dark bands on a bright background. Images (digitally photographed using UVP Bio Doc-It System) were processed using Scion Image software (http://www.scioncorp.com). Background-corrected integrated density (ID) was calculated in areas of constant size according to the formula: ID = N*(mean-background) where N is the number of pixels in the selected area and background is the modal gray value (pixels). After detection of chitinases, the gels were stained for detection of total proteins with 5 % (w/v) Coomassie brilliant blue R 250 in 7 % (v/v) acetic acid and 20 % (v/v) methanol and subsequently photographed.
Simple sequence repeat (SSR) marker analysis
DNA from roots was isolated according to Békésiová et al. (1999). SSR markers (SatK147, SacK149, SaatK150 and SattK152) for low Cd accumulation were applied to screen the soybean cultivars as described previously (Jegadeesan et al. 2010).
Statistical analyses
Each experiment was performed in three replicates. The data are expressed as the mean ± standard error (SEM). For sensitivity screenings, cluster analyses using Ward’s method based on Euclidean distance were performed. For analyses of stress responses, three-way analyses of variance (ANOVA) were performed with planned comparisons of the means. Stress type (with four levels—Al, As, Cd, NaCl), effect of stress (with two levels—control and stress) and variety (two levels—the cv. Kyivska 98 and the cv. Chernyatka) were considered as fixed effect factors. A P value below 0.05 was considered to be statistically significant. To identify natural groupings of objects and confirm the results of clustering analyses we performed principal component analysis (PCA). The relation between the observed changes was examined using Spearman’s correlation coefficient. Statistical analyses were completed with the statistical package STATISTICA 8 (StatSoft Inc. 2007).
Results
Effects of the applied stresses on the roots of soybean cultivars
Based on the literary data we assigned/defined ecologically relevant concentrations for different metals. Based on this we exposed soybean roots to Cd2+ as to typical heavy metal, metalloid arsenic As3+ and to Al3+ as light metal, respectively. In addition, as a stressor other than metals we applied NaCl. Screening of ten soybean cultivars confirmed the variability of tolerance to the applied stressors. Tolerance indexes dropped to 40 % based on fresh weights and 70 % based on dry weight (Online Resource 1). PCA analysis showed that this variability can be assigned to stress effect (58.58 %) and stressor type (above 21 % of variability) (Fig. 1a). Arsenic of a given dose expressed the strongest inhibitory effect on all cultivars (P ≤ 0.001), and cadmium was the least toxic to the roots (P ≤ 0.01). Impacts of Cd and As were similar, those of Al and salt were obviously different (Fig. 1a). Clustering analysis grouped the ten varieties into three clusters (Fig. 1b). The cluster 3 groups the most tolerant cultivars to all stress types studied. The remaining two clusters comprise more sensitive cultivars, while response to Al3+ distinguishes between them.
Effects of different metals and NaCl on root tolerance indexes of tested soybean cultivars. a Principal component analysis identified two directions (Factors 1 and 2) along which the data have the largest spread. b Clustering analysis grouped the cultivars into three clusters based on their overall tolerance/sensitivity to all the stress types applied. Asterisks indicate genotypes with high cadmium accumulation potential as identified by SSR marker analyses
Based on overall tolerance to all stress types, we selected the cv. Chernyatka from cluster 3 and the cv. Kyivska 98 from cluster 2 as representative of relatively tolerant and sensitive cultivars, respectively, for further analyses. SSR marker analyses revealed a contrasting genetic potential for cadmium uptake by roots for these cultivars, too; the cv. Kyivska 98 was giving a DNA profile marking to low-, and the cv. Chernyatka for high Cd accumulation capacity (Online Resource 2).
Roots of the selected soybean cultivars were further studied for effects of the applied doses of stresses. Since the dose 5 mg l−1 Cd2+ had a fairly modest impact on soybean root growth, in further analyses we applied 50 mg l−1 to set more pronounced (but not lethal) stress conditions (Konotop et al. 2012). Roots were thick and tissue browning was noted after Cd and As treatment (Fig. 2). On rhizodermis exposed to Cd, As and NaCl (but not to Al) further signs of occurring stress were histochemically detected such as accumulation of hydrogen peroxide, increased rate of membrane lipid peroxidation and increased cell death (Fig. 2). The inner tissue, however, was obviously not affected since spectrophotometrical data showed no changes in H2O2, MDA, and cell death levels (Fig. 3).
