Abstract
The NAC protein family is one of the novel classes of plant-specific transcription factors. In this study, two genes (BnNAC2 and BnNAC5) encoding the putative NAC transcription factors were identified in Brassica napus. Sequence analysis revealed that the deduced BnNAC proteins contain conserved N-terminal region (NAC domain) and highly divergent C-terminal domain. Yeast transactivation analysis showed that BnNAC2 could activate reporter gene expression, suggesting that BnNAC2 functions as a transcriptional activator. Quantitative RT-PCR analysis revealed that BnNAC2 was preferentially expressed in flowers, whereas BnNAC5 mRNAs accumulated at the highest level in stems. Further experimental results indicated that the two genes are high-salinity-, drought- and abscisic acid (ABA)-induced. Overexpression of BnNAC2 and BnNAC5 genes in yeast (Schizosaccharomyces pombe) remarkably inhibited the growth rate of the host cells, and enhanced the cells sensitive to high-salinity and osmotic stresses. Complementation test indicated that BnNAC5 could recover the defects such as salt-hypersensitivity and accelerated-leaf senescence of vni2 T-DNA insertion mutant. Several stress-responsive genes including COR15A and RD29A were enhanced in the complemented plants. These results suggest that BnNAC5 may perform the similar function of VNI2 in response to high-salinity stress and regulation of leaf aging.
Key message BnNAC2 and BnNAC5 are salt-, drought- and ABA-induced genes. Overexpression of BnNAC5 in Arabidopsis vni2 mutant recovered the mutant defects (salt-hypersensitivity and accelerated-leaf senescence) to the phenotype of wild type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought and high-salinity are the major abiotic stresses that affect plant growth and agricultural productivity (Chen et al. 2010). In order to respond the external stresses, a lot of genes are induced in plants, leading to physiological and metabolic changes to increase plant stress-tolerance. The expression of these stress-related genes in plants is mainly regulated by specific transcription factors (such as MYB, bZIP, WRKY, AP2/EREBP and NAC, etc.) which play important roles in eliciting stress responses (Singh et al. 2002; Vinocur and Altman 2005; Yamaguchi-Shinozaki and Shinozaki 2006). Previous study reported that TcWRKY53 was strongly induced by NaCl and drought stresses (Wei et al. 2008). AtMYB44 transgenic plants displayed the enhanced tolerance to drought and salt stresses, compared with wild type (Jung et al. 2008). Overexpression of CBF3 in Arabidopsis improved the transgenic plants freezing and dehydration tolerance (Liu et al. 1998). A study reported that ABF2 interacted with the ABA-responsive elements and was involved in ABA/stress responses, and its overexpression altered the expression of ABA/stress-regulated genes, which affected the whole plant survival rate under water-deficit conditions (Kim et al. 2004).
NAC (for N AM, A TAF1/2 and C UC2) protein family is a plant-specific transcription factor family. NAC protein has highly conserved DNA-binding domain in N-terminal, and highly variable C-terminal domain, which contains the transcriptional activation site (Souer et al. 1996). It has been reported that 105 and 75 NAC genes were identified in Arabidopsis and rice (Oryza sativa) genomes, respectively (Ooka et al. 2003). NAC proteins are involved in regulating a lot of plant development processes, such as flower development (Sablowski and Meyerowitz 1998), leaf senescence (John et al. 1997), embryo development (Duval et al. 2002), lateral root formation and development (Xie et al. 2000; He et al. 2005), secondary wall thickenings (Mitsuda et al. 2005). In addition, NAC proteins also participate in plant responses to abiotic and biotic stresses. Previous study revealed that three NAC genes (ANAC019, ANAC055, and ANAC072) in Arabidopsis were induced by drought, salinity and abscisic acid (ABA). Overexpression of these genes in Arabidopsis up-regulated the expression of the stress-inducible genes, resulting in the improved drought- and salt-tolerance of plants (Tran et al. 2004). Similarly, OsNAC6 is induced by abiotic stresses such as cold, drought, high salinity and jasmonic acid treatment, and overexpression of this gene in rice lead to the increased stress resistance of plants (Nakashima et al. 2007). Recently, a study revealed that an ABA-responsive NAC transcription factor VNI2 integrates ABA-mediated high-salinity stress signals into leaf aging by regulating a subset of COR and RD genes (Yang et al. 2011).
Arabidopsis is considered as a model dicotyledonous plant which genome has been fully sequenced, while rice (Oryza sativa) is a very important food crop and also as a good model for studies of monocotyledonous plants because of its small genome (430 Mb). A lot of abiotic stress-induced genes have been well characterized in the two plant species in the past years (Goff et al. 2002; Sasaki et al. 2002; Feng et al. 2002). On the other hand, a little is known about stress-induced genes in Brassica napus, especially about NAC genes so far. Hegedus et al. (2003) reported that nine BnNAC genes were differentially regulated in response to biotic and abiotic stresses including wounding, insect feeding, Sclerotinia sclerotiorum infection, cold shock and dehydration. Among them, BnNAC1-1, 5-1, 5-7, 5-8 and 5-11 were orthologous to Arabidopsis ATAF1 or ATAF2. Ectopic expression of these genes in Arabidopsis resulted in limited shoot apical meristem formation and cup-shaped or curled cotyledons, similar to Arabidopsis cuc1 and cuc2 mutants (Aida et al. 1997, 1999). BnNAC14 overexpression transgenic plants exhibited the large leaves, thickened stems and hyper-developed lateral root systems, like those observed in AtNAC1 transgenic plants (Xie et al. 2000). In our study, two NAC genes were identified in Brassica napus. The two NAC genes were induced by high-salinity, drought and ABA (abscisic acid). Overexpression of the two NAC genes in yeast (Schizosaccharomyces pombe) inhibited the growth rate of the host cells, and enhanced the cells sensitive to high-salinity and osmotic stresses. Overexpression of BnNAC5 in the T-DNA insertion mutant vni2 (SALK_143793) conferred a wild-type phenotype to the transgenic plants in response to high-salinity and in regulation of leaf aging.
