Abstract
Key message
Our study highlights the use of the DNA repair gene MtTdp2α as a tool for improving the plant response to heavy metal stress.
Abstract
Tyrosyl-DNA phosphodiesterase 2 (Tdp2), involved in the removal of DNA topoisomerase II-mediated DNA damage and cell proliferation/differentiation signalling in animal cells, is still poorly characterised in plants. The Medicago truncatula lines Tdp2α-13c and Tdp2α-28 overexpressing the MtTdp2α gene and control (CTRL) line were exposed to 0.2 mM CuCl2. The DNA diffusion assay revealed a significant reduction in the percentage of necrosis caused by copper in the aerial parts of the Tdp2α-13c and Tdp2α-28 plants while neutral single cell gel electrophoresis highlighted a significant decrease in double strand breaks (DSBs), compared to CTRL. In the copper-treated Tdp2α-13c and Tdp2α-28 lines there was up-regulation (up to 4.0-fold) of genes encoding the α and β isoforms of Tyrosyl-DNA phosphodiesterase 1, indicating the requirement for Tdp1 function in the response to heavy metals. As for DSB sensing, the MtMRE11, MtRAD50 and MtNBS1 genes were also significantly up-regulated (up to 2.3-fold) in the MtTdp2α-overexpressing plants grown under physiological conditions, compared to CTRL line, and then further stimulated in response to copper. The basal antioxidant machinery was always activated in all the tested lines, as indicated by the concomitant up-regulation of MtcytSOD and MtcpSOD genes (cytosolic and chloroplastic Superoxide Dismutase), and MtMT2 (type 2 metallothionein) gene. The role of MtTdp2α gene in enhancing the plant response to genotoxic injury under heavy metal stress is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are naturally present in traces in the soil but modern agricultural and industrial practices have so far contributed to release huge amounts of these elements as environmental pollutants. Heavy metal accumulation in plant tissues leads to genotoxic stress and genome instability, with the occurrence of chromosomal aberrations and micronuclei (Rodriguez et al. 2011). DNA repair genes have been reported to be up-regulated in response to heavy metal stress, such as the MSH3 and MLH1 (MutS and MutL Homologue, respectively) encoding enzymes involved in Mismatch Repair (MR) (Liu et al. 2008), OGG1 (8-oxoguanine DNA glycosylase/lyase) and FPG (Formamidopyrimidine-DNA glycosylase) (Macovei et al. 2011a), TFIIS involved in Transcription-Coupled-Nucleotide Excision Repair (TC-NER) (Macovei et al. 2010) and Tdp1 encoding tyrosyl-DNA phosphodiesterase 1 (Macovei et al. 2011b).
Tyrosyl-DNA phosphodiesterases (TDPs) are involved in the repair of DNA damage induced by DNA topoisomerases, key enzymes that regulate DNA topology (Pommier 2013). These enzymes release the covalent bond originating between the DNA molecule and stalled DNA topoisomerases in the presence of oxidised nucleotides or specific inhibitors. Tdp1 cleaves the 3′-phosphotyrosyl bond between DNA topoisomerase I (topo I) and DNA (Interthal et al. 2001), while Tdp2 is able to resolve the 5′-phosphotyrosyl bonds between stalled DNA topoisomerase II (topo II) and DNA (Cortes Ledesma et al. 2009). In animal models, Tdp2 is also known as a factor interacting with components of different signalling pathways (Varady et al. 2011). In plants, information concerning TDPs is still limited, although it is rapidly expanding. The small Tdp1 gene family was first identified in planta in the model legume M. truncatula by Macovei et al. (2010). Both the MtTdp1α and MtTdp1β genes were up-regulated in response to heavy metal and osmotic stress and during seed imbibition, when Reactive Oxygen Species (ROS) are accumulated and DNA repair genes are induced to maintain genome stability (Balestrazzi et al. 2011a, b; Ventura et al. 2012). The involvement of plant TDPs in the complex response of M. truncatula cell suspension cultures to ionising radiation, as a function of dose rate, has been recently demonstrated by Donà et al. (2013a). In M. truncatula, depletion of MtTdp1α gene impairs ribosome biogenesis, thus highlighting a role of MtTdp1α enzyme in nucleolar metabolism (Donà et al. 2013b). As regards the Tdp2 function in plants, M. truncatula lines overexpressing the MtTdp2α gene were characterised by increased biomass and chlorophyll content under physiological and osmotic stress conditions. Moreover, these lines showed reduced DNA damage accumulation in response to osmotic stress and up-regulation of both antioxidant and topology-related genes (Confalonieri et al. 2013).
The present work focuses on the molecular characterisation of MtTdp2α-overexpressing M. truncatula lines challenged with toxic copper concentrations. According to the reported data, MtTdp2α gene plays a relevant role in the DNA damage response against heavy metal stress and, thus, it could represent a promising tool for biotechnological applications meant to improve crop productivity.