Histochemical analyses for detection of signs of stress on soybean roots. Complexes of hydrogen peroxide (dark precipitates) are formed on rhizodermis. Elevated peroxidation of membrane lipids is visible as dark red coloration mostly on root tips. Dead cells are detected with Evans Blue staining. Cross sections of the root tips are shown; scale bars represent 1 mm (color figure online)
Spectrophotometric measurements of certain stress indicators. a The levels of hydrogen peroxide, b contents of malondialdehyde (MDA) as product of membrane lipid peroxidation and c uptake levels of Evans Blue indicating to dead cells are given. Roots of the sensitive cultivar Kyivska 98 (black bars) and the relatively tolerant cv. Chernyatka (gray bars) were treated with cadmium (Cd), arsenic (As) aluminum (Al), NaCl or water (C). Bars indicate standard errors of mean values of 3 replicates. No significant effect of applied stresses was detected at P ≤ 0.05
The two cultivars responded to the applied stresses similarly. Notably, the overall hydrogen peroxide and lipid peroxidation levels were higher in each case for the cv. Chernyatka comparing to the cv. Kyivska 98 (at P ≤ 0.01) (Fig. 3).
Pattern and activities of total chitinases
Proteins were first separated on SDS-PAGE and three chitinase isoforms were detected (Figs. 4, 5). The largest protein of 66 kDa was most responsive to stress and shown to accumulate in the tolerant genotype but declined in the sensitive cv. Kyivska 98 (P ≤ 0.01). The effect of NaCl was significant in the cv. Chernyatka. A similar but less pronounced pattern (P > 0.05) was observed for the isoform of 21 kDa. Also the middle-sized isoform showed inducibility (effects of Cd and As), but the detected changes were not significant. In general, similar responses of chitinases were observed for Al and NaCl, as well as for As and Cd (Fig. 6).
The gel profiles of chitinases from soybean roots. Total proteins were separated on SDS-containing PAGE and after renaturation the fractions with chitinase activity were identified (upper gels). Three isoforms of different size (in kDa) were detected. The samples were also separated under native conditions (lower gels). The basic chitinases detected were not further analyzed
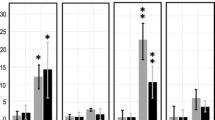
Accumulation levels of chitinase isoforms in soybean roots. Total chitinases as well as acidic/neutral chitinases were analyzed. Out of the acidic/neutral fractions a–e identified (see Fig. 4) the isoform C was polymorphic between the cultivar Kyivska 98 (black columns) and cv. Chernyatka (gray columns). Data (relative activity) represent integrated gel density values with respect to corresponding controls. Bars represent standard errors of mean values of 3 at least replicates. Asterisks indicate to significance of change with respect to controls (C) at P ≤ 0.05
Pattern and activities of chitinases separated on native gels (basic and acidic)
Chitinase patterns were further analyzed upon separation by both size- and charge. There could be four different basic chitinase fractions detected (Fig. 4). Unfortunately, they could not be quantified at the level of the detection system used. Among the acidic chitinase fractions (Figs. 4, 5) there were five isoforms detected in soybean roots (assigned as A–E), while only four of them were present in the tolerant cultivar. The distinguishing isoform C in cv. Kyivska 98 responded to all stress types applied except for NaCl. While As and Cd induced this isoform, Al caused its suppression.
Behavior of the isoform D was induced in the cv. Chernyatka (for Cd treatment at P ≤ 0.05) but reduced (though not significantly) to all four stressors in the cv. Kyivska 98 (Fig. 5). The isoform B strongly accumulated after treatment with Cd and As in both cultivars (Fig. 5). In contrast, the fractions A and E were not significantly affected (Fig. 5). Overview on significantly altered chitinase behavior is given in the Table 1.