Materials and methods
Plant growth conditions
Seeds of Brassica napus (cv. Zhongyou 821) were surface sterilized and germinated on half-strength Murashige and Skoog (MS) medium (pH 5.8) containing 0.8 % agar under a 16 h light/8 h dark cycle at 25 °C for 7 days. The 7-day-old seedlings were transferred onto MS medium containing 150 mM NaCl, 200 mM mannitol, or 100 μM abscisic acid (ABA) for treatment, respectively.
Quantitative RT-PCR analysis
Total RNA was isolated and purified from different tissues of B. napus according to the protocol of the RNeasy plant mini kit (Qiagen). Expression of the selected genes in roots was analyzed by real-time quantitative reverse transcriptase (RT)-PCR using the fluorescent intercalating dye SYBRGreen with a detection system (Opticon 2, MJ Research, Waltham, USA). A BnACT2 was used as a standard control in RT-PCR reactions. Two-step RT-PCR procedure was performed in all experiments using a method described earlier (Li et al. 2005). In brief, total RNAs, predigested with DNase I for removing any contaminated genomic DNA, were reversely transcribed into cDNAs that were used as templates in PCR reactions. PCR reaction was performed using real-time PCR Master Mix (Toyobo, Osaka, Japan) according to the manufacturer’s instructions, with gene-specific primers, BnNAC2: up-chain primer, 5′-GTCGACGAAACTTCCGAGGAC-3′, down-chain primer, 5′-ACAATCTTCCATGTTCATTTC-3′; BnNAC5 : up-chain primer, 5′-ATATGATAATGACCATATTGA-3′, down-chain primer, 5′-GAGTTATTAAAGATAGTCAAC-3′. The C t (cycle threshold), defined as the PCR cycle at which a statistically significant increase of reporter fluorescence is first detected, is used as a measure for the starting copy numbers of the target gene. Relative quantity of the target gene expression level was performed using the comparative C t method. For the accurate amplification of each specific target gene, we carefully designed the primer sets for each gene based on the sequences corresponding to 3′-untranslated region. To achieve optimal amplification, PCR conditions for each primer combination were optimized for annealing temperature, and PCR products were verified by melting curve analysis and confirmed on an agarose gel.
To assay the expression of several stress-responsive marker genes, total RNA was isolated from 2-week-old whole Arabidopsis seedlings, expression levels of COR15A, COR15B, RD29A, RD29B genes were determined by quantitative RT-PCR, using gene-specific primers: COR15A forward 5′-GCAGATGGTGAGAAAGCGAA-3′ and reverse 5′-GGCATCCTTAGCCTCTCCTG-3′; COR15B forward 5′-ATGACCTCAACGAAGCCACA-3′ and reverse 5′-GTGGCATTCTTAGCCTCTTCT-3′; RD29A forward 5′-TGAAAGGAGGAGGAGGAATGGTTGG-3′ and reverse 5′-ACAAAACACACATAAACATCCAAAGT-3′; RD29B forward 5′-CCAGATAGCGGAGGGGAAAGGACAT-3′ and reverse 5′-AAGTTCACAAACAGAGGCATCATAC-3′. AtACT2 gene was used as a standard control in RT-PCR reactions.
Isolation of BnNAC cDNAs
NAC domain proteins from Arabidopsis thaliana were used as query sequences for tBLASTn searches of the B. napus EST database (http://www.ncbi.nlm.nih.gov/). Vector sequences and low quality sequences were removed manually from the resulting hits. The remaining non-redundant B. napus assembled sequences with the highest similarity to the query sequences were kept as putative NAC domain genes.
From these gene predictions, gene-specific primers were designed for PCR to isolate the cDNA and its genomic DNA. BnNAC2: up-chain primer, 5′-CTTGGATCCATGGACATGAATCCCAACACT-3′, down-chain primer, 5′-GGGGAGCTCCTAAAACTGAAACATACTAGC-3′; BnNAC5: up-chain primer, 5′-GGGGGATCCATGGATAAGGTTAAACTTGTA-3′, down-chain primer, 5′-GGGGAGCTCTTAAAGATTTCGTCTGAAACT-3′. The PCR fragments were sequenced by Shanghai Sunny Biotechnology Co, Ltd.
DNA and protein sequence analysis
Sequences of the isolated B. napus BnNAC genes (cDNAs) and their deduced proteins were analyzed using DNAstar software (DNAstar Inc., Madison, WI, USA). Protein sequence homology analysis was performed with ClustalW (http://www.ebi.ac.uk/clustalw/), and phylogenetic analysis was employed to investigate the evolutionary relationships among BnNAC proteins. A Neighbor-Joining tree was generated in MEGA3.1. A bootstrap analysis with 1,000 replicates was performed to assess the statistical reliability of the tree topology.
Isolation of BnNAC2 promoter and construction of BnNAC2 promoter: GUS reporter vector
BnNAC2 5′-flanking sequence was isolated from B. napus using Genome Walking Kit (TaKaRa, Dalian, China). A 1,672 bp 5′-flanking fragment (including putative promoter and 5′-untranslated region) of BnNAC2 gene was amplified by PCR, using primer pair: up-chain primer, 5′-CTTGTCGACTTAAATAAATCCGCCGTGGCG-3′, down-chain primer, 5′-CTTGGATCCGGTTCTGGGGTTGGTTTAGTG-3′. A Sal I site and a BamH I site were introduced at 5′-terminal and 3′-terminal of BnNAC2 promoter, respectively. BnNAC2 promoter fragment was subcloned into Sal I/BamH I sites of pBI101 vector to generate the chimeric BnNAC2:GUS construct.
