Abstract
Phosphoglycerolipids are essential structural constituents of membranes and some also have important cell signalling roles. In this review, we focus on phosphoglycerolipids that are mediators in hormone signal transduction in plants. We first describe the structures of the main signalling phosphoglycerolipids and the metabolic pathways that generate them, namely the phospholipase and lipid kinase pathways. In silico analysis of Arabidopsis transcriptome data provides evidence that the genes encoding the enzymes of these pathways are transcriptionally regulated in responses to hormones, suggesting some link with hormone signal transduction. The involvement of phosphoglycerolipid signalling in the early responses to abscisic acid, salicylic acid and auxins is then detailed. One of the most important signalling lipids in plants is phosphatidic acid. It can activate or inactivate protein kinases and/or protein phosphatases involved in hormone signalling. It can also activate NADPH oxidase leading to the production of reactive oxygen species. We will interrogate the mechanisms that allow the activation/deactivation of the lipid pathways, in particular the roles of G proteins and calcium. Mediating lipids thus appear as master players of cell signalling, modulating, if not controlling, major transducing steps of hormone signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Importance of hormones for plant physiology and development
Hormones are molecules produced by plant cells in response to environmental stresses and during plant development. The chemical natures of plant hormones are diverse. Some are related to adenine (cytokinins), others are terpenoids (gibberellins; abscisic acid, ABA) or polyhydroxysteroids (brassinosteroids) or are related to phenol derivatives (salicylic acid, SA) or derived from oxidised fatty acid (jasmonate). Some peptide hormones have also been described. Some plant hormones are volatile such as ethylene and some jasmonates. All hormones have in common that they act at extremely low concentrations. The first step of their action initiates when they are recognised by a receptor, thus triggering intracellular transduction and amplification of the signal. This will ultimately lead to cellular and organ responses.
The importance of hormone in plant physiology can be briefly illustrated by a few examples. Pathogen attacks induce an increase in SA that then triggers a response leading to systemic acquired resistance. ABA is synthesised during drought stress and promotes stomatal closure and inhibits stomatal opening, thereby reducing transpiration and water loss. ABA therefore has a major role in protection against dehydration. In development, auxins, for instance, are well known to stimulate cell elongation, stimulate differentiation of phloem and xylem, mediate the tropic response of bending in response to gravity and light, to stimulate lateral root development and delay leaf senescence (Taiz and Zeiger 2010).
These few examples clearly illustrate that hormones intervene at many levels during the plant’s lifetime. As a consequence, studying hormone signal transduction has always been considered primordial in plant biology. Hormone production, signalling and responses can affect vegetative growth, plant health and development of flowers, fruits and seeds, all aspects that are of high importance for agriculture.
For all hormone signalling pathways, major advances were achieved using genetics, mainly but not exclusively with Arabidopsis, where the focus is on the regulation of gene expression, the protein/protein interactions, the protein post-translational modifications and, more recently, the role of noncoding RNAs. For instance, now classical studies of promoter analysis allowed the identification of ABA-responsive element motifs (ABRE; Marcotte et al. 1989); the first step is to identify ABRE binding factors and their regulators. The isolation of ABA-insensitive mutants made it possible to show the importance of protein phosphorylation status in ABA responses (Leung et al. 1994). Those major findings and the ease of molecular biology tools using protein encoding genes eclipsed the fact that lipids were found early on to be signal mediators in hormone transduction (Scherer and André 1989). Recent studies have nevertheless put the production and involvement of lipids in hormone signalling back in the spotlight. Nevertheless, knowledge on lipids needs to be better integrated into some of the genetically defined hormone pathways.
We attempt this here by reviewing the current knowledge of the role of phosphoglycerolipids in hormone signalling. For each hormone signalling cascade known to require a phosphoglycerolipid pathway, we will describe which specific lipid signalling pathways are activated or inhibited. Ideas on how these pathways, and the molecules they generate, act and interact will be discussed. In doing so, lipid mediators, and in particular phosphatidic acid, will be shown to be involved in controlling well-established hormone signal transducers such as protein phosphatases or NADPH oxidases. Finally, we will discuss how the pathways can be activated or inhibited downstream of hormone perception. This review will thus propose mediating lipids to be master players of cell signalling, modulating, if not controlling, major transducing steps of hormone signals.
Overview of phosphoglycerolipid metabolic pathways
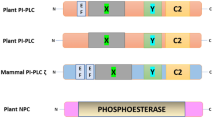
Phosphoglycerolipids are built from a glycerol backbone that is acylated by fatty acids on hydroxyls at positions sn-1 and sn-2. The hydroxyl group in the sn-3 position is esterified by phosphoric acid which is further esterified by another alcohol such as ethanolamine, serine, choline or myo-inositol (Fig. 1a). Signalling phosphoglycerolipids are generated through the action of phospholipases or lipid kinases (Fig. 1b). Structural phosphoglycerolipids such as phosphatidylcholine (PC), phosphatidylglycerol (PG) or phosphatidylethanolamine (PE) can be hydrolysed by phospholipase D (PLD, EC 3.1.4.4) into phosphatidic acid (PA). PA can also be formed in the reaction catalysed by diacylglycerol kinase (DGK, EC 2.7.1.107). The diacylglycerol (DAG) substrates of DGK can be generated by the action of phospholipase C enzymes (PLCs) of which there are two kinds. Phosphoinositide-specific PLC (PI-PLC, EC 3.1.4.11) hydrolyses phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) into DAG and inositol-1,4,5-triphosphate (IP3). IP3 can be phosphorylated into higher phosphorylated forms of soluble inositol by inositol kinases (IPK). Phosphatidylinositol-4-phosphate (PI4P) may be an in vivo substrate of PI-PLC in plants as it is known to be an in vitro substrate and PI4P is 10- to 100-fold more abundant than PI-4,5-P2 in plants (Delage et al. 2013). The so-called non-specific PLC (NPC, EC 3.1.4.3) hydrolyses structural phosphoglycerolipids into the corresponding phosphoalcohol and DAG (Pokotylo et al. 2013). Phospholipase A (PLA) catalyses the hydrolysis of ester bonds at the sn-1 and/or sn-2 positions of phosphoglycerolipids, thus liberating a free fatty acid (FFA) and forming a lysophosphoglycerolipid. PLAs may act specifically at the sn-1 or the sn-2 positions, and are named PLA1 (EC 3.1.1.32) or PLA2 (EC 3.1.1.4) accordingly (Ryu 2004; Scherer et al. 2010). PI4P and PI-4,5-P2 result from the sequential phosphorylations of phosphatidylinositol (PI) catalysed by PI-4-kinases (PI4K, EC 2.7.1.67) and PI4P-5-kinases (PI4P5K, EC 2.7.1.68). PI can also be phosphorylated into phosphatidylinositol 3-phosphate (PI3P) by a PI-3-kinase (PI3K, EC 2.7.1.137). PI3P can then be phosphorylated into PI(3,5)P2 (Delage et al. 2013). Finally, PA can be phosphorylated into diacylglycerol pyrophosphate (DGPP) by PA kinase (PAK) or dephosphorylated by phosphatidate phosphatase (PAP, EC. 3.1.3.4) or lipid phosphate phosphatases (LPP, EC 3.1.3.76). DGPP can also be a substrate of LPPs (Pleskot et al. 2012). Phosphates in the inositol group of either soluble polyphosphate-inositol or in lipid phosphoinositides can be hydrolysed by myo-inositol polyphosphate phosphatases (EC 3.1.3.57) and PI phosphatases (EC 3.1.3.64/66) that are more or less specific for the soluble or lipid inositol forms (Williams et al. 2005).