Discussion
The soybean cultivars exerted variability in response to the stresses applied similarly as described previously (Metwally et al. 2005; Mészáros et al. 2013). Decrease in fresh and dry weight of roots after exposure to the applied stresses was a stable sign of toxicity for most of the cultivars examined. It can partially be explained by interfering with mitosis, cell wall synthesis, damage to the Golgi as well as by changes in the polysaccharide metabolism (Krupa and Baszynski 1995). The values of TIs distinguished between the impacts of Cd2+, As3+, Al3+, and NaCl. Ions of the heavy metal Cd and of the metalloid As revealed noticeably distinctive effects from the light metal Al (Fig. 1a), possibly because of their chemical properties. While Cd2+ and As3+ bind ligands containing sulfur and nitrogen, Al3+ forms stable complexes with ligands containing oxygen, therefore, their targets and impacts might differ (Appenroth 2010). On the other hand, the impact of NaCl differed from the three metals. Tolerance indexes to different stress types enabled clustering of soybean cultivars according to their overall sensitivity to stresses. This distribution did not coincide with the Cd accumulation potential of individual cultivars confirming that metal tolerance and accumulation are genetically (partially) independent traits (Macnair et al. 1999; Willems et al. 2010).
Two cultivars with contrasting stress tolerances were subjected to further in-depth studies (Table 1). The roots of the relatively tolerant cv. Chernyatka and the sensitive cv. Kyivska 98 were stressed in a similar way, except that a higher Cd2+ dose was applied to evince a more pronounced response. Despite their contrasting sensitivity, visually there were no obvious differences between the roots of the two cultivars (Fig. 2). On roots exposed to Cd2+ and As3+ we observed thickening and tissue browning, which may be a result of severe oxidative stress (Fojtova and Kovarik 2000). Oxidative stress mediates defense responses such as suberization or lignification of the cell wall, and these result in loss of capacity to receive nutrients and lead to repression of root growth (Schützendübel and Polle 2002; Balestri et al. 2014). Browning and blackening of tissues is considered by Fecht-Christoffers et al. (2003) to be a result of the increased content of polyphenols. In contrast, we noted no clear signs of stress by Al and NaCl in either cultivar. However, symptoms caused by the presence of Al3+ ions are difficult to characterize clearly, since some of them appear after a few seconds, while others are detectable only after prolonged exposure to aluminum (Kochian et al. 2005).
Accumulation of hydrogen peroxide and a certain degree of cell mortality were recognized on the rhizodermis of stressed roots. In Cd2+- and As3+-treated plants lipid peroxidation was also enhanced. Though these features may well reflect the current oxidative stress in plants, their internal levels remained almost unaltered after application of the given stress types and doses. Similar (Skorzynska-Polit and Krupa 2006; Yang et al. 2007; Piršelová et al. 2011; Mészáros et al. 2013) but also opposing observations were made by other authors (Pan et al. 1991; Mascher et al. 2002). These contradictions can result from variable activity and/or efficiency of ROS detoxification mechanisms (Martinez Dominguez et al. 2010). The absence of lipid peroxidation (at least in metal stressed plants) is explained by Fe2+ ions bound to the membrane that are not subject to attack by H2O2 molecules and, therefore, ·OH radicals cannot be generated (Boscolo et al. 2003). Finally, death is a later symptom of toxicity (Yamamoto et al. 2001), and its expression would probably be detectable after a prolonged exposure. Chandran et al. (2008) have reported that genes associated with cell death, senescence and cell wall degradation were induced by aluminum after 48 h in a sensitive alfalfa line. These features of stress were observed on the root surface only. More importantly, the two cultivars studied differed in the basic levels of stress markers such as MDA or hydrogen peroxide (Fig. 3). Previously it has been speculated that relatively higher basic levels of membrane lipid peroxidation in the tolerant cultivar (comparing to sensitive one) might indicate to a more efficient detoxification mechanism and/or to a strategy of constitutive “standby” state defense (Bruce et al. 2007; Mészáros et al. 2013). Nevertheless, the restraint FW and DW clearly show that roots were injured by the applied stresses, thus defense responses are likely to occur in both cultivars.