Arabidopsis transformation and histochemical assay of GUS activity
Arabidopsis transformation was performed by the floral-dip method. Seeds were harvested and stored at 4 °C. Arabidopsis seeds were sterilized in 75 % (v/v) ethanol for 1 min and then in 10 % NaClO solution for 6 min, followed by washing with sterile water. The seeds germinated on MS medium with 50 mg/L kanamycin for selecting transformants. Transgenic seedlings harboring BnNAC2p:GUS were stained in 5-bromo-4-chloro-3-indolylglucuronide (X-gluc) solution overnight according to the method described earlier (Li et al. 2002). The stained plant materials were then cleared and fixed by rinsing with 70 % (v/v) ethanol, and the samples were examined and photographed under a Leica MZ16f stereomicroscope (Leica, Germany).
Transcription activation assay in yeast
To investigate the transcriptional activity of BnNAC proteins, the coding sequences of BnNAC2 and BnNAC5 cDNAs were amplified using a forward primer containing an EcoR I site (BnNAC2: 5′-GGGGAATTCATGGACATGAATCCCAACACT-3′, BnNAC5: 5′-GGGGAATTCATGGATAAGGTTAAACTTGTA-3′) and a reverse primer containing a Sac I site (BnNAC2: 5′-GGGGAGCTCCTAAAACTGAAACATACTAGC-3′, BnNAC5: 5′-GGGGGATCCATGGATAAGGTTAAACTTGTA-3′), and cloned into pGBKT7 vector (Clontech, Palo Alto, CA, USA) to generate reporter plasmids, namely, pBD-Bn-NAC2 and pBD-Bn-NAC5. The two reporter plasmids were then individually transformed into yeast strain AH109 containing HIS3 and LacZ reporter genes. Yeast transformants were streaked on selective medium lacking tryptophan and adenine to assay transcriptional activity. β-Galactosidase activity was also assayed by colony-lift filter assay, using 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-gal) as substrate (Zhang et al. 2010).
Overexpression of BnNAC genes in fission yeast
The coding sequences of BnNAC2 and BnNAC5 genes, amplified from their cDNAs by PCR with Pfu DNA polymerase, were cloned into yeast vector pREP5N with Sal I/Not I sites, respectively. Afterwards, the constructed vectors and pREP5N empty vector were transferred into fission yeast (Schizosaccharomyces pombe) cells by electroporation (Bio-Rad MicroPulser Electroporation Apparatus, Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instruction. Transformants were then selected on minimal medium (MM) supplemented with 75 mg L−1 adenine and 2 μM uracil and 100 mg L−1 ampicillin at 30 °C. Ten colonies of each transformant were randomly picked out to grow in liquid MM with 2 μM thiamine, which represses the nmt-1 promoter activity, until mid-log phase in a shaker (220 rpm, 30 °C). Subsequently, yeast cells were harvested and washed three times with MM without thiamine to de-repress the promoter, and then incubated in the same medium without thiamine (induction medium) for 20 h (220 rpm, 30 °C). About 1 ml of the cultured cells was respectively transferred into 20 ml of fresh thiamine-free MM medium (OD600 = 0.1) for further incubation. When the culture grew to stationary phase (OD600 reached 2.0), 5 μl of the culture was spotted onto thiamine-free MM solid medium with 250 mM NaCl and 400 mΜ mannitol. After 48 h, cell growth status was observed. Meanwhile, 1 ml of the culture was transferred and cultivated in 20 ml of fresh thiamine-free MM medium with 250 mM NaCl, 400 mΜ mannitol, respectively (OD600 = 0.1). The culture was taken of every 3 h to measure their OD when treated with NaCl and mannitol till 9 h, and then calculated cell growth rate, using empty pREP5N transformants as controls.
Identification of Arabidopsis vni2 mutant and complementation of BnNAC5
The vni2 mutant (SALK_143793) (also called vni2-1, Yang et al. 2011) was obtained from the SALK lines collection. The homozygous vni2 mutant, which was selected by PCR-based genomic identification with gene-specific primers, was confirmed by RT-PCR. The seedlings of vni2 mutant were transferred on MS with 150 mM NaCl for phenotypic confirmation. The coding sequence of BnNAC5 was cloned into the modified pCAMBIA1301 vector with hygromycin resistance and introduced into vni2 mutant. The transformed seeds were selected on MS medium containing 250 mg/L hygromycin.
The vni2/35S:BnNAC5 transgenic, vni2, and wild-type Arabidopsis seeds germinated in MS medium for 7 days and then were transferred onto MS with 150 mM NaCl and cultured for 3 days. Subsequently, green leaf rate and chlorophyll content in leaves of vni2/35S:BnNAC5 transgenic, vni2 mutant, and wild-type plants were measured, respectively. In brief, chlorophylls in leaves were extracted with 80 % acetone, and the extracted solution was incubated at 4 °C for 2 h in complete darkness. Chlorophyll content was assayed by measuring absorbance at 645, 652, and 663 nm using a spectrophotometer. To observe the phenotype of adult Arabidopsis plants, the vin2/35S:BnNAC5 transgenic, vni2 mutant, and wild-type seedlings were transplanted in soil for growth to maturation.