Structure of glycerophospholipids (a) and metabolic pathways generating phosphoglycerolipid signal mediators (b). Each phosphoglycerolipid class differs according to the nature of the polar head. The structure of inositol is indicated; its hydroxyl at position 1 is the one engaged in forming the phosphodiester bond; the other hydroxyls can be phosphorylated leading to different phosphoinositides. PI phosphatidylinositol, PI4P phosphatidylinositol-4-phosphate, PI3P phosphatidylinositol-3-phosphate, PI4,5P2 phosphatidylinositol-4,5-bisphosphate, DAG diacylglycerol, IP3 inositol triphosphate, IP6 inositol hexakisphosphate, PA phosphatidic acid, DGPP diacylglycerolpyrophosphate, FFA free fatty acid, PI4KIII type-III phosphatidylinositol-4-kinase, PI3K phosphatidylinositol-3-kinase, PI3P5K phosphatidylinositol-3-phosphate-5-kinase, PI4P5K phosphatidylinositol-4-phosphate-5-kinase, PI-PLC phosphoinositide-specific phospholipase C, NPC non-specific phospholipase C, DGK diacylglycerol kinase, IPK inositol phosphate kinase, LPP lipid phosphate phosphatase, PAP PA-phosphatase, PAK PA kinase, PLA phospholipase A, PLD phospholipase D
Gene expression in response to hormones
A great deal of data implicating phosphoglycerolipids in hormone signal transduction is coming from transcriptome analysis (Lin et al. 2004). If a gene encoding an enzyme of the phosphoglycerolipid signalling pathways is upregulated in response to a hormone, it could mean that the encoded protein has a role in transducing the hormone signal. We analysed the relative levels of expression of genes encoding enzymes involved in phosphoglycerolipid pathways in Arabidopsis thaliana using Genevestigator (Hruz et al. 2008). We chose experiments in which RNA was sampled no later than 4 h after hormone treatment (Fig. 2). The transcript level of most genes encoding enzymes involved in the phosphoglycerolipid pathway changed in response to hormone. Within individual families of enzymes, some isoforms are induced and some repressed by the same hormone treatment, which suggests that different isoforms have different roles. For instance, PLDβs, γ1, γ3, ζ1 and α1 are repressed by ABA. They are also repressed by the cytokinin zeatin, but induced by SA. The fact that ABA and zeatin have the same effect, which is opposite to that of SA, cannot be generalised though. PLDδ and ζ2 are induced by ABA but are repressed by zeatin. It is striking that transcript levels of some PLA1 and plastid LPP genes are very much controlled by hormones, while their role in hormone transduction in plants is as yet poorly documented (Fig. 2).
Relative expression of genes encoding enzymes involved in phosphoglycerolipid signalling. The hormone, treatment duration and Gene Expression Omnibus code for each set of experimental data used in this analysis are as follows: zeatin, 3 h (AT-00239); SA, 4 h (AT-0039); methyl jasmonate (MeJA), 3 h (AT-00110); auxin indole-3-acetic acid (IAA), 1 h (AT-00407); gibberellic acid (GA), 3 h (AT-00110); ethylene precursor 1-aminocyclopropane-1-carboxylic-acid (ACC), 3 h (AT-00110); ABA, 3 h on leaf (AT-00433); and ABA, 3 h on guard cell (AT-00433). The AGI references for gene names are in Supplementary Table 1. Genes of each enzyme class were then sorted out after a Pearson’s correlation clustering analysis in Genevestigator to evidence genes with close expression pattern in response to hormone treatments
Involvement of phosphoglycerolipids in hormone signalling
While transcriptome studies can indicate possible involvement, it is important to show that enzymes of these pathways are actually active and/or activated or inhibited in response to hormones. Mostly in vivo lipid labelling has been used for which suspension cells are the most amenable and relevant models. The availability of mutant plants is helpful to further narrow down the exact role of the in vivo activities measured.
Abscisic acid
The most documented description of phosphoglycerolipids in hormone signal transduction is for ABA. In Arabidopsis thaliana suspension cells, ABA plasmalemma perception results in a transient stimulation of phospholipase D (PLD) activity that is necessary for the activation of anion channels in the pathway leading to RAB18 expression (Hallouin et al. 2002). This PLD activity leads to a transient increase in PA that is followed by an increase in DGPP, showing that a PAK is active. While exogenous PA activates plasmalemma anion currents, it does not trigger the expression of RAB18 or RD29A. On the contrary, exogenous DGPP mimics ABA in stimulating similar changes in both anion currents and gene expression. This suggests that DGPP could be the lipid mediator resulting from ABA activation of the PLD pathway in Arabidopsis suspension cells (Zalejski et al. 2005, 2006).
Guard cells generate reactive oxygen species (ROS) in response to ABA, which triggers stomatal closure. Inhibiting the production of PI3P or PI4P, or sequestrating them by the expression of PI3P-binding or PI4P-binding domains, inhibits ABA-induced stomatal closure (Park et al. 2003). The role of PI4P could be linked to its role as a precursor of substrates for PI-PLC. Indeed, endogenous IP3 levels were found to increase within 2 min of ABA application to stomata (Lee et al. 1996). The induction of some ABA-responsive genes was reduced in transgenic lines expressing an antisense AtPLC1 (Sanchez and Chua 2001). The signalling molecules generated by the PI-PLC pathway activated in stomata in response to ABA are probably soluble phosphorylated inositols (polyphosphoinositols) as microinjection of IP3 into stomata initiates their closure (Gilroy et al. 1990). Reducing the level of polyphosphoinositols by overexpressing inositol polyphosphate phosphatases decreases the induction of some ABA-responsive genes (Sanchez and Chua 2001). By contrast, Arabidopsis fiery1 (fry1), in which an inositol polyphosphate 1-phosphatase is mutated, accumulates more IP3 than the wild type upon ABA treatment with stronger induction of ABA-responsive genes (Xiong et al. 2001).
PLDs are also involved in ABA signal transduction in guard cells. PLDs can use primary alcohols as substrates to form a phosphatidylalcohol (in the so-called transphosphatidylation reaction) to the detriment of PA production. Secondary or tertiary alcohols are not substrates of the PLD-catalysed reaction. n-Butanol (n-ButOH) inhibits ABA-induced stomatal closure, pointing to a role for PLD-produced PA in this process. Similarly, in an Arabidopsis pldα1 pldδ double mutant, ABA-induced stomatal closure was suppressed. Accumulation of ROS and nitric oxide (NO), alkalisation of the cytosol, and inhibition of inward-rectifying K+ channel currents that occur in wild-type ABA-treated guard cells are all inhibited in this double mutant. However, the calcium response to ABA is not altered in double-mutant guard cells (Uraji et al. 2012). While the above results suggest PLDs are upstream of the NO response, PLDδ could also be downstream of NO as pldδ mutants fail to close their stomata in response to NO (Distéfano et al. 2012). The mode of action of the PA produced by PLDα1 in response to ABA is detailed in “Phosphatidic acid targets”.
PA and DGPP levels increase when seeds are treated with ABA. In Arabidopsis, LPP-coding genes AtLPP2 and AtLPP3 are expressed during seed germination. AtLPP2 insertion mutants are hypersensitive to ABA in terms of germination inhibition. This is correlated with greater accumulation of PA during mutant germination compared to WT, showing that PA relays the ABA effect on germination. Similarly, in rice, the expression of α-amylase, a key step in germination, is inhibited when seeds are treated with PA. Double-mutant analysis in Arabidopsis showed that ABA-insensitive 4 (ABI4) is epistatic to AtLPP2. PA thus appears to be upstream of the activity of ABI4, an AP2-type transcription factor. The origin of PA involved in ABA inhibition of germination might be due to PLD activities. In Oryza sativa, mutation of PLDβ1 inhibits the repression of germination by ABA and leads to a reduction in the repression of α-amylase by ABA (Li et al. 2007). PI-PLC could also participate in ABA inhibition of germination. Germination of seeds overexpressing the AtPLC1 gene is not inhibited by ABA (Sanchez and Chua 2001). Here again the active molecule generated by PI-PLC in seeds might be polyphosphoinositol. Seeds with a mutated inositol polyphosphate phosphatase gene, which have increased levels of IP3, have an enhanced sensitivity to ABA (Gunesekera et al. 2007).