Materials and methods
Plant material and treatments
Medicago truncatula Gaertn. cv. Jemalong (M9-10a genotype) plants were grown in vitro in sterile vessels (Micropoli, Cesano Boscone, Italy) on a medium containing macrosalts and microsalts MS (Murashige and Skoog 1962), vitamin SH (Schenk and Hildebrandt 1972), 20 g/L sucrose and 4 g/L Gelrite™ (Duchefa Biochemie, Haarlem, The Netherlands) and maintained in a climate chamber at 22–24 °C with a 16-h light/8-h dark cycle photoperiod and a photosynthetic photon flux of 65–70 μMol/m2/s under a cool white fluorescenct lamp (Confalonieri et al. 2013). For heavy metal stress treatments, 0.2 mM CuCl2 (Sigma-Aldrich, Milan, Italy) was added to the above reported growth substrate as described by Macovei et al. (2010). For molecular analyses, tissues were collected from plants and stored in liquid N2.
Chlorophyll content
To determine the chlorophyll content, three leaflets per treatment were excised from 10-day-old M. truncatula plants grown in vitro in presence/absence of 0.2 mM CuCl2 and analysed as follows. Extraction with 1 ml of 80 % (v/v) acetone was performed and the total chlorophyll content was determined by measuring the absorbance of the supernatant at 663 and 646 nm (Wellburn 1994). Spectrum profiles of leaf pigments were determined performing a spectrophotometric assay on ethanol extracts of leaf tissues of untreated and treated (0.2 mM CuCl2) plantlets. Spectrum measurements were carried out between 400 and 550 nm using a V-530 spectrophotometer (Jasco Europe S.r.l., Cremella, Italy).
Quantitative real-time polymerase chain reaction
QRT-PCR was carried out with the SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories S.r.l., Segrate, Italy) in a final volume of 20 μl according to supplier’s indications, and using a Rotor-Gene 6000 PCR apparatus (Corbett Robotics, Brisbane, Australia). Amplification conditions were as follows: initial denaturation step at 95 °C for 30 s, and subsequently 95 °C for 5 s, 59 °C for 30 s, 72 °C for 30 s (40 cycles). Quantification was carried out using the M. truncatula ELF1α (GenBank Accession N° EST317575) as reference gene. Gene-specific oligonucleotide primers for the M. truncatula MtTdp2α (Phytozome Database Accession N° Medtr8g146980), MtTdp1α (XM_003622639), MtTdp1β (GenBank Accession N° AC141864.7), MtMRE11 (Phytozome Database Accession N° Medtr2g081100), MtRAD50 (Phytozome Database Accession N° Medtr3g084300), MtNBS1 (Phytozome Database Accession N° Medtr5g076180), MtcytSOD (Cu/Zn-SOD isoform) (Phytozome Database Accession N° Medtr7g114240), MtcpSOD (Cu/Zn-SOD isoform) (Phytozome Database Accession N° Medtr4g076170), MtMT2 (AC147202.14) coding sequences were designed using the Real-Time PCR Primer Design program from GenScript (https://www.genscript.com/ssl-bin/app/primer) (Supplemental Table S1). For each oligonucleotide set, a no-template water control was used. Ct values and QRT-PCR efficiency values, obtained by the Rotor-Gene 6000 Series Software 1.7 (Corbett Robotics, Brisbane, Australia), were analysed and statistically validated using the REST2009 Software V2.0.13 (Qiagen GmbH, Hilden, Germany) (Pfaffl et al. 2002).
DNA diffusion assay
The DNA diffusion assay (Singh 2003) was performed to evaluate cell death (PCD versus necrosis). Three leaflets per treatment were excised from 10-day-old M. truncatula plants grown in vitro in presence/absence of 0.2 mM CuCl2 and analysed. Nuclei were extracted as described by Donà et al. (2013b). Agarose-precoated slides were prepared by spreading 1 ml of 1 % agarose on each slide and drying them at room temperature. Aliquots (300 μl) of nuclei suspension were mixed with 200 μl of 1 % low melting point agarose (Sigma-Aldrich, Milan, Italy) in phosphate-buffered saline (PBS) at 37 °C and gently transferred onto slides. The gel was covered with a cover glass, slides were cooled on ice for 1 min. Cover glasses were removed and slides were immersed in lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris HCl pH 7.5) for 20 min at room temperature. After lysis, slides were washed twice in neutral solution TBE (89 mM Tris Base, 89 mM Boric Acid, 2 mM EDTA, pH 8.3) for 5 min, rinsed in 70 % ethanol (v/v) for 10 min at room temperature. Slides were air dried and stored at room temperature, stained with 20 μl DAPI (4′,6-diamidino-2-phenylindole, Sigma-Aldrich, 1 μg ml−1) and covered with a cover slip for 10 min. One hundred nuclei were analysed per slide. Cells undergoing PCD or necrosis were distinguished from viable cells as indicated by Singh (2003). In case of PCD, cell nuclei own a homogeneous outline without any clear boundary due to nucleosomal-sized DNA diffusing into the agarose. Necrotic cell nuclei are bigger and poorly defined with a non-homogeneous halo appearance. For each treatment, three replicated samples were analysed in two independent experiments.