Results
Isolation and characterization of BnNAC2 and BnNAC5 genes
In order to identify novel members of NAC family in Brassica napus, we use Arabidopsis NAC proteins as query probes to search the published B. napus EST database (http://www.arabidopsis.org/), we obtained six full-length cDNA sequences encoding B. napus NAC-like proteins, and consequently designated as BnNAC1 to BnNAC6. Among them, BnNAC2 and BnNAC5 (Accession Numbers in GenBank: JF957836 and JF957837) were selected for further study. We amplified the complete sequences of BnNAC2 and BnNAC5 cDNAs from total RNAs of Brassica napus by RT-PCR, using gene-specific primers. The two cDNAs encodes novel NAC-like proteins in B. napus. Subsequently, we isolated BnNAC2 and BnNAC5 genes from B. napus genome by PCR. By comparing the gene and its cDNA sequences, it was revealed that both BnNAC genes contain three extrons and two introns (Fig. 1). The first two exons encodes N-terminal region and the last one encodes C-terminal domain. BnNAC2 encodes a NAC-like protein with 271 amino acids, and BnNAC5 deduces a NAC domain polypeptide of 250 amino acids. As shown in Fig. 2, high sequence similarities between BnNAC2/5 and the other plant NAC proteins are found in N-terminus, which contained several distinguishable blocks of heterogeneous amino acids or gaps and are divided into five subdomains. On the other hand, C-terminus of BnNAC2/5 proteins, serving as transcription activation domain, showed relatively high diversity from the other plant NAC proteins.
Structure of BnNAC2 and BnNAC5 genes. Exons are denoted by black boxes. Introns, 5′-flanking region and 3′-untranslated region are denoted by lines. The lengths of the introns in base pairs are indicated. The number at the boundaries of each exon indicates the codon at which the intron is located. The translation initiation and termination codons are shown. aa amino acids
Sequence alignment of BnNAC2 and BnNAC5 proteins with other NAC domain proteins. BnNAC2 and BnNAC5 are identified in this work, and the other NAC proteins, including Arabidopsis thaliana AtNAM (AF123311), ATAF1 (NP_171677), ATAF2 (NP_680161), CUC1 (AB049069), CUC2 (AB002560), AtNAC2 (At3g15510), and VNI2/ANAC83 (At5g13180) are selected from GenBank. Residues that are identical in all (white on black background) or most (black on gray background) proteins are highlighted. The five subdomains of NAC domain are denoted by lines and with a, b, c, d, and e
Phylogenetic analysis of BnNAC2 and BnNAC5 proteins
To investigate divergence of BnNAC2/5 proteins with other NAC proteins during evolution, we analyzed phylogenetic relationship of BnNAC2/5 proteins with eight Arabidopsis NAC proteins, one tomato NAC protein (SENU5) and five B. napus NAC proteins by an MEG3.1 program. As shown in Fig. 3, 16 NACs available could be divided into three subgroups in the tree. BnNAC2 and BnNAC5 locate on the first subgroup of the tree. BnNAC2 occupies a distinct branch that is basal to the NAP and AtNAC2 clade, indicating that divergence in these three proteins occurred relatively late. Similarly, BnNAC5 and ANAC83 (VNI2) form another branch, and SENU5 is basal to this clade, suggesting BnNAC5 are more closely related to ANAC83 (VNI2) and SENU5 than to the others.
Phylogenetic relationship of BnNAC2 and BnNAC5 proteins with the other NAC proteins in plants. The minimum evolution tree was constructed in MEGA3.1 from 1,000 bootstrap replicates. BnNAC2 and BnNAC5 in this work; SENU5 from tomato; BnNAC1-1, BnNAC3, BnNAC5-1, BnNAC14, BnNAC18 from Brassica napus, the rest proteins from Arabidopsis thaliana. The accession numbers of the known NAC proteins in GenBank are as follows: AtNAM (AF123311), ATAF1 (NP_171677), ATAF2 (NP_680161), NAP (AJ222713), CUC1 (AB049069), CUC2 (AB002560), AtNAC2 (At3g15510), VNI2/ANAC83 (At5g13180), BnNAC1-1 (AY245879), BnNAC3 (AY245880), BnNAC5-1 (AY245881), BnNAC14 (AY245885), BnNAC18 (AY245886), SENU5 (Z75524)
Transcriptional activity of BnNAC2 and BnNAC5 proteins
The NAC transcription factors contain conserved N-terminal DNA-binding domain and variable C-terminal transcriptional regulation region (TRR), which can act as either a repressor or activator in regulatory pathways (Tran et al. 2004). In order to determine whether BnNAC2 and BnNAC5 proteins have transcription activation activity, yeast one-hybrid system was employed. The cDNA fragments containing the C-terminal domains of BnNAC2/5 were cloned into pGBKT7 which contains the GAL4-binding domain. To assay their ability to activate transcription of HIS3 and ADE2 reporter genes from GAL4 upstream activation sequence, we transformed AH109 yeast strain with the fusion plasmids pBD-BnNAC2 and pBD-BnNAC5. All the transformed yeast cells grew well in SD medium lacking tryptophan (−Trp) (Fig. 4a). In contrast, pBD-BnNAC2 transformant yeast cells were able to grow well on selection medium lacking tryptophan and adenine (−Trp and −Ade), whereas cells containing pBD-BnNAC5 and pBD did not grow on the same medium (Fig. 4b). Yeast cells with expressing pBD-BnNAC2 on SD medium without histidine and adenine turned blue at the presence of 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-Gal), indicating that the reporter gene LacZ was activated. However, cells with expressing pBD-BnNAC5 did not show any blue color (Fig. 4c). These results suggest that BnNAC2 protein functions as a transcriptional activator, while BnNAC5 lacks the activity of transcriptional activation.