Salicylic acid
In Arabidopsis suspension cells, we showed that SA stimulation led to the rapid activation of PLD. In the presence of primary alcohols that quench PA formation by PLDs, transcriptomic changes due to the hormone were strongly affected (Krinke et al. 2009). Interestingly, in the same model, SA also activated a type-III PI4K, leading to the formation of PI4P and PI-4,5-P2. There was considerable overlap between PLD-controlled genes and PI4K-controlled genes. Amongst the genes controlled similarly by both pathways were WRKY38 and PR1. This led us to hypothesise that SA activates PI4K, increasing PI4P and PI-4,5-P2, with the latter lipid activating some PLD isoforms. However, NPR1 induction by SA was abolished by including n-ButOH but not by inhibiting type-III PI4K, suggesting that in response to SA, both PI-4,5-P2-dependent PLDs and PI-4,5-P2-independent PLDs are brought into play (Krinke et al. 2007a, 2009). Identifying the PLDs activated in response to SA will help in validating this hypothesis. In Arabidopsis, the PI-4,5-P2-dependent PLDs, PLDβ2 and PLDγs, are induced by SA (see “Gene expression in response to hormones”), which might point to them having a role in SA transduction.
Do the pathways involved in the suspension cell SA response have the same role in plants? Possibly. The addition of primary alcohols blocks PR1 expression in response to SA in Arabidopsis plantlets (Janda et al., unpublished). PI-PLC could also have a role in SA signal transduction in plants. PR1 is overexpressed in Arabidopsis plants expressing a mammalian inositol polyphosphate 5-phosphatase in control conditions (Perera et al. 2008).
It is also important to understand how SA activates PI4K. In in vitro enzymatic assays of microsomes extracted from Capsicum chinense Jacq. suspension cells 30 min after SA treatment, it was found that SA provoked an increase in lipid kinase activities leading to PI4P and PI-4,5-P2 and a decrease in PI-PLC activity (Altuzar-Molina et al. 2011). This fits well with what we observed in vivo in Arabidopsis suspension cells. The very short period in which these in vitro changes occur after SA treatment most likely implies that PI4K and/or PI-PLC are subject to post-translational modifications, which will be confirmed by further investigation.
Auxins
As early as 1989, Scherer and André showed that when auxins were added to soybean suspension cells prelabelled with radioactive ethanolamine or choline, an increase in radioactive lysoPE or lysoPC occurred within 5 min. This was a strong indication that a PLA was activated. Interestingly, some PLA2 inhibitors were found to inhibit the growth of etiolated Arabidopsis hypocotyls, an auxin-driven mechanism (Holk et al. 2002), as well as the auxin-induced activation of the promoter of the DR5 gene (Scherer et al. 2007). Plant PLA2 enzymes are encoded either by patatin-related PLA (pPLA) genes or by secreted PLA2 (sPLA2) genes. All single Arabidopsis pPLA mutants show delays in the up-regulation of early auxin-induced genes compared to WT. Thus, ppla mutants are considered to be auxin-signalling mutants (Labusch et al. 2013). Overexpression of AtsPLA2β promotes cell elongation, whereas silencing of AtsPLA2β expression retards it. AtsPLA2β is also rapidly induced by auxin treatments. To explain the elongation phenotypes, it is proposed that AtsPLA2β has a role in transducing auxin (Lee et al. 2003). PLAs are not the only phospholipases that have been shown to be involved in passing on the auxin signal. In cucumber, a PLD was activated, and PI4P and PI-4,5-P2 also accumulated within 1 min of auxin treatment. Interestingly, the auxin-induced accumulation of these signalling lipids is dependent on auxin-induced NO production (Lanteri et al. 2008).
When considering auxin signalling, it is important to bear in mind that auxin has an asymmetrical distribution. This asymmetry is achieved through polar transport of auxin by influx or efflux auxin carriers. PINs are the best-characterised proteins controlling auxin efflux. Auxin distribution is altered in an inositol polyphosphate 5-phosphatase 13 mutant (Wang et al. 2009), whereas PIN distribution is affected in AtPLA2α and AtPIP5K2 mutants (Lee et al. 2010). It has also been suggested that an AtPLDζ2 mutant was affected in PIN cycling (Li and Xue 2007). Finally, auxin synthesis might be controlled through PI-PLC. Myo-inositol and indole-3-acetonitrile, a precursor of the auxin indole-3-acetic acid, were up-regulated in the rotunda1-1 (ron1-1) mutant. RON1 gene is identical to FRY1, mentioned in “Abscisic acid”, which encodes an enzyme with inositol polyphosphate 1-phosphatase activity (Robles et al. 2010).
Which molecules act as mediators
Phosphoglycerolipid pathways generate molecules that can act as mediators by binding and thus altering the activity of effectors, or targets. Altering may imply activating or inhibiting the target and also sequestering it in a membrane, thus preventing the effector from reaching its site of action. Identifying these targets is a field of research in full swing. Although some targets have been identified, their roles in hormone signal transduction may not necessarily have been described. We will summarise current knowledge of the targets of molecules generated by phosphoglycerolipid pathways, always bearing in mind how these pathways may act in hormone signal transduction and how new actors may be identified.
Phosphatidic acid targets
PA is the common product of PLC/DGK and PLD pathways. Besides its involvement in hormone signalling as reviewed above, it has been shown to be involved in response to various abiotic and biotic stresses. Some PA binding proteins have been found in plants (Dubots et al. 2012; Wang et al. 2006). Table 1 lists a selection of plant proteins that bind PA and also have a role in signalling. The list is not exhaustive as protein kinases, protein phosphatases, lipid kinases and lipid phosphatases also bind PA in a way that modifies their activity.
The role of some of these proteins has been well described in studies of ABA responses. PLDα1 produces PA that binds to the ABI1 protein phosphatase 2C, a negative regulator of ABA action. This interaction inhibits the function of ABI1 by inhibiting its phosphatase activity and tethering it to the plasma membrane. PA formation by PLDα1 thus alleviates the inhibiting effect of ABI1 and allows gene expression through ATHB6. PA produced by PLDα1 has also been shown to interact with and stimulate NADPH oxidases. Interestingly, the interaction between PA and ABI1 is not required for the ABA-induced production of ROS and NO but is important for mediating the ROS effect on stomatal closure (Zhang et al. 2009). Finally another PA binding protein related to ABA signal transduction is sphingosine kinase (SPHK). The interplay between PLD and SPHK pathways could be complex. PA is involved in the activation of SPHK, but activation of PLDα1 requires SPHK activity, suggesting that SPHK and PLDα1 are co-dependent in amplification of the ABA response-mediating stomatal closure in Arabidopsis (Guo et al. 2012).
It would be interesting to know whether the same protein targets play the same roles in other hormone signalling pathways involving the production of PA. Kalachova et al. (2013) showed that Arabidopsis NADPH oxidase AtRbohD is also downstream of PA production in guard cells challenged with SA.
The other proteins listed in Table 1 have not been directly linked to hormone signal transduction, but they are very good candidates as mediators of hormone signals that lead to PA accumulation. Many of these proteins belong to multigene families, so other members of the families may also be involved in hormone signalling.
Diacylglycerol
Very few proteins that bind DAG have been identified in plants (Dong et al. 2012). In mammals, DAG binds protein kinase C (PKC) isoforms through their cysteine-rich C1 domain. Higher plants lack genes encoding PKCs. However, some kinases having the enzymatic properties of PKC have been detected, and some of them are activated by DAG, such as a Brassica campestris kinase (Nanmori et al. 1994). Plant genomes also have genes encoding proteins with C1 domains. For instance, the Arabidopsis thaliana genome encodes 30 confirmed or putative C1 domain proteins (Supplemental figure S1), including 2 DGKs. It is possible that, for some of these proteins, the C1 domain is an actual DAG binding domain. More data are necessary to decipher the exact role of DAG in hormone signal transduction.