Single cell gel electrophoresis (SCGE)
Three leaflets per treatment were excised from 10-day-old M. truncatula plants grown in vitro in presence/absence of 0.2 mM CuCl2 and analysed. Nuclei were extracted from M. truncatula cells as described by Donà et al. (2013b). The suspension containing purified nuclei and a solution of 1 % low melting point agarose in PBS at 37 °C were mixed in equal volume. Two drops of the resulting suspension were then pipetted onto agarose-precoated slides and solidified on ice. For neutral SCGE, slides were then incubated 20 min at room temperature in high salt lysis buffer (2.5 M NaCl, 100 mM Tris–HCl pH 7.5, 100 mM EDTA) to disrupt the nuclear membrane and subsequently electrophoresed 8 min at 1 V/cm in TBE. After electrophoresis, slides were washed in 0.4 M Tris–HCl pH 7.5 three times for 5 min, rinsed in 70 % ethanol (v/v) three times for 5 min at 4 °C and dried overnight at room temperature. Subsequently, slides were stained with 20 μL DAPI (1 μgMmL, Sigma-Aldrich). For each slide, one hundred nucleoids were scored, using a fluorescence microscope with an excitation filter of 340–380 nm and a barrier filter of 400 nm. Nucleoids were classified and results were expressed in arbitrary units according to Collins (2004).
Statistical analysis
Three replicated plantlets from each treatment combination were randomly selected for tissue analysis. Results were subjected to Analysis of Variance (ANOVA) and the means were compared by Holm-Sidak test. Percentage data were transformed to arcsin√x before statistical analysis. Statistical significance of differences was determined using Student’s t test (P < 0.05).
Results
Chlorophyll content in MtTdp2α-overexpressing M. truncatula plants exposed to copper
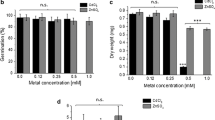
To assess the response to heavy metal toxicity, the Tdp2α-13c and Tdp2α-28 lines and the control (CTRL) line carrying the empty vector were grown in vitro for 10 days in presence/absence of 0.2 mM CuCl2. The latter was able to induce visible toxic effects on growth rates in M. truncatula plants (Macovei et al. 2010). The amount of MtTdp2α was measured by QRT-PCR in the CTRL, Tdp2α-13c and Tdp2α-28 lines grown in presence/absence of copper. Under physiological conditions, the overexpression of MtTdp2α gene was 6.8-fold (Tdp2α-13c) and 21.7-fold (Tdp2α-28), compared to CTRL line. Exposure to copper caused a further enhancement in the amount of MtTdp2α transcript not only in the CTRL (3.9-fold) but also in the MtTdp2α-overexpressing lines (3.1-fold, Tdp2α-13c; 1.8-fold, Tdp2α-28) (Fig. 1a). The estimated increase in MtTdp2α mRNA was lower in the Tdp2α-28 line characterised by higher overexpression of the gene of interest.
Expression profiles of MtTdp2α gene assessed by QRT-PCR (a) and total chlorophyll content (b) in leaves of 10-day-old plants (CTRL and transgenic lines Tdp2α-13c and Tdp2α-28) grown in vitro in presence/absence of 0.2 mM CuCl2 for 10 days. For each treatment combination, data represent the mean values ± SD of at least two independent experiments each of them carried out using three technical replications. a Significant difference between copper treatment and physiological conditions. b Significative difference between Tdp2α-overexpressing line and CTRL line
The chlorophyll content was analysed in leaflets of the CTRL, Tdp2α-13c and Tdp2α-28 lines exposed to 0.2 mM CuCl2 (Fig. 1b). In the untreated CTRL line, the amount of total chlorophyll was 0.86 ± 0.03 mg ml−1 gFW−1 while a non significant (P = 0.108) increase occurred in the Tdp2α-13c line (1.03 ± 0.21 mg ml−1 gFW−1) and contrarily a significant (P < 0.0001) increase (0.99 ± 0.06 mg ml−1 gFW−1) was detected in the Tdp2α-28 line. Under heavy metal stress, no significant changes (P = 0.0453) in chlorophyll content were observed in the CTRL line (0.75 ± 0.16 mg ml−1 gFW−1) while a significant increase was present in the Tdp2α-13c line (1.35 ± 0.13 mg ml−1 gFW−1) (Fig. 1b). The observed response might be due to compensatory mechanisms for copper-mediated stress occurring at the photosynthetic apparatus, already described in M. truncatula (Macovei et al. 2010). The absorbance spectra of leaf carotenoids were also determined and, as shown in Supplemental Fig. S1, the Tdp2α-13C and Tdp2α-28 lines exposed to heavy metal stress were able to maintain significantly (P < 0.05) higher levels of these antioxidant compounds, compared to CTRL. As for the average plant biomass, exposure to copper caused an estimated decrease of 60 % in the CTRL line. The recorded average biomass of the Tdp2α-13c and Tdp2α-28 plants was reduced under heavy metal stress up to 28 % (data not shown).