Three chitinase fractions of different sizes were detected in soybean roots after their size-based separation. We could not confirm a significant response to the applied stresses for any of them. Our previous time-course study on arsenic-exposed roots of the same cultivars (Mészáros et al. 2013) displayed a sound activation of the two larger isoforms by arsenic depending on time of exposure and cultivar. Chitinases as secondary defense compounds during metal stress are hypothesized to be induced by an altered oxidative status of cells (Mészáros et al. 2013). Therefore, at latter time points chitinases could similarly be impelled by other metal types, too. Chitinases might adjust cell wall elasticity to decrease its permeability for metals and contribute to water retention in the cells (Amaya et al. 1999).
Activity data of total chitinase isoforms showed that their behavior under As and Cd stress coincides and differs from behavior under Al and NaCl stress. This clustering pattern agrees with the PCA results and points to specific regulation of distinct defense paths specific to certain groups of metals. These in turn activate the responsive chitinase isoforms with possible physiological relevance in relation to metal sensitivity/tolerance. Metal specific response as well as induction of chitinases de novo has been observed previously (Békésiová et al. 2008). However, in those cases much higher metal doses were applied and the newly accumulated isoforms might play a role in other processes (for example apoptosis) rather than defense (Passarinho et al. 2001).
Total activities of chitinases can have limited value for concluding since they comprise activities of several individual isoforms. Moreover, the behavior of individual isoforms might differ from the overall pattern. Under conditions used in this work a minimum of five individual acidic or neutral and four basic or neutral individual isoforms were detected in soybean roots. None appeared newly induced by either of the stresses. Most interestingly, one acidic isoform (C) was present (solely) in the sensitive cv. Kyivska 98. To our knowledge there are few literary data on polymorphic chitinase enzyme patterns in plants. Zur et al. (2013) has recently reported on different chitinase patterns and activity in a susceptible and resistant wheat cultivar infected with a fungal pathogen. In contrast, no chitinase polymorphism was detected in two soybean varieties with contrasting sensitivity to root-knot nematodes (Qiu et al. 1997) and in five soybeans with variable root nodulation potential (Xie et al. 1999). The physiological relevance of the number of different chitinases in plants is unknown. High chitinase activities in different plant species have been associated with disease (stress) tolerance (Marek et al. 2000; Metwally et al. 2005). On the other hand, in this work and in that of Zur et al. (2013) the sensitive genotypes were equipped with higher numbers of chitinases, while the polymorphic isoforms were stress responsive.
The distinguishing soybean isoform C detected here was responsive to each tested metal type. Further, after Al3+ stress it revealed opposing behavior as compared to Cd2+ and As3+ conditions. Of the acidic soybean chitinases the isoforms B and D were induced by Cd2+ and/or As3+. The overall behavior of acidic chitinases (excluding the distinguishing isoform C) differed in the two cultivars since the individual isoforms responded differently to the applied stress types (Fig. 6). While activities of chitinases were more similar for the control, NaCl and Al3+ conditions in both cultivars, they were different for As3+ and Cd2+. It remains unclear if this is an effect of metal type, dosage, genetic background or their combination. Nonetheless, the phenomenon of polymorphism of chitinases in plants has rarely been reported, and might result from evolutionary adaptation of susceptible cultivars to an unfavorable environment. This idea, however, has to be tested on a much broader set of plant species/cultivars.
In conclusion, the impacts of different stressors on a set of soybean varieties enabled clustering of the studied genotypes based on their overall sensitivity or tolerance. This might indicate that the plant defense equipment, once efficient against several stresses, is advantageous against other stresses, too. Our data show that tolerance and responses of the selected soybean cultivars against the tested (mainly metal) stresses coincide with the behavior of total chitinases. More in-depth analyses of acidic and neutral chitinase isoforms revealed that individual chitinases specifically respond to different metal types, depending on the cultivar. We identified a polymorphism between the chitinase profiles of the tolerant and sensitive soybean cultivar. The additional isoform in the sensitive cultivar was clearly stress responsive and might evolve as an evolutionary adaptation to environment. Though chitinases are considered to act primarily during pathogen attack by hydrolyzing microbial cell walls (Moravčíková et al. 2007; Sels et al. 2008), apparently they might provide an advantage for plants during other types of stresses (including metals), too. Polymorphism of chitinases observed in our and a few previous studies indicates that the pattern of chitinases could be informative for the identification of sensitive cultivars.