Analysis of transactivation activity of BnNAC2 and BnNAC5 proteins in yeast. Fusion proteins of pBD-BnNAC2, pBD-BnNAC5 and pBD were expressed in yeast strain AH109. The transformants were streaked on the SD/−Trp−Ade medium. The plates were incubated for 2 days and subjected to an X-gal assay. a Yeast transformants were streaked on SD/−Trp medium (SD minimal medium lacking Trp). b Yeast transformants were streaked on SD/−Trp−Ade medium (SD minimal medium lacking Trp and Ade). c Flash-freezing filter assay of the β-galactosidase activity
Expression patterns of BnNAC2 and BnNAC5 genes in Brassica napus
To investigate the expression patterns of BnNAC2/5 genes in different tissues of B. napus, quantitative RT-PCR analysis was performed. The results showed that BnNAC2 mRNAs were predominantly accumulated in flowers, whereas the moderate to weak signals of its expression were detected in other tissues such as roots, cotyledons, stems, hypocotyls and leaves. BnNAC5 transcripts were accumulated at the highest level in stems, at moderate levels in cotyledons and leaves, but at low levels in other tissues (Fig. 5).
Quantitative RT-PCR analysis of expression of BnNAC2 and BnNAC5 genes in B. napus tissues. Total RNAs were isolated from roots (1), hypocotyls (2), cotyledons (3), stems (4), leaves (5), and flowers (6) of B. napus respectively. Relative value of the expression of the identified genes in B. napus tissues was shown as percentage of BnACT2 expression activity. Mean values and standard errors (bar) were shown from three independent experiments
To determine the precise expression profiling of the BnNAC2 gene in B. napus, the 1,672 bp BnNAC2 5′-flanking region (the putative promoter fragment and 5′-untranslated region) before the translational initiation codon (ATG) was cloned upstream GUS reporter gene in pBI101 vector, giving rise to BnNAC2p:GUS chimeric gene. BnNAC2p:GUS construct was introduced into Arabidopsis by Agrobacterium tumefaciens-mediated transformation. Expression of GUS gene controlled under BnNAC2 promoter in transgenic Arabidopsis plants was examined by histochemical assay (Fig. 6), using non-transformed plants as negative control. In the early seedling development, no GUS expression was detected in the 3-day-old seedlings (Fig. 6a). As seedlings further developed, strong GUS activity was observed clearly in the root tip regions and shoot meristems, but no or weak GUS staining was found in the other tissues of the 7-day-old transgenic seedlings (Fig. 6b). High level of GUS expression was also detected in the veins of rosette leaves of the 15-day-old seedlings (Fig. 6c). As leaves further developed, however, GUS activity was gradually declined to undetectable level in the senescent leaves (Fig. 6d). When plants grew to maturation, strong GUS staining was found only in the axils of bracts (Fig. 6e), sepal (Fig. 6f) and abscission zone of mature siliques (Fig. 6f, g). These results are consistent with the RT-PCR data, suggesting that BnNAC2 may mainly function in early seedling development and flower development of B. napus.
Histochemical assay of expression of GUS gene driven by BnNAC2 promoter in transgenic Arabidopsis plants. GUS staining was not detected in the 3-day-old seedling (a), but strong GUS activity was observed clearly in the root tip regions and the shoot meristems of 7-day-old seedling (b) and in the veins of rosette leave of a 15-day-old plant (c). No GUS activity was detected in the senescent leaf (d), whereas relatively high levels of GUS expression were found in the axils of bracts (e), sepals (f) and the abscission zone of mature siliques (f, g). Bar 5 mm
Expression of BnNAC2 and BnNAC5 genes are up-regulated by NaCl, mannitol and abscisic acid (ABA)
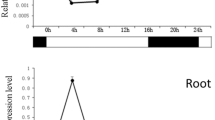
To investigate whether the two BnNAC genes are induced by high-salinity, drought and abscisic acid (ABA), quantitative RT-PCR analysis was performed using gene-specific primers. The expression of both BnNAC genes was induced by 150 mM NaCl treatment. BnNAC2 expression was up-regulated at the highest level at 3 h after NaCl treatment, whereas BnNAC5 reached its peak value at 12 h after NaCl treatment. Similarly, BnNAC2/5 expression was also up-regulated by 200 mM mannitol treatment. BnNAC2 activity reached to its peak value at late stage (12 h), whereas BnNAC5 mRNAs were accumulated at the highest level at early stage (1 h) of the osmotic stress. Furthermore, the expression of the two genes was also induced by ABA. The transcripts of both genes were accumulated at the highest levels at 6 h after ABA treatment (Fig. 7). These results suggested that the BnNAC2 and BnNAC5 genes may be involved in B. napus response to salt and osmotic stresses and ABA signaling.
Quantitative RT-PCR analysis of expression of BnNAC2 and BnNAC5 genes under NaCl, mannitol and abscisic acid (ABA) treatments. Total RNAs were isolated from 7-day-old roots treated with 150 mM NaCl, 200 mM mannitol, or 100 μM ABA for 1, 3, 6, 12 h respectively. ck, untreated roots (control). Relative value of the expression of the identified genes in B. napus roots was shown as percentage of BnACT2 expression activity. Mean values and standard errors (bar) were shown from three independent experiments
Overexpression of BnNAC2 and BnNAC5 in yeast enhances cell sensitivity to high-salinity and osmotic stresses
To investigate whether both genes play roles in response to high-salinity and drought stresses, BnNAC2 and BnNAC5 genes were ectopically expressed in fission yeast (Schizosaccharomyces pombe). The coding sequences of BnNAC genes were cloned into pREP5N vector, placing the genes under the control of an nmt-1 promoter, which is activated in the absence of exogenous thiamine. The transformed yeast cell lines with expressing BnNAC2 or BnNAC5 and control lines containing empty vector (pREP5N) were randomly selected to analyze cell growth under 250 mM NaCl and 400 mM mannitol treatments, respectively, using the same cell lines grown in normal conditions (minimal solid medium without supplemental NaCl, mannitol) as controls. As shown in Fig. 8b, when they grew in non-induction medium, the growth rate of BnNAC2/5 transformed cell lines was as same as control lines. However, when they grew in the same thiamine-free medium (induction medium), BnNAC2 and BnNAC5 transformed cells grew slower than that of control lines (Fig. 8a). As treated with NaCl and mannitol, the transformed cells with expressing BnNAC2 or BnNAC5 genes on induction medium were more sensitive than those controls (Fig. 8c, e), whereas they showed similar growth rates on non-induction medium (Fig. 8d, f). Statistical analysis revealed there were significant differences between the transformed cell lines and controls (Fig. 9).