Phosphorylated inositols
IP3 is produced through the action of PI-PLC on PIP2. In mammals, IP3 is well documented to bind to some calcium channels, thus allowing the release of calcium from internal stores into the cytosol. No IP3-binding channels have been found in land plants (Krinke et al. 2007b), although they do occur in Chlamydomonas (Wheeler and Brownlee 2008). It has been suggested that the more highly phosphorylated forms of inositol could be mediators in higher plants. For instance, inositol hexakisphosphate (IP6, also named phytic acid) has been shown to act as a more potent endomembrane calcium-release agent than IP3 in guard cells of Vicia faba (Lemtiri-Chlieh et al. 2003).
The auxin receptor transport inhibitor response 1 (TIR1) was unexpectedly found to have crystallised with IP6 (Tan et al. 2007), as was the jasmonate receptor COI1 (Sheard et al. 2010). Does this mean that they are polyphosphoinositide receptors and that PI-PLC signalling controls auxin and jasmonate perception? If so, this would be very interesting. However, we need to keep in mind that the PI-PLC pathway is not the only source of phosphorylated inositols. Glucose-6-P can be converted into IP3 by myo-inositol monophosphate synthase and IP3 can then be sequentially phosphorylated (Raboy 2009).
Phosphoinositides
Several phosphoinositide-binding domains are known, such as the pleckstrin homology (PH), FYVE, HEAT, C2 and Phox homology (PX) domains (Catimel et al. 2008). These domains are more or less specific for phosphoinositides as some can also bind to other anionic phospholipids or proteins. The Arabidopsis thaliana genome encodes ca. 168 proteins with at least one of these domains (Supplemental figure S2). Many of these proteins are involved in signal transduction, in cell fate determination or intracellular trafficking. For most of these proteins, however, it has yet to be established whether they act in hormonal signal transduction.
Some phosphoinositides control cellular processes. In animal cells, a wide range of channels are controlled by PI-4,5-P2. In Nicotiana tabacum protoplasts isolated from cultured cells with genetically altered plasma membrane levels of PIP2, the net steady-state outward K+ current was inversely related to the plasma membrane PIP2 level. Increasing PIP2 levels in controls or in ABA-treated cells with high PI levels, using the PI-PLC inhibitor U73122, decreased the outward-rectifying potassium channel (NtORK) activity. These results would be consistent with the negatively charged PI-4,5-P2 in the inner plasma membrane leaflet inhibiting NtORK (Ma et al. 2009).
Tubby and Tubby-like proteins (TLPs) were first discovered in mammals where they are involved in the development and function of neuronal cells. The binding of the carboxyl-terminal Tubby domain to PI-4,5-P2 sequestrates these proteins to the plasma membrane. When PI-PLC is activated, PIP2 is hydrolysed and thus TLPs are released from the plasma membrane. TLPs are conserved across eukaryotic kingdoms. Hydrogen peroxide stimulates the release of AtTLP3 from the plasma membrane in a mechanism involving PI-PLC (Reitz et al. 2012). Therefore, Tubby proteins could be a link between PI-PLC and oxidative stress, and hence to hormone signalling. However, in Arabidopsis, no phytohormone treatment (with methyljasmonate, ABA, SA, auxin, brassinosteroid, or GA) leads to relocalisation of AtTLP3. Therefore, the possible role of other Arabidopsis TLPs in hormone transduction in conjunction with PI-PLC is uncertain.
Lysophosphoglycerolipids and free fatty acids
Lysophosphoglycerolipids and FFA are released by the action of PLA. Both types of molecules have mediating roles. For instance, oxidised fatty acids in potato suspension cells in vivo and saturated fatty acids in vitro stimulate protein kinase activity. Unsaturated fatty acids inhibit MP2C, a protein phosphatase, while polyunsaturated fatty acids can modulate K+ channels. Oleic acid activates PLDδ. The lysophosphoglycerolipid lysoPE inhibits a PLD in fruit senescence. It has been postulated that lysoPC could activate an H+/Na+ exchange vacuolar transporter, thus mobilising the vacuolar proton pool for pH signalling (references in Scherer et al. 2010). Clearly, more work is necessary to identify other targets of FFA and lysophosphoglycerolipids.
Conclusion
Open questions
Clearly lipid signalling is of major consequence in plant hormone signal transduction. Yet many questions remain open that will define the immediate future of this fascinating research field.
Lipid signalling in the signal transduction of other hormones
Phosphoglycerolipid signalling is now well documented for ABA, SA and auxins. Does it also play a role in the transduction of other hormone signals? Very promising data suggest so. Methyljasmonate has been shown to activate PLD in Brassica napus, Taxus cuspidata and Silybum marianum cells (Profotová et al. 2006; Yang et al. 2008; Madrid and Corchete 2010; Sánchez-Sampedro et al. 2009). In Arabidopsis plantlets, mechanical wounding known to induce jasmonate led to a 4- to 5-fold increase in IP3 with concomitant depletion in phosphoinositides. No such IP3 increase was observed in dde2-2 mutant plants that are deficient in JA biosynthesis (Mosblech et al. 2008). Cytokinins activate PLD as a step in the control of gene expression in Arabidopsis (Romanov et al. 2002) and Catharanthus roseus suspension cells (Amini et al. 2008). In Amaranthus seedlings, a PI-4,5-P2-dependent PLD controls the accumulation of amaranthin (a pigment) that follows cytokinin application (Kravets et al. 2010). Ethylene activates PLD in carrot suspension cells (Lee et al. 1998). PLDα-deficient transgenic plants have slower rates of senescence induced by ethylene than WT (Fan et al. 1997). Gibberellic acid (GA) leads to a rapid and transient IP3 accumulation in barley aleurone (Villasuso et al. 2003). Interestingly, in rice seeds, ABA prevents the gibberellin-induced formation of IP3, although ABA alone does not alter the IP3 level (Kashem et al. 2000). Brassinosteroid treatment of Arabidopsis seedlings leads to increased expression of NPC3 and NPC4 monitored by GUS activity in leaves (Wimalasekera et al. 2010). LysoPE, a product of PLA2 activity, collaborates with brassinosteroids during stimulation of Arabidopsis root growth and development (Jeong et al. 2012).
How are the lipid signalling pathways activated or inhibited in response to hormones?
The upstream events leading to the regulation of phospholipid signalling pathways are far less well understood than the downstream processes.
Calcium has an important role in controlling the activity of enzymes involved in lipid signalling. PI-PLCs are strictly dependent on calcium for their activity, except AtPLC4 that retains 20 % of its activity with no calcium. Most PLDs need calcium for their activity and only PLDζ isoforms have shown to be calcium independent. PLDα enzymes need higher concentrations of calcium than other calcium-dependent PLDs, which may indicate that this is an important regulatory mechanism. Some PLAs, both pPLA2 and sPLA2 isoforms, have shown to be calcium dependent (Chen et al. 2011).
Some enzymes of the phosphoglycerolipid pathway may be post-translationally modified in a way that modulates their activities. In Brassica oleracea, a PIP2-dependent PLD is phosphorylated and this phosphorylation might positively regulate its activity (Novotná et al. 2003). Arabidopsis PLDα1 also seems to be regulated by this mechanism (Novotná, unpublished results). Arabidopsis patatin-related PLA2 pPLA-IIδ and pPLA-IIε are phosphorylated by calcium-dependent protein kinases in vitro (Rietz et al. 2010). Regulation by phosphorylation is probably a major component of the activation/inhibition of lipid pathways. The PhosPhAt database curates the sequences of experimentally detected phosphorylated peptides obtained in Arabidopsis phosphoproteomics experiments (http://phosphat.mpimp-golm.mpg.de/download.html; Durek et al. 2010), and includes many that originate from enzymes of the phosphoglycerolipid pathway (Supplemental Table 2). However, the significance of these phosphorylations in relation to hormone signalling has not been investigated.