Abbreviations
- Al:
-
Aluminum
- As:
-
Arsenic
- Cd:
-
Cadmium
- MDA:
-
Malondialdehyde
- SSR:
-
Simple sequence repeat
References
Amaya I, Botella MA, de la Calle M, Medina MI, Heredia A, Bressan RA, Hasegawa PM, Quesada MA, Valpuesta V (1999) Improved germination under osmotic stress of tobacco plants overexpressing a cell wall peroxidase. FEBS Lett 457:80–84
Appenroth K-J (2010) Definition of “heavy metals” and their role in biological systems. In: Sherameti I, Varma A (eds) Soil heavy metals. Springer, Berlin Heidelberg, pp 19–29
Baker CJ, Mock NM (1994) An improved method for monitoring cell-death in cell-susspension and leaf disc assays using Evans Blue. Plant Cell Tiss Org Cult 39:7–12
Balestri M, Ceccarini A, Forino LMC, Zelko I, Martinka M, Lux A, Ruffini Castiglione M (2014) Cadmium uptake, localization and stress-induced morphogenic response in the fern Pteris vittata. Planta 239:1055–1064
Békésiová I, Nap JP, Mlynárová L (1999) Isolation of high quality DNA and RNA from leaves of the carnivorous plant Drosera rotundifolia. Plant Mol Biol Rep 17:269–277
Békésiová B, Hraška S, Libantová J, Moravčíková J, Matušíková I (2008) Heavy-metal stress induced accumulation of chitinase isoforms in plants. Mol Biol Rep 35:579–588
Boscolo PRS, Menossi M, Jorge RA (2003) Aluminum-induced oxidative stress in maize. Phytochemistry 62:181–189
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Chandran D, Sharopova N, VandenBosch KA, Garvin DF, Samac DA (2008) Physiological and molecular characterization of aluminum resistance in Medicago truncatula. BMC Plant Biol 8:89
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Corrales I, Poschenrieder C, Barcelo J (2008) Boron-induced amelioration of aluminium toxicity in a monocot and a dicot species. J Plant Physiol 165:504–513
Dana MM, Pintor-Toro JA, Cubero B (2006) Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol 142:722–730
Dobroviczká T, Piršelová B, Mészáros P, Blehová A, Libantová J, Moravčiková J, Matušíková I (2013) EffectS of cadmium and arsenic ions on content of photosynthetic pigments in the leaves of Glycine max (L.) Merrill. Pak J Bot 45:105–110
Drličková G, Vaculik M, Matejkovič P, Lux A (2013) Bioavailability and toxicity of arsenic in maize (Zea mays L.) grown in contaminated soils. Bull Environ Contam Toxicol 91:235–239
Fecht-Christoffers MM, Braun HP, Lemaitre-Guillier C, VanDorsselaer A, Horst WJ (2003) Effect of manganese toxicity on the proteome of the leaf apoplast in cowpea. Plant Physiol 133:1935–1946
Fojtova M, Kovarik A (2000) Genotoxic effect of cadmium is associated with apoptotic changes in tobacco cells. Plant Cell Environ 23:531–537
Gaur N, Flora G, Yadav M, Tiwari A (2014) A review with recent advancements on bioremediation-based abolition of heavy metals. Environ Sci Process Impacts 16:180–193
Grennan AK (2011) Metallothioneins, a diverse protein family. Plant Physiol 155:1750–1751
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Hurkman WJ, Tanaka CK (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81:802–806
Jegadeesan S, Yu K, Poysa V, Gawalko E, Morrison MJ, Shi C, Cober E (2010) Mapping and validation of simple sequence repeat markers linked to a major gene controlling seed cadmium accumulation in soybean Glycine max (L.) Merr. Theor Appl Genet 121:283–294
Karabal E, Yucel M, Oktem HA (2003) Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci 164:925–933
Kieffer P, Dommes J, Hoffmann L, Hausman JF, Renaut J (2008) Quantitative changes in protein expression of cadmium-exposed poplar plants. Proteomics 8:2514–2530
Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274:175–195
Konotop Y, Mészáros P, Spiess N, Mistríková V, Piršelová B, Libantová J, Moravčíková J, Taran N, Hauptvogel P, Matušíková I (2012) Defense responses of soybean roots during exposure to cadmium, excess of nitrogen supply and combinations of these stressors. Mol Biol Rep 39:10077–10087