Evaluation of cell viability, programmed cell death and necrosis in response to heavy metal stress
Occurrence of cell death was monitored in leaves of M. truncatula plants grown in vitro in presence/absence of 0.2 mM CuCl2, using the DNA diffusion assay. Three distinct nuclear morphologies were evidenced, corresponding to viable cells, PCD and necrosis events (Supplemental Fig. S2). Statistical significance of differences was determined using Student’s t test (P < 0.05). As shown in Supplemental Table S2, under physiological conditions, the percentage of viable cells in the CTRL leaves was 75.0 % while slight different values were recorded for Tdp2α-13c (72.5 %) and Tdp2α-28 (81.0 %). PCD was predominant in CTRL (15.0 %), Tdp2α-13c (19.5 %) and Tdp2α-28 (15.0 %) while the necrosis events, estimated 10.0 % in the CTRL line, were only slightly reduced in Tdp2α-13c (8.0 %) and significantly decreased in Tdp2α-28 (4.0 %) lines. This feature became more evident under heavy metal stress (Supplemental Table S2). In the CTRL leaves exposed to copper there was an overall reduction in cell viability (57.5 %) (Supplemental Table S2). However, the PCD events only slightly changed (17.5 %), compared to the untreated sample while necrosis accounted for 26.5 %. By contrast, both Tdp2α-13c and Tdp2α-28 lines treated with copper maintained almost unaltered viability values (75.0 %, Tdp2α-13c; 79.0 %, Tdp2α-28) when compared to the untreated sample. Under oxidative stress, the estimated percentage of PCD events was 17.0 % (Tdp2α-13c) and 15.5 % (Tdp2α-28) while necrosis accounted for 8.0 % (Tdp2α-13c) and 5.5 % (Tdp2α-28). The observed decrease in cell death events well correlated with the level of MtTdp2α gene overexpression. When comparing CTRL and the MtTdp2α-overexpressing lines, the calculate decrease in necrosis frequency was 25 % (Tdp2α-13c) and 20 % (Tdp2α-28).
Double strand break accumulation in MtTdp2α-overexpressing and CTRL lines
The level of Double strand breaks (DSBs) was evaluated in leaf tissues of CTRL, Tdp2α-13c and Tdp2α-28 lines challenged with 0.2 mM CuCl2 using neutral SCGE (Fig. 2). Morphologies of nuclei extracted from viable, PCD and necrotic cells are shown in Suppl. Fig. S2. In the absence of copper, the amount of DSBs in leaf tissues was 91.0 ± 11.0 a.u. while a significantly (P < 0.0001) lower level of DNA damage was recorded in the Tdp2α-13c and Tdp2α-28 lines (59.0 ± 7.0 and 57.3 ± 4.1 a.u., respectively). In response to copper treatment, the amount of DSBs was significantly (P < 0.0001) enhanced (129.3 ± 8.5 a.u.) in CTRL leaves in comparison with the untreated sample and a similar response was observed in the Tdp2α-13c and Tdp2α-28 lines, which showed a DSB level of 96.0 ± 9.0 a.u. and 74.5 ± 0.6 a.u., respectively (Fig. 2). Heavy metal-induced DNA damage increased up to 1.0-fold in the CTRL line while there was a limited increase (0.7- and 0.4-fold, respectively) in the Tdp2α-13c and Tdp2α-28 lines. The Tdp2α-13c line with lower levels of MtTdp2α gene overexpression, accumulated significantly higher DSB amounts, compared to the Tdp2α-28 line.
Comet images of intact (untreated control) and damaged (0.2 mM CuCl2) DNA from M. truncatula leaf tissues (a) and level of DSBs measured by neutral SCGE in leaves excised from 10-day-old Tdp2α-13c, Tdp2α-28 and control (CTRL) plantlets grown in vitro in presence/absence of 0.2 mM CuCl2 (b). For each treatment combination, data represent the mean values ± SD of at least two independent experiments each of them carried out using three technical replications. a.u. arbitrary units. a Significant difference between copper treatment and physiological conditions. b Significative difference between Tdp2α-overexpressing line and CTRL line
Expression profiles of Tdp1 genes
To assess the overall contribution in the plant response to heavy metal stress resulting from Tyrosyl-DNA phosphodiesterases, the expression profiles of MtTdp1α and MtTdp1β genes were also investigated in leaves of CTRL and MtTdp2α-overexpressing plants. As shown in Fig. 3, the MtTdp1α gene was significantly (P < 0.0001) up-regulated (9.7-fold) in the CTRL leaves exposed to 0.2 mM CuCl2. The Tdp2α-13c line, showing a significant (P = 0.0116) increase in MtTdp1α transcript levels (2.3-fold compared to CTRL) under physiological conditions, was responsive to copper since there was a further significant (P < 0.0001) enhancement (3.1-fold compared to the untreated sample). The Tdp2α-28 line resulted into a significant (P < 0.0001) accumulation of the MtTdp1α transcript (3.5-fold compared to the untreated sample) only under heavy metal stress (Fig. 3). The MtTdp1β gene was significantly (P < 0.0001) up-regulated (4.2-fold) in the CTRL leaves exposed to 0.2 mM CuCl2. The Tdp2α-13c line, showed a significant (P < 0.0001) increase in MtTdp1β transcript levels (5.7-fold compared to CTRL) in response to copper while the Tdp2α-28 line resulted into a significant (P < 0.0001) accumulation of the MtTdp1β transcript (2.1-fold compared to the untreated sample) under heavy metal stress (Fig. 3). The reported data indicate that the Tdp1 function plays a key role in preserving genome stability in response to copper. The Tdp2α-28 line, showing the highest levels of MtTdp2α transcript, revealed lower accumulation of MtTdp1α and MtTdp1β transcripts compared to Tdp2α-13c line.