An important mechanism in phosphoglycerolipid signalling seems to be the coupling with heterotrimeric G protein (Tuteja and Sopory 2008). Potential G-protein activators and inhibitors, such as toxins, nonhydrolyzable guanine nucleotide analogues, and alcohols, affect plant PLD (Munnik et al. 1995; Ritchie and Gilroy 2000), and PLD can also control G proteins. The direct interaction between AtPLDα1 and the α-subunit of heterotrimeric G proteins (GPA1) has been characterised using protein co-precipitation. PLDα1 binds to GPA1 and activates the intrinsic GTPase activity that converts active Gα-GTP to inactive Gα-GDP. In turn, Gα-GDP binds to PLDα1 and attenuates its activity. Thus, the PLDα1–GPA1 interaction mutually regulates both partners (Mishra et al. 2006). An intriguing possibility that follows on from this observation is that PLD might be regulated by G protein-coupled receptors. G proteins also regulate PI-PLC. In tobacco cells overexpressing either Arabidopsis G protein-coupled receptor (GCR1) or the Gα-subunit of the heterotrimeric G protein, PI-PLC is active and IP3 levels are elevated (Apone 2003). The G-protein activator Mas7 is able to trigger α-amylase secretion in barley aleurone just as strongly as GA does. U73122 inhibits this Mas7 effect, linking the Mas7 effect with PI-PLC activity (Villasuso et al. 2003). Pisum sativum Gα interacts with the C-terminal C2 domain of pea PLCδ (Misra et al. 2007). It is still not known whether all PI-PLC and PLD isoforms are dependent on G protein activity.
An intriguing aspect of lipid signalling regulation may be the role played by the interaction of phospholipases and signalling phospholipids with components of the cytoskeleton. In tobacco, monomeric G-actin inhibits and polymeric F-actin enhances the PIP2-dependent activity of NtPLDβ1, while PIP2-independent PLDα activity and oleate-activated PLDδ activity are unaffected by either form of actin (Pleskot et al. 2010). PA has also been shown to modulate actin dynamics both in vitro and in vivo by binding to capping protein (Li et al. 2012; Pleskot et al. 2012).
Finally, it should be noted that the different phosphoglycerolipid pathways are likely to be intertwined. For example, plant PLDs need PI-4,5-P2 as cofactors, the exceptions being the α-type PLDs. Therefore, PI-PLC activity might indirectly modulate PLD activity, by modulating the level of phosphoinositide cofactors.
What is the link with known hormone receptors?
The first step in signalling is when a receptor perceives the hormone. However, when phosphoglycerolipid signalling is known to be involved in a specific hormone signalling pathway, the link to the hormone receptor(s) has not been made. It has recently been proved that the PYR/PYL/RCAR family of soluble proteins in Arabidopsis are ABA-binding receptor-like proteins. Upon ABA binding, these PYR/PYL/RCAR receptors bind to and inhibit PP2C allowing the phosphorylation and activation of SnRK2 proteins. It is not certain how PA fits into this scenario given that PA interacts with ABI1. Is PLDα1 activated upon ABA binding to PYR/PYL/RCAR soluble receptors? Or does PLDα1 activation involve a yet-to-be-discovered receptor? We note that a membrane impermeant ABA–bovine serum albumin conjugate, which cannot enter the cell, is able to activate PLD in Arabidopsis suspension cells (Hallouin et al. 2002). Different proteins are known to bind SA, such as catalase, ascorbate peroxidase, chloroplast carbonic anhydrase, methyl salicylate esterase, some α-ketoglutarate dehydrogenases and some glutathione S-transferases (Tian et al. 2012). NPR1 (Wu et al. 2012) and its paralogues NPR3 and NPR4 (Fu et al. 2012) are proteins that have also been shown to bind SA. Thus, it needs to be elucidated whether or not lipid signalling is connected to these “receptors”, and if so how (whether directly or via other proteins such as G proteins). Auxin is perceived either by ABP1 or TIR1/auxin-signalling F box proteins. It has been suggested that the receptor responsible for pPLA activation by auxin is ABP1, but more experimental data are necessary to establish this (Scherer et al. 2012).
How lipid mediators act
More work is needed to discern the modes of action of the lipid mediators. There is now quite a long list of PA binding proteins, but it is unlikely to be exhaustive. No DGPP binding proteins have been identified so far either in plants or in other biological kingdoms. Nevertheless, the specific role of DGPP as a hormone signal transduction player seems beyond doubt since DGPP has effect that PA has not (Zalejski et al. 2006). Finding DGPP binding proteins is therefore a challenge ahead.
Most of the molecules generated by these pathways are lipids and stay in membranes. This gives rise to two questions. First, how do these lipids affect the physical state of the membrane—its fluidity, flexibility, or capacity to curve. PA, for instance, is considered to be a cone-shaped lipid. Lysophospholipids have inverted cone shapes. FFAs make membranes more fluid. Do such changes in the physical state of membranes have a role in hormone signal transduction as part of lipid signalling? It is believed that vesicle budding is favoured by PLA2 activity, because its action on a single leaflet of the bilayer asymmetrically increases the number of inverted cone-shaped lysophospholipids, thus enhancing membrane curvature towards the said leaflet side (Brown et al. 2003). PLD products of PA could have similar effects. PA is a fusogenic lipid (Jenkins and Frohman 2005). Vesicle formation, mobility and fusion are necessary for hormone signalling as exemplified by the trafficking of PIN proteins in auxin responses. Discovering the importance of lipid mediators not through binding proteins but through membrane effects will be a fascinating field of research.
In conclusion, phosphoglycerolipids appear to be major players in hormone transduction. The data obtained so far suggest they are involved in the signalling of most, if not all, plant hormones. Much more data will be necessary to understand how these pathways are regulated and how they act to transduce hormone signals. The same phosphoglycerolipid players are involved in the responses to different hormones and this could explain in part the crosstalk between hormones. However, the exact timing, duration and amplitude of the phosphoglycerolipid responses, and their interacting partners, probably differ going some way to explain the specificity of hormone responses.