Krupa Z, Baszynski T (1995) Some aspects of heavy-metals toxicity towards photosynthetic apparatus—direct and indirect effects on light and dark reactions. Acta Biol Plantarum 17:177–190
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lindberg S, Greger M (2002) Plant genotypic differences under metal deficient and enriched conditions. In: Prasad MNV, Strzaka K (eds) Physiology and chemistry of metal toxicity and tolerance in plants. Kluwer, Amsterdam, pp 368–394
Lindberg S, Griffiths G (1993) Aluminum effects on ATPase activity and lipid composition of plasma membranes in sugar beet roots. J Exp Bot 44:1543–1550
Macnair MR, Bert V, Huitson SB, Saumitou-Laprade P, Petit D (1999) Zinc tolerance and hyperaccumulation are genetically independent characters. Proc Royal Soc B Biol Sci 266:2175–2179
Marek SM, Roberts CA, Karr AL, Sleper DA (2000) Seedling development, and ethephon. Crop Sci 40:713–716
Martinez Dominguez D, Cordoba Garcia F, Canalejo Raya A, Torronteras Santiago R (2010) Cadmium-induced oxidative stress and the response of the antioxidative defense system in Spartina densiflora. Physiol Plant 139:289–302
Mascher R, Lippmann B, Holzinger S, Bergmann H (2002) Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163:961–969
Mészáros P, Rybanský L, Hauptvogel P, Kuna R, Libantová J, Moravčíková J, Piršelová B, Tirpaková A, Matušíková I (2013) Cultivar-specific kinetics of chitinase induction in soybean roots during exposure to arsenic. Mol Biol Rep 40:2127–2138
Metwally A, Safronova VI, Belimov AA, Dietz KJ (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativum. J Exp Bot 56:167–178
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moravčíková J, Libantová J, Heldák J, Salaj J, Bauer M, Matušíková I, Gálová Z, Mlynárová L (2007) Stress-induced expression of cucumber chitinase and Nicotiana plumbaginifolia beta-1,3-glucanase genes in transgenic potato plants. Acta Biol Plantarum 29:133–141
Nakamura Y, Ki Ohba, Ohta H (2012) Participation of metal transporters in cadmium transport from mother rat to fetus. J Toxicol Sci 37:1035–1044
Pan SQ, Ye XS, Kuc J (1991) A technique for detection of chitinase, beta-1,3-glucanase, and protein- patterns after a single separation using polyacrylamide-gel electrophoresis or isoelectrofocusing. Phytopathology 81:970–974
Passarinho PA, Van Hengel AJ, Fransz PF, de Vries SC (2001) Expression pattern of the Arabidopsis thaliana AtEP3/AtchitIV endochitinase gene. Planta 212:556–567
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot 52:199–223
Pérez-Chaca MV, Rodríguez-Serrano M, Molina AS, Pedranzani HE, Zirulnik F, Sandalio LM, Romero-Puertas MC (2014) Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant Cell Environ 37:1672–1687
Piršelová B, Matušíková I (2011) Plant defense against heavy metals: the involvement of pathogenesis-related (PR) proteins. In: Awaad AS, Kaushik G, Govil JN (eds) Recent progress in medicinal plant: mechanism and action of phytoconstituents. Studium Press LLC, USA, pp 179–205
Piršelová B, Kuna R, Libantová J, Moravčíková J, Matušíková I (2011) Biochemical and physiological comparison of heavy metal-triggered defense responses in the monocot maize and dicot soybean roots. Mol Biol Rep 38:3437–3446
Qiu J, Hallmann J, Kokalis-Burelle N, Weaver DB, Rodriguez-Kabana R, Tuzan S (1997) Activity and differential induction of chitinase isozymes in soybean cultivars resistant or susceptible to root-knot nematodes. J Nematol 29:523–530
Rout GR, Samantaray S, Das P (2001) Aluminium toxicity in plants: a review. Agronomie 21:3–21
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Sels J, Mathys J, De Coninck BMA, Cammue BPA, De Bolle MFC (2008) Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol Biochem 46:941–950