Expression profiles of the MtTdp1α and MtTdp1β genes in the MtTdp2α-overexpressing lines Tdp2α-13c, Tdp2α-28 and control (CTRL) line assessed by QRT-PCR. Leaves were excised from 10-day-old plants grown in vitro in presence/absence of 0.2 mM CuCl2. For each treatment combination, data represent the mean values ± SD of at least two independent experiments each of them carried out using three technical replications. a Significant difference between copper treatment and physiological conditions. b Significative difference between Tdp2α-overexpressing line and CTRL line
Expression profiles of genes involved in DSB sensing in MtTdp2α-overexpressing lines
The expression profiles of MtMRE11, MtRAD50 and MtNBS1 genes were evaluated by QRT-PCR in leaf tissues of CTRL and MtTdp2α-overexpressing lines exposed to copper. As shown in Fig. 4, the MtMRE11 gene was significantly (P < 0.0001) up-regulated (4.5-fold) in the CTRL leaves exposed to 0.2 mM CuCl2. The Tdp2α-13c line, showing a significant (P < 0.0001) increase in MtMRE11 transcript levels (2.0-fold compared to CTRL) under physiological conditions, was responsive to copper since there was a further significant (P < 0.0001) enhancement (1.6-fold compared to the untreated sample). The Tdp2α-28 line resulted into a significant (P < 0.0001) up-regulation of MtMRE11 gene, both under physiological conditions (1.3-fold) and copper stress (1.5-fold). The MtRAD50 gene was significantly (P < 0.0001) up-regulated (4.9-fold) in the CTRL leaves exposed to 0.2 mM CuCl2. Both the Tdp2α-13c and Tdp2α-28 lines showed a significant (P < 0.0001) increase in MtRAD50 transcript levels (2.5-fold in both lines) under physiological conditions. Exposure to copper triggered a further transcript accumulation (1.4- and 1.2-fold compared to the untreated sample). The MtNBS1 gene was significantly (P < 0.0001) up-regulated (5.0-fold) in the CTRL leaves exposed to 0.2 mM CuCl2. The Tdp2α-13c line, showing a significant (P < 0.0001) increase in MtNBS1 transcript levels (2.7-fold compared to CTRL) under physiological conditions, was responsive to copper since there was a further significant (P < 0.0001) enhancement (1.6-fold compared to the untreated sample). A similar response was observed for the Tdp2α-28 line which showed a significant (P = 0.0082) increase in MtNBS1 transcript levels (1.6-fold compared to CTRL) under physiological conditions, and a further significant (P < 0.0001) enhancement (2.2-fold compared to the untreated sample) (Fig. 4).
Expression profiles of the MtMRE11, MtRAD50 and MtNBS1 genes in MtTdp2α-overexpressing lines Tdp2α-13c, Tdp2α-28 and control (CTRL) line assessed by QRT-PCR. Leaves were excised from 10-day-old plants grown in vitro in presence/absence of 0.2 mM CuCl2. For each treatment combination, data represent the mean values ± SD of at least two independent experiments each of them carried out using three technical replications. a Significant difference between copper treatment and physiological conditions. b Significative difference between Tdp2α-overexpressing line and CTRL line
Expression profiles of antioxidant genes
A preliminary search in Phytozome and NCBI databases revealed the presence of different superoxide dismutase (SOD) isoforms in M. truncatula. Results from this analysis are summarised in see Supplemental Table S3 where all isoforms (Cu/Zn SOD, Fe SOD and Mn SOD) and relative subcellular localization are indicated. In the present work, the expression profiles of the MtcytSOD (Medtr7g114240) and MtcpSOD (Medtr4g057240) genes, encoding a cytosolic and a chloroplastic isoform, respectively, were evaluated. Moreover, the MtMT2 gene encoding a type 2 metallothionein was investigated. As shown in Fig. 5, the MtcytSOD gene was significantly (P < 0.0001) up-regulated (11.1-fold) in the CTRL leaves exposed to 0.2 mM CuCl2. The Tdp2α-13c line, showing a significant (P = 0.0051) increase in MtcytSOD transcript levels (2.5-fold compared to CTRL) under physiological conditions, was highly responsive to copper since there was a further significant (P < 0.0001) enhancement (6.5-fold compared to the untreated sample). Similarly, the Tdp2α-28 line resulted into a significant (P = 0.0002) accumulation of the MtcytSOD transcript (3.0-fold compared to the untreated sample) under physiological conditions and a further up-regulation (3.4-fold compared to the untreated sample) under heavy metal stress (Fig. 5). As for the MtcpSOD gene, there was a significant (P < 0.0001) up-regulation only under heavy metal stress in all the tested lines, with an estimated increase of 5.0-fold (CTRL), 5.5-fold (Tdp2α-13c) and 5.2-fold (Tdp2α-28). Similarly, the MtMT2 gene showed a significant (P < 0.0001) up-regulation only under heavy metal stress in all the tested lines, with an increase of 4.2-fold (CTRL), 2.4- fold (Tdp2α-13c) and 1.8-fold (Tdp2α-28).