Analysis of growth rate of yeast cells with Overexpressing BnNAC2 and BnNAC5 genes under normal conditions and under NaCl and mannitol treatments. Yeast cells harboring empty pREP5N vector (upper row), constructed pREP5N–BnNAC2 (middle row) and pREP5N–BnNAC5 (bottom row) were cultured on induction medium (a, c, e) or non-induction medium (b, d, f) without or with NaCl and mannitol treatments, respectively. CK, yeast cells were cultured on MM medium without NaCl and mannitol (a, b); NaCl, yeast cells were cultured on minimal medium with 250 mM NaCl (c, d); Mannitol, yeast cells were cultured on minimal medium with 400 mΜ mannitol (e, f). Five microliters of serial decimal dilutions were spotted onto different medium and incubated at 30 °C for 2 days
Statistical analysis of the Cell growth rate of yeast cells by different treated. Cell growth rate of yeast cells, harboring empty plasmid (pREP5N) or constructed vectors (pREP5N–BnNAC2/5) cultured in different conditions. Yeast cells were cultured in minimal medium (a), 250 mM NaCl (c) and 400 μM mannitol (e) under non-induction condition. Yeast cells were cultured in minimal medium (b), 250 mM NaCl (d) and 400 μM mannitol (f) under induction condition. Mean values and standard errors (bar) from three independent experiments. Single asterisk represents significant difference between transformed cell lines and controls (P < 0.05) in t test. Double asterisks represent very significant difference between transformed cell lines and controls (P < 0.01) in t test
Phenotypic defects of vni2 are rescued by BnNAC5
The vni2 knockout mutant (SALK_143793) (also called vni2-1, Yang et al. 2011) was obtained from the SALK lines collection. The homozygous vni2 mutant was identified by PCR and then confirmed that VNI2 transcripts were undetectable in mutant lines. To evaluate whether BnNAC5 rescues the phenotypic defects of vni2, BnNAC5 gene was introduced in the homozygous vni2 mutant. Over 20 homozygous lines (T2 and T3 generations) of the transgenic vni2/35S:BnNAC5 plants with expressing BnNAC5 were obtained. The expression levels of BnNAC5 gene in these vni2/35S:BnNAC5 transgenic progeny plants were examined by RT-PCR analysis using gene-specific primers (Fig. 10h). As seedlings grew in MS medium, there was no difference among the vni2/35S:BnNAC5 transgenic plants, wild type, and vni2 mutant (Fig. 10a). After 7-day-old seedlings were transferred and cultured on MS medium with 150 mM NaCl for 3 days, however, vni2/35S:BnNAC5 plants displayed a similar phenotype with wild type. In contrast, the vni2 knockout mutant showed a salt-hypersensitivity phenotype (Fig. 10b). We further determined the total chlorophyll content in leaves and green leaf rate of the vni2/35S:BnNAC5 transgenic, vni2 mutant, and wild-type plants under NaCl treatment. Measurement and statistical analysis indicated that the total chlorophyll content and green leaf rate of vni2 mutant plants were declined more rapidly than those of the vni2/35S:BnNAC5 transgenic plants and wild type (Fig. 10c, d). When the transgenic plants further developed to maturation, leaf aging was clearly delayed in vni2/35S:BnNAC5 plants, like wild type, whereas vni2 mutant showed obvious leaf senescent phenotype (Fig. 10e–g). The above data suggested that BnNAC5 may perform a similar function of VNI2 in plant response to high-salinity stress and in regulation of leaf aging.
BnNAC5 rescues the phenotypic defects of vni2 mutant (SALK_143793). a vni2/35S:BnNAC5 transgenic, wild type, and vni2 mutant seedlings were cultured in MS medium. b vni2/35S:BnNAC5 transgenic, wild type, and vni2 mutant seedlings were cultured in MS medium with 150 mM NaCl. c Percentage of green leaves of vni2/35S:BnNAC5 transgenic, wild type, and vni2 mutant seedlings with 150 mM NaCl treatment. d Chlorophyll content in leaves of vni2/35S:BnNAC5 transgenic, wild type, and vni2 mutant seedlings with 150 mM NaCl treatment. e–g Phenotype observation of 8-week-old adult Arabidopsis plants grown in soil under normal conditions (22 °C, 16 h light/8 h dark). e Wild-type plants, f vni2 mutant, g vni2/35S:BnNAC5 transgenic plants. h BnNAC5 expression in the vni2/35S:BnNAC5 transgenic plants was analyzed by RT-PCR, using RNA samples extracted from 2-week-old seedlings grown on MS medium and Arabidopsis ACTIN2 as quantification control. Single asterisk represents significant difference between the vni2/35S:BnNAC transgenic lines and vni2 mutant (P < 0.05) in t test. Double asterisks represent very significant difference between the vni2/35S:BnNAC5 transgenic lines and vni2 mutant (P < 0.01) in t test. WT, wild type; vni2, vni2 mutant; BnNAC5/vni2, the vni2/35S:BnNAC5 transgenic lines
We also analyzed the expression levels of several stress-responsive marker genes, COR15A, COR15B, RD29A and RD29B, in vni/35S:BnNAC5 transgenic plants, wild type, and vni2 mutant. The experimental results indicated that the transcript levels of these genes were significantly enhanced in vni2/35S:BnNAC5 transgenic plants, compared with those in the vni2 mutant and wild type (Fig. 11). These data suggest that BnNAC5 may be correlated to the stress-regulation pathway, or directly regulated downstream stress- response genes, as did VNI2 (Yang et al. 2011).