References
Altuzar-Molina AR, Armando Munoz-Sanchez J, Vazquez-Flota F, Racagni-Di Palma G, Hernández-Sotomayor SM (2011) Phospholipidic signaling and vanillin production in response to salicylic acid and methyl jasmonate in Capsicum chinense J. cells. Plant Physiol Biochem 49:151–158. doi:10.1016/j.plaphy.2010.11.005
Amini A, Glévarec G, Andreu F, Reverdiau P, Rideau M, Crèche J (2008) Effects of phosphatidic acid on cytokinin signal transduction in periwinkle cells. J Plant Growth Regul 27:394–399. doi:10.1007/s00344-008-9058-3
Anthony RG, Henriques R, Helfer A, Mészáros T, Rios G, Testerink C, Munnik T, Deák M, Koncz C, Bögre L (2004) A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in Arabidopsis. EMBO J 23:572–581. doi:10.1038/sj.emboj.7600068
Apone F (2003) The G-protein-coupled receptor GCR1 regulates DNA synthesis through activation of phosphatidylinositol-specific phospholipase C. Plant Physiol 133:571–579. doi:10.1104/pp.103.026005
Brown WJ, Chambers K, Doody A (2003) Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic 4:214–221. doi:10.1034/j.1600-0854.2003.00078.x
Camoni L, Di Lucente C, Pallucca R, Visconti S, Aducci P (2012) Binding of phosphatidic acid to 14-3-3 proteins hampers their ability to activate the plant plasma membrane H+-ATPase. IUBMB Life 64:710–716. doi:10.1002/iub.1058
Catimel B, Schieber C, Condron M, Patsiouras H, Connolly L, Catimel J, Nice EC, Burgess AW, Holmes AB (2008) The PI(3,5)P2 and PI(4,5)P2 interactomes. J Proteome Res 7:5295–5313. doi:10.1021/pr800540h
Chen G, Snyder CL, Greer MS, Weselake RJ (2011) Biology and biochemistry of plant phospholipases. Crit Rev Plant Sci 30:239–258. doi:10.1080/07352689.2011.572033
Delage E, Puyaubert J, Zachowski A, Ruelland E (2013) Signal transduction pathways involving phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: convergences and divergences among eukaryotic kingdoms. Prog Lipid Res 52:1–14. doi:10.1016/j.plipres.2012.08.003
Distéfano AM, Scuffi D, García-Mata C, Lamattina L, Laxalt AM (2012) Phospholipase Dδ is involved in nitric oxide-induced stomatal closure. Planta 236:1899–1907. doi:10.1007/s00425-012-1745-4
Dong W, Lv H, Xia G, Wang M (2012) Does diacylglycerol serve as a signaling molecule in plants? Plant Signal Behav 7:472–475. doi:10.4161/psb.19644
Dubots E, Botte C, Boudiere L, Yamaryo-Botté Y, Jouhet J, Maréchal E, Block MA (2012) Role of phosphatidic acid in plant galactolipid synthesis. Biochimie 94:86–93. doi:10.1016/j.biochi.2011.03.012
Durek P, Schmidt R, Heazlewood JL, Jones A, MacLean D, Nagel A, Kersten B, Schulze WX (2010) PhosPhAt: the Arabidopsis thaliana phosphorylation site database. An update. Nucleic Acids Res 38:D828–D834. doi:10.1093/nar/gkp810
Fan L, Zheng S, Wang X (1997) Antisense suppression of phospholipase D alpha retards abscisic acid- and ethylene-promoted senescence of postharvest Arabidopsis leaves. Plant Cell 9:2183–2196. doi:10.1105/tpc.9.12.2183
Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, Dong X (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486:228–232. doi:10.1038/nature11162
Gilroy S, Read ND, Trewavas AJ (1990) Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature 346:769–771. doi:10.1038/346769a0
Gunesekera B, Torabinejad J, Robinson J, Gillaspy GE (2007) Inositol polyphosphate 5-phosphatases 1 and 2 are required for regulating seedling growth. Plant Physiol 143:1408–1417. doi:10.1104/pp.106.089474
Guo L, Mishra G, Taylor K, Wang X (2011) Phosphatidic acid binds and stimulates Arabidopsis sphingosine kinases. J Biol Chem 286:13336–13345. doi:10.1074/jbc.M110.190892
Guo L, Mishra G, Markham JE, Li M, Tawfall A, Welti R, Wang X (2012) Connections between sphingosine kinase and phospholipase D in the abscisic acid signaling pathway in Arabidopsis. J Biol Chem. doi:10.1074/jbc.M111.274274
Hallouin M, Ghelis T, Brault M, Bardat F, Cornel D, Miginiac E, Rona JP, Sotta B, Jeannette E (2002) Plasmalemma abscisic acid perception leads to RAB18 expression via phospholipase D activation in Arabidopsis suspension cells. Plant Physiol 130:265–272. doi:10.1104/pp.004168
Holk A, Klumpp L, Scherer GFE (2002) A cell wall protein down-regulated by auxin suppresses cell expansion in Daucus carota (L.). Plant Mol Biol 50:295–305. doi:10.1023/A:1016052613196
Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008:420747. doi:10.1155/2008/420747
Huang S, Gao L, Blanchoin L, Staiger CJ (2006) Heterodimeric capping protein from Arabidopsis is regulated by phosphatidic acid. Mol Biol Cell 17:1946–1958. doi:10.1091/mbc.E05-09-0840
Jenkins GM, Frohman MA (2005) Phospholipase D: a lipid centric review. Cell Mol Life Sci 62:2305–2316. doi:10.1007/s00018-005-5195-z
Jeong S, Park CH, Kim MK, Nam S, Hong J, Kim SK (2012) Effect of lysophosphatidylethanolamine and brassinosteroids on development of Arabidopsis roots. J Plant Biol 55:178–184. doi:10.1007/s12374-011-9202-7
Kalachova T, Iakovenko O, Kretinin S, Kravets V (2013) Involvement of phospholipase D and NADPH-oxidase in salicylic acid signaling cascade. Plant Physiol Biochem. doi:10.1016/j.plaphy.2013.02.006
Kashem MA, Itoh K, Iwabuchi S, Hori H, Mitsui T (2000) Possible involvement of phosphoinositide-Ca2+ signaling in the regulation of α-amylase expression and germination of rice seed (Oryza sativa L.). Plant Cell Physiol 41:399–407. doi:10.1093/pcp/41.4.399
Klimecka M, Szczegielniak J, Godecka L, Lewandowska-Gnatowska E, Dobrowolska G, Muszyńska G (2011) Regulation of wound-responsive calcium-dependent protein kinase from maize (ZmCPK11) by phosphatidic acid. Acta Biochim Pol 58:589–595
Kravets VS, Kolesnikov YS, Kretynin SV, Getman IA, Romanov GA (2010) Rapid activation of specific phospholipase(s) D by cytokinin in Amaranthus assay system. Physiol Plant 138:249–255. doi:10.1111/j.1399-3054.2009.01324.x
Krinke O, Ruelland E, Valentova O, Vergnolle C, Renou JP, Taconnat L, Flemr M, Burketová L, Zachowski A (2007a) Phosphatidylinositol 4-kinase activation is an early response to salicylic acid in Arabidopsis suspension cells. Plant Physiol 144:1347–1359. doi:10.1104/pp.107.100842
Krinke O, Novotná Z, Valentová O, Martinec J (2007b) Inositol trisphosphate receptor in higher plants: is it real? J Exp Bot 58:361. doi:10.1093/jxb/erl220
Krinke O, Flemr M, Vergnolle C, Collin S, Renou JP, Taconnat L, Yu A, Burketová L, Valentová O, Zachowski A, Ruelland E (2009) Phospholipase D activation is an early component of the salicylic acid signaling pathway in Arabidopsis cell suspensions. Plant Physiol 150:424–436. doi:10.1104/pp.108.133595
Labusch C, Shishova M, Effendi Y, Li M, Wang X, Scherer GFE (2013) Changes in pattern and timing of expression for early auxin-induced genes in phospholipase A mutants imply their involvement in the regulation of auxin responses. Mol Plant (in press)
Lanteri ML, Laxalt AM, Lamattina L (2008) Nitric oxide triggers phosphatidic acid accumulation via phospholipase D during auxin-induced adventitious root formation in cucumber. Plant Physiol 147:188–198. doi:10.1104/pp.107.111815
Lee Y, Choi YB, Suh S, Lee J, Assmann SM, Joe CO, Kelleher JF, Crain RC (1996) Abscisic acid-induced phosphoinositide turnover in guard cell protoplasts of Vicia faba. Plant Physiol 110:987–996. doi:10.1104/pp.110.3.987
Lee SH, Chae HS, Lee TK, Kim SH, Shin SH, Cho BH, Cho SH, Kang BG, Lee WS (1998) Ethylene-mediated phospholipid catabolic pathway in glucose-starved carrot suspension cells. Plant Physiol 116:223–229. doi:10.1104/pp.116.1.223