Expression profiles of the MtcytSOD, MtcpSOD and MtMT2 genes in the MtTdp2α-overexpressing lines Tdp2α-13c, Tdp2α-28 and control (CTRL) line assessed by QRT-PCR. Leaves were excised from 20-day-old plants grown in vitro in presence/absence of 0.2 mM CuCl2. For each treatment combination, data represent the mean values ± SD of at least two independent experiments each of them carried out using three technical replications. a Significant difference between copper treatment and physiological conditions. b Significative difference between Tdp2α-overexpressing line and CTRL line
Discussion
Heavy metal pollution originating from anthropogenic sources represents a concern for the environment and the nutritional quality of plant-derived foods. Due to the complexity of DNA repair pathways and antioxidant processes activated in planta, detailed investigations are required to sort out genes involved in the removal of heavy metal-mediated DNA damage. The role played by tyrosyl-DNA phosphodiesterases in protecting plants against the genotoxic damage caused by copper has been demonstrated in the present report, using transgenic M. truncatula plants overexpressing the MtTdp2α gene. To our knowledge, this is the first case in which the overexpression in planta of a specific DNA repair gene reinforces the genotoxic stress response.
The protective effects against copper toxicity resulting from the overexpression of MtTdp2α gene were clearly evidenced by scoring the frequency of cell death events. In both Tdp2α-13c and Tdp2α-28 lines, cell viability remained significantly higher than in CTRL line, concomitant with the reduction in the percentage of necrotic cells. Interestingly, a lower frequency of necrosis was also recorded in the same MtTdp2α-overexpressing lines exposed to osmotic stress (Confalonieri et al. 2013). Since necrosis arises when high stress levels are provided (Reape and McCabe 2008), the reduced amount of necrotic cells found in the MtTdp2α-overexpressing lines clearly suggests that the tested copper concentration was perceived as a mild stress treatment in these tissues, contrarily to what was observed in the CTRL plants. It is worth noting that the Tdp2α-28 line, characterised by the highest levels of overexpression, also showed the highest percentage of viable cells together with the most consistent reduction in frequency of necrosis.
SCGE confirmed that both Tdp2α-13c and Tdp2α-28 lines accumulated significantly lower amounts of DSBs compared to CTRL, even in the absence of heavy metal. The same DSB amount was detected in both the MtTdp2α-overexpressing lines, despite the different levels of transgene overexpression, in agreement with Confalonieri et al. (2013). Under physiological conditions, the M. truncatula cells may own a damage threshold under which no further DNA repair takes place, an aspect which deserves a deeper investigation. As for DNA damage levels in relation to MtTdp2α overexpression, the Tdp2α-28 line stands as the best performing. The ability of MtTdp2α-overexpressing lines to buffer DSB accumulation under osmotic stress was demonstrated by Confalonieri et al. (2013).
Up-regulation of the endogenous MtTdp2α gene was observed in response to copper in all the tested lines, however, the extent of up-regulation was limited in the Tdp2α-13c and Tdp2α-28 lines, compared to the CTRL line. It is possible that the enhanced amounts of MtTdp2α mRNA contribute to improve the plant response to copper-induced genotoxic injury. Although information concerning the contribution of the Tdp1 family members in heavy metal stress response in plants is scanty, these genes were demonstrated to be copper responsive (Macovei et al. 2010; Balestrazzi et al. 2011a).
Another interesting point is related to the ability of the MtTdp2α-overexpressing lines to sense DNA damage accumulation. This capacity should be possibly enhanced since all the three components of the MRN complex, a crucial sensor of DSBs in both animal and plant cells, were up-regulated under physiological conditions and then further stimulated in presence of copper. The MRN (MRE11-RAD50-NBS1) complex plays a key role as a sensor of DSBs, able to activate the DSB-induced cell cycle checkpoints and recognise DSB-repair effectors (Lamarche et al. 2010). MRE11 is a multifunctional nuclease active as 3′-5′ exonuclease in DSB repair, while the RAD50 protein characterised by ATPase and DNA binding activity interacts with MRE11. NBS1 directs the nuclear localization of the complex (Daoudal-Cotterell et al. 2002). The expression profiles of MNR components hereby reported are in agreement with those presented by Confalonieri et al. (2013) in the CTRL, Tdp2α-13c and Tdp2α-28 lines exposed to osmotic stress. Copper-induced ROS accumulation, particularly the production of hydroxyl radical (OH.) results in enhanced levels of single strand breaks (SSBs) and DSBs (Chiu et al. 1995). When copper binds directly to DNA, the presence of hydrogen peroxide (H2O2) seems to be required for the genotoxic effect to occur (Moriwaki et al. 2008). The enhanced expression of the DSB sensing/repair genes observed in MtTdp2α-overexpressing M. truncatula lines under physiological conditions well correlates with the reduced level of DSBs revealed by SCGE. On the other hand, it should be hypothesised that heavy metal stress did not result in MtMRE11, MtRAD50 and MtNBS1 mRNA accumulation as high as that found in CTRL due to the low DSB levels occurring in the MtTdp2α-overexpressing M. truncatula lines.