Quantitative RT-PCR analysis of expression of stress-responsive genes in vni2/35S:BnNAC5 transgenic Arabidopsis plants. Total RNA was isolated from 2-week-old whole seedlings. Expression levels of COR15A, COR15B, RD29A, RD29B genes were determined by quantitative RT-PCR, using ACTIN2 as a quantification control. Single asterisk represents significant difference between the vni2/35S:BnNAC5 transgenic lines and vni2 mutant (P < 0.05) in t test. WT, wild type; vni2, vni2 mutant; BnNAC5/vni2-1, -2 and -3, the vni2/35S:BnNAC5 transgenic lines
Discussion
NAC proteins are plant-specific transcriptional factors, which are involved in regulating plant development, response to biotic and abiotic stresses, and are also related to cell senescence and death (John et al. 1997; Olsen et al. 2005; Balazadeh et al. 2010; Yang et al. 2011). In this study, we isolated two novel genes (BnNAC2 and BnNAC5) encoding NAC proteins in B. napus. Sequence comparison between the genomic DNA and its cDNA revealed that each BnNAC gene consists of three exons and two introns. The first two exons encode the conserved NAC domain, whereas the last exon encodes the highly divergent C-terminal transcriptional activation domain, as did the classical NAC genes. BnNAC2 and BnNAC5 displayed different tissues expression patterns. BnNAC2 transcripts were predominantly accumulated in flowers, while BnNAC5 was mainly expressed in stems, suggesting that the two genes might perform different functions in plant development. In Arabidopsis, AtNAC1 is preferentially expressed in roots and plays an important role in the regulation of lateral root development (Xie et al. 2000). It has been reported that the NAC transcription factors regulate plant response to drought stress through both ABA-dependent and ABA-independent pathways (Tran et al. 2004). AtNAC2 mRNAs are also accumulated at its highest level in root tissues, and overexpression of this gene in Arabidopsis stimulates the development of lateral roots in the transgenic plants (He et al. 2005). A later study revealed that AtNAC2 (also called ANAC092) controls a gene regulatory network during salt-promoted senescence (Balazadeh et al. 2010). Arabidopsis VNI2 gene encoding a NAC transcription factor is induced by high-salinity in an ABA-dependent manner, and its spatial and temporal expression profiling is correlated with leaf aging and senescence (Yang et al. 2011). In Brassica napus, expressions of the nine NAC genes are differently regulated by biotic and abiotic stresses (Hegedus et al. 2003). Similarly, our study indicated that both BnNAC2 and BnNAC5 were induced by high-salinity, drought and ABA. Overexpression of BnNAC5 in vni2 mutant could rescue plant salt-hypersensitive phenotype, suggesting that BnNAC5 may play a VNI2-similar role in response to abiotic stress.
To further characterize the isolated BnNACs, the transcriptional activities of BnNAC2 and BnNAC5 proteins were investigated, using a yeast system. Our results showed BnNAC2 protein functions as a transcriptional activator, whereas BnNAC5 have no such an activity. In order to illuminate this result, we isolated VNI2 (ANAC083), which shared the homology of 91 % in amino acid levels with BnNAC5. By comparing the amino acid sequences of both proteins, we found that there are several amino acid difference in C-terminal between BnNAC5 and VNI2. This amino acid divergence may affect the transcriptional activity. Our experimental results showed that VNI2 protein did not show the transcriptional activity (data not shown), similar to BnNAC5. However, the transcriptional activity of BnNAC5 may be enhanced in response to high-salinity stress and in regulation of leaf aging. In NAC family, some members function as transcription activators, while others act as repressors. For example, rice NAC6 is a transcription activator (Nakashima et al. 2007), while CBNAC, a CaM binding NAC protein, has been found to be a transcriptional repressor in Arabidopsis (Kim et al. 2007). Recently, a study reported that NARD (NAC Repression Domain, a highly conserved sequence in NAC) not only represses the transactivation ability of NAC proteins, but also influence that of transcription factors from different families, including Dof, WRKY and AP2/DRE. The transcriptional activation ability of the proteins may depend on the interaction between the NARD and activation domain (Hao et al. 2010). Thus, we supposed that the role of NARD might be less than that of activation domain in BnNAC2, leading to this protein as a transcriptional activator. In contrast, the NARD function might be stronger than that of activation domain in BnNAC5, and consequently, this protein would appear like a transcriptional repressor, but it needs further evidence to sustain the hypothesis.
NAC genes play important roles in plant growth, development, and hormone signaling (Olsen et al. 2005). They are also involved in plant stress response. ANAC019, ANAC055, and RD26/ANAC072 in Arabidopsis thaliana are induced by drought, high salinity and/or abscisic acid (ABA), and overexpression of these genes up-regulates the expression of several stress-related genes, resulting in the enhancement of plant tolerance to drought stress (Tran et al. 2004). To investigate whether BnNAC proteins are involved in high-salinity and osmotic stresses, we chose fission yeast (S. pombe) cell, a relative simple system for study. The results showed that overexpression of BnNAC2/5 in yeast resulted in cells more sensitive to high-salinity and osmotic stresses than those controls. Furthermore, an Arabidopsis T-DNA insertion mutant vni2 (also called vni2-1) was employed for investigating the role of BnNAC5. Complementation test indicated that BnNAC5 could recover the phenotypic defects of vni2 mutant, suggesting that BnNAC5 is involved in response to high-salinity stress in a similar manner of VNI2. It has been demonstrated that AtNAC2 regulates salt-promoted senescence (Balazadeh et al. 2010), VNI2 serves as a molecular link to integrate plant response to environmental stresses into modulation of leaf longevity (Yang et al. 2011), and ANAC083 is involved in the control of senescence processes (John et al. 1997). Likewise, our results showed that growth of yeast cells was remarkably inhibited by overexpression of BnNAC2/5 genes under normal conditions and high-salinity and osmotic treatments, implying that overexpression of BnNAC genes may promote yeast cell senescence. In addition, BnNAC5 could recover the salt-hypersensitivity and accelerated-leaf senescence of vni2 mutant to the wild-type phenotype. The vni2/35S:BnNAC5 transgenic plants showed significantly higher leaf chlorophyll content and green leaf rate than those of vni2 mutant under NaCl treatment, and displayed a phenotype of the delayed leaf senescence, compared with that of vni2 mutant. These results suggest that BnNAC5 may perform the similar function of VNI2 in regulating leaf longevity against high-salinity stress. Thus, our data will facilitate the understanding of the roles of B. napus NAC genes in response to high-salinity stress and in regulation leaf aging.