Lee HY, Bahn SC, Kang YM, Lee KH, Kim HJ, Noh EK, Palta JP, Shin JS, Ryu SB (2003) Secretory low molecular weight phospholipase A2 plays important roles in cell elongation and shoot gravitropism in Arabidopsis. Plant Cell 15:1990–2002. doi:10.1105/tpc.014423
Lee OR, Kim SJ, Kim HJ, Hong JK, Ryu SB, Lee SH, Ganguly A, Cho HT (2010) Phospholipase A(2) is required for PIN-FORMED protein trafficking to the plasma membrane in the Arabidopsis root. Plant Cell 22:1812–1825. doi:10.1105/tpc.110.074211
Lemtiri-Chlieh F, MacRobbie EAC, Webb AAR, Manison NF, Brownlee C, Skepper JN, Chen J, Prestwich GD, Brearley CA (2003) Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc Natl Acad Sci USA 100:10091–10095. doi:10.1073/pnas.1133289100
Leung J, Bouvierdurand M, Morris PC, Guerrier D, Chefdor F, Giraudat J (1994) Arabidopsis Aba response gene Abi1—features of a calcium-modulated protein phosphatase. Science 264:1448–1452. doi:10.1126/science.7910981
Li G, Xue HW (2007) Arabidopsis PLDzeta2 regulates vesicle trafficking and is required for auxin response. Plant Cell 19:281–295. doi:10.1105/tpc.106.041426
Li G, Lin F, Xue HW (2007) Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLD beta 1 in seed germination. Cell Res 17:881–894. doi:10.1038/cr.2007.77
Li J, Henty-Ridilla JL, Huang S, Wang X, Blanchoin L, Staiger CJ (2012) Capping protein modulates the dynamic behavior of actin filaments in response to phosphatidic acid in Arabidopsis. Plant Cell 24:3742–3754. doi:10.1105/tpc.112.103945
Lin WH, Ye R, Ma H, Xu ZH, Xue HW (2004) DNA chip-based expression profile analysis indicates involvement of the phosphatidylinositol signaling pathway in multiple plant responses to hormone and abiotic treatments. Cell Res 14:34–45. doi:10.1038/sj.cr.7290200
Lou Y, Ma H, Lin WH, Chu ZQ, Mueller-Roeber B, Xu ZH, Xue HW (2006) The highly charged region of plant beta-type phosphatidylinositol 4-kinase is involved in membrane targeting and phospholipid binding. Plant Mol Biol 60:729–746. doi:10.1007/s11103-005-5548-x
Ma X, Shor O, Diminshtein S, Yu L, Im YJ, Perera I, Lomax A, Boss WF, Moran N (2009) Phosphatidylinositol (4,5)bisphosphate inhibits K+-efflux channel activity in NT1 tobacco cultured cells. Plant Physiol 149:1127–1140. doi:10.1104/pp.108.129007
Madrid E, Corchete P (2010) Silymarin secretion and its elicitation by methyl jasmonate in cell cultures of Silybum marianum is mediated by phospholipase D-phosphatidic acid. J Exp Bot 61:747–754. doi:10.1093/jxb/erp339
Marcotte WR, Russell SH, Quatrano RS (1989) Abscisic acid-responsive sequences from the Em gene of wheat. Plant Cell 1:969. doi:10.2307/3868997
McLoughlin F, Galvan-Ampudia CS, Julkowska MM, Caarls L, van der Does D, Laurière C, Munnik T, Haring MA, Testerink C (2012) The Snf1-related protein kinases SnRK2.4 and SnRK2.10 are involved in maintenance of root system architecture during salt stress. Plant J 72:436–449. doi:10.1111/j.1365-313X.2012.05089.x
Mishra G, Zhang W, Deng F, Zhao J, Wang X (2006) A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312:264–266. doi:10.1126/science.1123769
Misra S, Wu Y, Venkataraman G, Sopory SK, Tuteja N (2007) Heterotrimeric G-protein complex and G-protein-coupled receptor from a legume (Pisum sativum): role in salinity and heat stress and cross-talk with phospholipase C. Plant J 51:656–669. doi:10.1111/j.1365-313X.2007.03169.x
Mosblech A, König S, Stenzel I, Grzeganek P, Feussner I, Heilmann I (2008) Phosphoinositide and inositolpolyphosphate signalling in defense responses of Arabidopsis thaliana challenged by mechanical wounding. Mol Plant 1:249–261. doi:10.1093/mp/ssm028
Munnik T, Arisz SA, De Vrije T, Musgrave A (1995) G protein activation stimulates phospholipase D signaling in plants. Plant Cell 7:2197–2210. doi:10.1105/tpc.7.12.2197
Nanmori T, Taguchi W, Kinugasa M, Oji Y, Sahara S, Fukami Y, Kikkawa U (1994) Purification and characterization of protein kinase C from a higher plant, Brassica campestris L. Biochem Biophys Res Commun 203:311–318. doi:10.1006/bbrc.1994.2183
Novotná Z, Linek J, Hynek R, Martinec J, Potocký M, Valentová O (2003) Plant PIP2-dependent phospholipase D activity is regulated by phosphorylation. FEBS Lett 554:50–54. doi:10.1016/S0014-5793(03)01093-7
Park KY, Jung JY, Park J, Hwang JU, Kim YW, Hwang I, Lee Y (2003) A role for phosphatidylinositol 3-phosphate in abscisic acid-induced reactive oxygen species generation in guard cells. Plant Physiol 132:92–98. doi:10.1104/pp.102.016964
Perera IY, Hung CY, Moore CD, Stevenson-Paulik J, Boss WF (2008) Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase exhibit increased drought tolerance and altered abscisic acid signaling. Plant Cell 20:2876–2893. doi:10.1105/tpc.108.061374
Pleskot R, Potocký M, Pejchar P, Linek J, Bezvoda R, Martinec J, Valentová O, Novotná Z, Zárský V (2010) Mutual regulation of plant phospholipase D and the actin cytoskeleton. PLant J 62:494–507. doi:10.1111/j.1365-313X.2010.04168.x
Pleskot R, Pejchar P, Bezvoda R, Lichtscheidl IK, Wolters-Arts M, Marc J, Zárský V, Potocký M (2012) Turnover of phosphatidic acid through distinct signaling pathways affects multiple aspects of pollen tube growth in tobacco. Front Plant Sci 3:54. doi:10.3389/fpls.2012.00054
Pokotylo I, Pejchar P, Potocký M, Kocourková D, Krčková Z, Ruelland E, Kravets V, Martinec J (2013) The plant non-specific phospholipase C gene family. Novel competitors in lipid signalling. Prog Lipid Res 52:62–79. doi:10.1016/j.plipres.2012.09.001
Pribat A, Sormani R, Rousseau-Gueutin M, Julkowska MM, Testerink C, Joubès J, Castroviejo M, Laguerre M, Meyer C, Germain V, Rothan C (2012) A novel class of PTEN protein in Arabidopsis displays unusual phosphoinositide phosphatase activity and efficiently binds phosphatidic acid. Biochem J 441:161–171. doi:10.1042/BJ20110776
Profotová B, Burketová L, Novotná Z, Martinec J, Valentová O (2006) Involvement of phospholipases C and D in early response to SAR and ISR inducers in Brassica napus plants. Plant Physiol Biochem 44:143–151. doi:10.1016/j.plaphy.2006.02.003
Raboy V (2009) Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci 177:281–296. doi:10.1016/j.plantsci.2009.06.012
Reitz MU, Bissue JK, Zocher K, Attard A, Hückelhoven R, Becker K, Imani J, Eichmann R, Schäfer P (2012) The subcellular localization of Tubby-like proteins and participation in stress signaling and root colonization by the mutualist Piriformospora indica. Plant Physiol 160:349–364. doi:10.1104/pp.112.201319
Rietz S, Dermendjiev G, Oppermann E, Tafesse FG, Effendi Y, Holk A, Parker JE, Teige M, Scherer GF (2010) Roles of Arabidopsis patatin-related phospholipases a in root development are related to auxin responses and phosphate deficiency. Mol Plant 3:524–538. doi:10.1093/mp/ssp109
Ritchie S, Gilroy S (2000) Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane. Plant Physiol 124:693–702. doi:10.1104/pp.124.2.693
Robles P, Fleury D, Candela H, Cnops G, Alonso-Peral MM, Anami S, Falcone A, Caldana C, Willmitzer L, Ponce MR, Van Lijsebettens M, Micol JL (2010) The RON1/FRY1/SAL1 gene is required for leaf morphogenesis and venation patterning in Arabidopsis. Plant Physiol 152:1357–1372. doi:10.1104/pp.109.149369
Romanov GA, Kieber JJ, Schmülling T (2002) A rapid cytokinin response assay in Arabidopsis indicates a role for phospholipase D in cytokinin signalling. FEBS Lett 515:39–43. doi:10.1016/S0014-5793(02)02415-8