The plant antioxidant system includes several enzymes that respond in a coordinated manner when ROS are generated under stress conditions. SOD catalyses the dismutation of O2 − to O2 and H2O2 while metallothioneins have been recognised as efficient ROS scavengers able to prevent DNA damage (Balestrazzi et al. 2009). The plant tolerance to heavy metals is mediated by the antioxidant network and among the enzymatic components SOD plays a key role. Copper toxicity targets chloroplasts by affecting the photosynthetic electron transport and altering the thylakoid membrane structure (Vinit-Dunand et al. 2002). The Cu/Zn-SOD isoforms are found in cytosol and chloroplast, the cytSOD being responsive to 0.2 mM CuCl2 in aerial parts of M. truncatula plants (Macovei et al. 2011). Confalonieri et al. (2013) reported the up-regulation of MtcytSOD gene in the aerial parts of Tdp2α-13c and Tdp2α-28 plants grown in vitro under physiological conditions and this finding was then confirmed in the present work. However, a significant and consistent up-regulation of MtcytSOD gene was observed in all the tested lines exposed to copper, suggesting that the heavy metal treatment caused the induction of the basal antioxidant defence, independent on MtTdp2α gene overexpression. The enhancement of this specific DNA repair function may provide cells with the ability to face genotoxic injury inside the nuclear compartment. On the contrary, outside the nucleus in the surrounding cytosol the incoming copper-mediated oxidative damage triggers the antioxidant machinery. This hypothesis is also strengthened by the response observed with the MtcpSOD gene which was as well up-regulated in all the tested lines, independent on MtTdp2α gene overexpression. Thus, the reported data underline the occurrence of compartment-specific antioxidant defences and repair mechanisms in the MtTdp2α-overexpressing cells, in agreement with the current literature (Kodicha and Stochaj 2012).
The MtMT2 gene was up-regulated only in the Tdp2α-28 line under physiological conditions, in agreement with Confalonieri et al. (2013) while there was significant up-regulation in all the tested lines when copper was provided. The role of metallothionein in preventing the genotoxic injury caused by copper has been demonstrated by Balestrazzi et al. (2009) using transgenic white poplar plants overexpressing the PsMTa2 gene. However, differently from the transgenic white poplar plants overexpressing the PsMTa2 gene, the M. truncatula lines hereby investigated possess enhanced DNA repair gene expression which ensures a proper protection within the nuclear compartment. Based on the reported data, it seems that the basal antioxidant defence activated by M. truncatula cells, with and without MtTdp2α gene overexpression, includes metallothionein.
The overall picture emerging from the present work strongly support for the use of MtTdp2α gene as a tool for improving the plant response to heavy metal stress and provides further evidence that the overexpression of this DNA repair gene enhances the plant response to genotoxic injury resulting from different stress agents. Thus, biotechnological approaches involving the overexpression of MtTdp2α gene are expected to significantly reinforce the agronomical performance of elite crop varieties under adverse field conditions. However, the reported data also highlight the compartment-specific organisation of the cell response to oxidative injury, a complex as well as flexible way to control the molecular events inside and outside the nucleus. This point needs to be carefully evaluated when designing novel strategies to improve the plant tolerance to environmental stresses, which might possibly require the coordinated interplay between DNA repair functions and ROS scavengers within and outside the nuclear compartment.