Sethy SK, Ghosh S (2013) Effect of heavy metals on germination of seeds. J Nat Sci Biol Med 4:272–275
Singh PK, Tewari RK (2003) Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L. plants. J Environ Biol 24:107–112
Skorzynska-Polit E, Krupa Z (2006) Lipid peroxidation in cadmium-treated Phaseolus coccineus plants. Arch Environ Con Tox 50:482–487
Sobrino-Plata J, Meyssen D, Cuypers A, Escobar C, Hernández LE (2014) Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil 377:369–381
Song WY, Mendoza-Cózatl DG, Lee Y, Schroeder JI, Ahn SN, Lee HS, Wicker T, Martinoia E (2014) Phytochelatin-metal(loid) transport into vacuoles shows different substrate preferences in barley and Arabidopsis. Plant Cell Environ 37:1192–1201
Tamari Y, Takada A, Tsuji H, Kusaka Y (1988) Determination of ppb level of arsenic(V) based on fluorescence quenching of thorium-morin chelate. Anal Sci 4:277–280
Tamás L, Šimonovičová M, Huttová J, Mistrík I (2004) Aluminium stimulated hydrogen peroxide production of germinating barley seeds. Environ Exp Bot 51:281–288
Tamás L, Dudíková J, Durčeková K, Halugková Lu, Huttová J, Mistrík I, Ollé M (2008) Alterations of the gene expression, lipid peroxidation, proline and thiol content along the barley root exposed to cadmium. J Plant Physiol 165:1193–1203
Trudel J, Asselin A (1989) Detection of chitinase activity after polyacrylamide gel electrophoresis. Anal Biochem 178:362–366
Willems G, Frerot H, Gennen J, Salis P, Saumitou-Laprade P, Verbruggen N (2010) Quantitative trait loci analysis of mineral element concentrations in an Arabidopsis halleri x Arabidopsis lyrata petraea F-2 progeny grown on cadmium-contaminated soil. New Phytol 187:368–379
Xie ZP, Staehelin C, Wiemken A, Broughton WJ, Muller J, Boller T (1999) Symbiosis-stimulated chitinase isoenzymes of soybean (Glycine max (L.) Merr.). J Exp Bot 50:327–333
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Yang Y-J, Cheng L-M, Liu Z-H (2007) Rapid effect of cadmium on lignin biosynthesis in soybean roots. Plant Sci 172:632–639
Yu ZJ, Yang XD, Wang K (2006) Metal ions induced heat shock protein response by elevating superoxide anion level in HeLa cells transformed by HSE-SEAP reporter gene. Toxicology 223:1–8
Zur I, Gołebiowska G, Dubas E, Golemiec E, Matušíková I, Libantová J, Moravčíková J (2013) β-1,3-glucanase and chitinase activities in winter triticales during cold hardening and subsequent infection by Microdochium nivale. Biologia (Poland) 68:241–248
Acknowledgments
This work was supported by grants from the Slovak Grant Agency VEGA No. 2/0062/11 and 1/0509/12. Financial support for P. Socha was provided by the Operational Programme Research and Development for the project: “Implementation of the research of plant genetic resources and its maintaining in the sustainable management of Slovak republic” (ITMS: 26220220097), co-financed from the resources of the European Union Fund for Regional Development. The support of COST Action FA 13006 is also acknowledged.
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Kathryn K. Kamo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1
Tolerance indexes of roots of tested soybean cultivars in response to the applied stressors. Supplementary material 1 (DOC 709 kb)
Electronic supplementary material 2
DNA-marker based characterization of soybean cultivars for cadmium accumulation potential. Supplementary material 2 (DOC 1,050 kb)
Rights and permissions
About this article
Cite this article
Mészáros, P., Rybanský, Ľ., Spieß, N. et al. Plant chitinase responses to different metal-type stresses reveal specificity. Plant Cell Rep 33, 1789–1799 (2014). https://doi.org/10.1007/s00299-014-1657-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1657-9