Ryu SB (2004) Phospholipid-derived signaling mediated by phospholipase A in plants. Trends Plant Sci 9:229–235. doi:10.1016/j.tplants.2004.03.004
Sanchez JP, Chua NH (2001) Arabidopsis PLC1 is required for secondary responses to abscisic acid signals. Plant Cell 13:1143–1154. doi:10.1105/tpc.13.5.1143
Sánchez-Sampedro MA, Fernández-Tárrago J, Corchete P (2009) Elicitation of silymarin in cell cultures of Silybum marianum: effect of subculture and repeated addition of methyl jasmonate. Biotechnol Lett 31:1633–1637. doi:10.1007/s10529-009-0043-0
Scherer GF, André B (1989) A rapid response to a plant hormone: auxin stimulates phospholipase A2 in vivo and in vitro. Biochem Biophys Res Commun 163:111–117. doi:10.1016/0006-291X(89)92106-2
Scherer GFE, Zahn M, Callis J, Jones AM (2007) A role for phospholipase A in auxin-regulated gene expression. FEBS Lett 581:4205–4211. doi:10.1016/j.febslet.2007.07.059
Scherer GFE, Ryu SB, Wang X, Matos AR, Heitz T (2010) Patatin-related phospholipase A: nomenclature, subfamilies and functions in plants. Trends Plant Sci 15:693–700. doi:10.1016/j.tplants.2010.09.005
Scherer GFE, Labusch C, Effendi Y (2012) Phospholipases and the network of auxin signal transduction with ABP1 and TIR1 as two receptors: a comprehensive and provocative model. Front Plant Sci. doi:10.3389/fpls.2012.00056
Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, He SY, Rizo J, Howe GA, Zheng N (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468:400–405. doi:10.1038/nature09430
Taiz L, Zeiger E (2010) Plant physiology, 5th edition. Sinauer Associates Inc, Sunderland
Takemiya A, Shimazaki K (2010) Phosphatidic acid inhibits blue light-induced stomatal opening via inhibition of protein phosphatase 1. Plant Physiol 153:1555–1562. doi:10.1104/pp.110.155689
Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446:640–645. doi:10.1038/nature05731
Testerink C, Larsen PB, Van der Does D, van Himbergen JA, Munnik T (2007) Phosphatidic acid binds to and inhibits the activity of Arabidopsis CTR1. J Exp Bot 58:3905–3914. doi:10.1093/jxb/erm243
Tian M, Von Dahl CC, Liu PP, Friso G, van Wijk KJ, Klessig DF (2012) The combined use of photoaffinity labeling and surface plasmon resonance-based technology identifies multiple salicylic acid-binding proteins. Plant J 72:1027–1038. doi:10.1111/tpj.12016
Tuteja N, Sopory SK (2008) Plant signaling in stress. Plant Signal Behav 3:79–86. doi:10.4161/psb.3.2.5303
Uraji M, Katagiri T, Okuma E, Ye W, Hossain MA, Masuda C, Miura A, Nakamura Y, Mori IC, Shinozaki K, Murata Y (2012) Cooperative function of PLD delta and PLD alpha 1 in abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 159:450–460. doi:10.1104/pp.112.195578
Villasuso AL, Molas ML, Racagni G, Abdala G, Machado-Domenech E (2003) Gibberellin signal in barley aleurone: early activation of PLC by G protein mediates amylase secretion. Plant Growth Regul 41:197–205. doi:10.1023/B:GROW.0000007505.37356.89
Wang X, Devaiah SP, Zhang W, Welti R (2006) Signaling functions of phosphatidic acid. Prog Lipid Res 45:250–278. doi:10.1016/j.plipres.2006.01.005
Wang Y, Lin WH, Chen X, Xue HW (2009) The role of Arabidopsis 5PTase13 in root gravitropism through modulation of vesicle trafficking. Cell Res 19:1191–1204. doi:10.1038/cr.2009.105
Wheeler GL, Brownlee C (2008) Ca2+ signalling in plants and green algae—changing channels. Trends Plant Sci 13:506–514. doi:10.1016/j.tplants.2008.06.004
Williams ME, Torabinejad J, Cohick E, Parker K, Drake EJ, Thompson JE, Hortter M, Dewald DB (2005) Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress-response pathway. Plant Physiol 138:686–700. doi:10.1104/pp.105.061317
Wimalasekera R, Pejchar P, Holk A, Martinec J, Scherer GF (2010) Plant phosphatidylcholine-hydrolyzing phospholipases C NPC3 and NPC4 with roles in root development and brassinolide signaling in Arabidopsis thaliana. Mol Plant 3:610–625. doi:10.1093/mp/ssq005
Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Reports 1:639–647. doi:10.1016/j.celrep.2012.05.008
Xiong L, Lee Bh, Ishitani M, Lee H, Zhang C, Zhu JK (2001) FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev 15:1971–1984. doi:10.1101/gad.891901
Yang S, Lu SH, Yuan YJ (2008) Lipidomic analysis reveals differential defense responses of Taxus cuspidata cells to two elicitors, methyl jasmonate and cerium (Ce4+). Biochim Biophys Acta 1781:123–134. doi:10.1016/j.bbalip.2007.11.005
Yu L, Nie J, Cao C, Jin Y, Yan M, Wang F, Liu J, Xiao Y, Liang Y, Zhang W (2010) Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol 188:762–773. doi:10.1111/j.1469-8137.2010.03422.x
Zalejski C, Zhang Z, Quettier A-L, Maldiney R, Bonnet M, Brault M, Demandre C, Miginiac E, Rona JP, Sotta B, Jeannette E (2005) Diacylglycerol pyrophosphate is a second messenger of abscisic acid signaling in Arabidopsis thaliana suspension cells. Plant J 42:145–152. doi:10.1111/j.1365-313X.2005.02373.x
Zalejski C, Paradis S, Maldiney R, Habricot Y, Miginiac E, Rona JP, Jeannette E (2006) Induction of abscisic acid-regulated gene expression by diacylglycerol pyrophosphate involves Ca2+ and anion currents in Arabidopsis suspension cells. Plant Physiol 141:1555–1562. doi:10.1104/pp.106.080218
Zhang W, Qin C, Zhao J, Wang X (2004) Phospholipase D alpha 1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 101:9508–9513. doi:10.1073/pnas.0402112101
Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X (2009) Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21:2357–2377. doi:10.1105/tpc.108.062992
Acknowledgments
The authors acknowledge funding by Czech Science Foundation (grant no. 501/11/1654; OV, MJ, JM), by CNRS and AVCR bilateral convention (MSMT, grant no. 7AMB12FR018; JM, ER, OV, MJ) and the French Agence Nationale de la Recherche (Programme Blanc PANACEANT09_517917; ER). ND is a recipient of a Hubert Curien-Tassili PhD fellowship and MJ is a recipient of a Specific University Research PhD fellowship (MSMT No. 21/2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kumar.
A contribution to the Special Issue: Plant Hormone Signaling.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1. List of genes and corresponding AGI used for Genevestigator analysis.
Supplemental Table 2. Phosphorylated residues of enzymes of glycerophospholipid pathways. The phosphorylated peptides for each enzyme were obtained from the PhosPhAt database. Only sequences for experimentally obtained phosphorylated peptides are shown with phosphorylated residues in red.
Supplementary Fig. 1. Phylogenic tree of proteins with a C1 domain. Proteins were retrieved using the Prosite database (http://prosite.expasy.org/). Sequences of reviewed or unreviewed peptides were obtained with Uniprot (http://www.uniprot.org/). Trees were built using the SeaView program (Gouy, M. Guindon, S. & Gascuel, O. (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27(2):221-224) with the maximum likelihood constraint. Protein names are indicated where known, otherwise gene nomenclature is used.
Supplementary Fig. 2. Phylogenic tree of the proteins with C2, PH, PX, FYVE or HEAT domains. The protocol is identical to the one used for C1 domain containing proteins. For the C2 domain, only reviewed sequences were considered.
Rights and permissions
About this article
Cite this article
Janda, M., Planchais, S., Djafi, N. et al. Phosphoglycerolipids are master players in plant hormone signal transduction. Plant Cell Rep 32, 839–851 (2013). https://doi.org/10.1007/s00299-013-1399-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1399-0