References
Balestrazzi A, Botti S, Zelasco S, Biondi S, Franchin C, Calligari P, Racchi M, Turchi A, Lingua G, Berta G, Carbonera D (2009) Expression of the PsMT A1 gene in white poplar engineered with the MAT system is associated with heavy metal tolerance and protection against 8-hydroxy-2′-deoxyguanosine mediated-DNA damage. Plant Cell Rep 28:1179–1192
Balestrazzi A, Confalonieri M, Macovei A, Carbonera D (2011a) Seed imbibition in Medicago truncatula Gaertn.: expression profiles of DNA repair genes in relation to PEG-mediated stress. J Plant Physiol 168:706–713
Balestrazzi A, Confalonieri M, Macovei A, Donà M, Carbonera D (2011b) Genotoxic stress and DNA repair in plants: emerging functions and tools for improving crop productivity. Plant Cell Rep 30:287–295
Chiu SM, Xue LY, Friedman LR, Oleinick NL (1995) Differential dependence of chromatin structure for copper and iron ion induction of DNA double-strand breaks. Biochem 34:2653–2661
Collins AR (2004) The comet assay for DNA damage and repair. Mol Biotechnol 26:249–261
Confalonieri M, Faè M, Balestrazzi A, Donà M, Macovei A, Valassi A, Giraffa G, Carbonera D (2013) Enhanced osmotic stress tolerance in Medicago truncatula plants overexpressing the DNA repair gene MtTdp2α (tyrosyl-DNA phosphodiesterase 2). Plant Cell Tiss Organ Cult J Plant Biotech. doi:10.1007/s11240-013-0395-y
Cortes Ledesma FC, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW (2009) A human 5′-tyrosyl-DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 461:674–678
Daoudal-Cotterell S, Gallego ME, White CI (2002) The plant Rad50-MRE11 protein complex. FEBS Lett 516:164–166
Donà M, Ventura L, Balestrazzi A, Buttafava A, Carbonera D, Confalonieri M, Giraffa G, Macovei A (2013a) Dose-dependent reactive species accumulation and preferential double strand breaks repair are featured in the γ-ray response in Medicago truncatula cells. Plant Mol Biol Rep. doi:10.1007/s11105-013-0635-7
Donà M, Confalonieri M, Minio A, Biggiogera M, Buttafava A, Raimondi E, Delledonne M, Ventura L, Sabatini ME, Macovei A, Giraffa G, Carbonera D, Balestrazzi A (2013b) RNA-Seq analysis discloses early senescence and nucleolar dysfunction triggered by Tdp1α depletion in Medicago truncatula. J Exp Bot 64:1941–1951
Interthal H, Pouliot JJ, Champoux JJ (2001) The tyrosyl-DNS phosphodiesterase Tdp1 is member of the phospholipase D superfamily. Proc Natl Acad Sci USA 98:12009–12014
Kodicha M, Stochaj U (2012) Nuclear transport: a switch for the oxidative stress-signaling circuit? J Signal Transd 2012:1–8
Lamarche BJ, Orazio NI, Weitzman MD (2010) The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett 584:3682–3695
Liu W, Yang YS, Francis D, Rogers HJ, Li P, Zhang Q (2008) Cadmium stress alters gene expression of DNA mismatch repair related genes. Chemosphere 73:1138–1144
Macovei A, Balestrazzi A, Confalonieri M, Carbonera D (2010) The Tdp1 (tyrosyl-DNA phosphodiesterase) gene family in Medicago truncatula Gaertn.: bioinformatic investigation and expression profiles in response to copper- and PEG-mediated stress. Planta 232:393–407
Macovei A, Balestrazzi A, Confalonieri M, Buttafava A, Carbonera D (2011a) The TFIIS and TFIIS-like genes from Medicago truncatula are involved in oxidative stress response. Gene 470:20–30
Macovei A, Balestrazzi A, Confalonieri M, Faè M, Carbonera D (2011b) New insights on the barrel medic MtOGG1 and MtFPG functions in relation to oxidative stress response in planta and during seed imbibition. Plant Physiol Biochem 49:1040–1050
Moriwaki H, Osborne MR, Phillips DH (2008) Effects of mixing metal ions on oxidative DNA damage mediated by a Fenton-type reduction. Toxicol In Vitro 22:36–44
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:73–79
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 9:e36
Pommier Y (2013) Drugging topoisomerases: lessons and challenges. ACS Chem Biol 8:82–95
Reape TJ, McCabe PF (2008) Commentary: the cellular condensation of dying plant cells: programmed retraction or necrotic collapse? Plant Sci 207:135–139
Rodriguez E, Azevedo R, Fernandes P, Santos C (2011) Cr(VI) induces DNA damage, cell cycle arrest and polyploidization: a low cytometric and comet assay study in Pisum sativum. Chem Res Toxicol 24:1040–1047
Schenk RV, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204
Singh NP (2003) Apoptosis by DNA diffusion assay. In: Blumenthal R (ed) Methods in molecular medicine-chemiosensitivity. Humana Press, New York, pp 78–94
Varady G, Sarkadi B, Fatyol K (2011) TTRAP is a novel component of the non-canonical TRAF6-TAK1 TGF-beta signaling pathway. PLoS ONE 6:e25548
Ventura L, Donà M, Macovei A, Carbonera D, Buttafava A, Mondoni A, Rossi G, Balestrazzi A (2012) Understanding the molecular pathways associated with seed vigor. Plant Physiol Biochem 60:196–206
Vinit-Dunand F, Epron D, Alaoui-Sossè B, Badot PM (2002) Effects of copper on growth and on photosynthesis of mature and expanding leaves in cucumber plants. Plant Sci 163:53–58
Wellburn AR (1994) The spectral determination of chlorophylls a and b as well as total carotenoids using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Acknowledgments
This research was supported by grants from University of Pavia and Consiglio per la Ricerca e la Sperimentazione in Agricoltura (C.R.A.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Feher.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faè, M., Balestrazzi, A., Confalonieri, M. et al. Copper-mediated genotoxic stress is attenuated by the overexpression of the DNA repair gene MtTdp2α (tyrosyl-DNA phosphodiesterase 2) in Medicago truncatula plants. Plant Cell Rep 33, 1071–1080 (2014). https://doi.org/10.1007/s00299-014-1595-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1595-6