References
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9:841–857
Aida M, Ishida T, Tasaka M (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126:1563–1570
Balazadeh S, Siddiqui H, Allu AD, Matallana-Ramirez LP, Caldana C, Mehrnia M, Zanor MI, Köhler B, Mueller-Roeber B (2010) A gene regulatory network controlled by the NAC transcription factor NAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J 62:250–264
Chen L, Ren F, Zhong H, Jiang WM, Li XB (2010) Identification and expression analysis of genes induced by high-salinity and drought stresses in Brassica napus. Acta Biochim Biophys Sin 42:154–164
Duval M, Hsieh T, Kim S, Thomas T (2002) Molecular characterization of AtNAM: a member of the Arabidopsis NAC domain superfamily. Plant Mol Biol 50:237–248
Feng Q, Zhang YJ, Hao P, Wang SY, Fu G, Huang YC et al (2002) Sequence and analysis of rice chromosome 4. Nature 420:316–320
Goff SA, Ricke D, Lan TH, Presting G et al (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296:92–100
Hao YJ, Song QX, Chen HW, Zou HF, Wei W, Kang XS, Ma B, Zhang WK, Zhang JS, Chen SY (2010) Plant NAC-type transcription factor proteins contain a NARD domain for repression of transcriptional activation. Planta 232:1033–1043
He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY (2005) AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J 44:903–916
Hegedus D, Yu M, Baldwin D, Gruber M, Sharpe A, Parkin I, Whitwill S, Lydiate D (2003) Molecular characterization of Brassica napus NAC domain transcriptional activators induced in response to biotic and abiotic stress. Plant Mol Biol 53:383–397
John I, Hackett R, Cooper W, Drake R, Farrell A, Grierson D (1997) Cloning and characterization of tomato leaf senescence-related cDNAs. Plant Mol Biol 33:641–651
Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ (2008) Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 146:623–635
Kim S, Kang JY, Cho DI, Park JK, Kim SY (2004) ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J 40:75–87
Kim HS, Park OB, Yoo HJ, Jung MS, Lee MS, Han JH, Kim EK, Kim HS, Lim OC, Yun D-J, Chung SW (2007) Identification of a calmodulin-binding NAC protein (CBNAC) as a transcriptional repressor in Arabidopsis. J Biol Chem 282:36292–36302
Li XB, Cai L, Cheng NH, Liu JW (2002) Molecular characterization of the cotton GhTUB1 gene that preferentially expressed in fiber. Plant Physiol 130:666–674
Li XB, Fan XP, Wang XL, Cai L, Yang WC (2005) The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation. Plant Cell 17:859–875
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Shinozaki KY, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Mitsuda N, Seki M, Shinozaki K, Takagi MO (2005) The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17:2993–3006
Nakashima K, Tran LSP, Nguyen DV, Fujita M, Maruyama K, Todaka D, Ito Y, Hayashi N, Shinozaki K, Shinozaki KY (2007) Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J 51:617–630
Olsen OE, Myklebust G, Engebretsen L, Holme I, Bahr R (2005) Exercises to prevent lower limb injuries in youth sports: cluster randomized controlled trial. Br Med J 330:449–452
Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K, Matsubara K, Osato N, Kawai J, Carninci P, Hayashizaki Y, Suzuki K, Kojima K, Takahara Y, Yamamoto K, Kikuchi S (2003) Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res 10:239–247
Sablowski RWM, Meyerowitz EM (1998) A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell 92:93–103
Sasaki T, Matsumoto T, Yamamoto K et al (2002) The genome sequence and structure of rice chromosome. Nature 420:312–316
Singh K, Foley RC, Onate-Sanchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5:430–436
Souer E, Houwelingen AV, Kloos D, Mol J, Koes R (1996) The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 85:159–170
Tran LSP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Shinozaki KY (2004) Isolation and functional analysis of Arabidopsis stress inducible NAC transcription factors that bind to a drought responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16:2481–2498
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Plant Biol 16:123–132
Wei W, Zhang Y, Han L, Guan Z, Chai T (2008) A novel WRKY transcriptional factor from Thlaspi caerulescens negatively regulates the osmotic stress tolerance of transgenic tobacco. Plant Cell Rep 27:795–803
Xie Q, Frugis G, Colgan D, Chua NH (2000) Arabidopsis NAC1 transduces auxin signal posterior of TIR1 to promote lateral root development. Genes Dev 14:3024–3036
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yang SD, Seo PJ, Yoon HK, Park CM (2011) The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell 23:2155–2168
Zhang ZT, Zhou Y, Li Y, Shao SQ, Li BY, Shi HY, Li XB (2010) Interactome analysis of the six cotton 14-3-3s that are preferentially expressed in fibres and involved in cell elongation. J Exp Bot 61:3331–3344
Acknowledgments
This work was supported by the project from the Ministry of Agriculture of China for transgenic research (Grant No. 2009ZX08009-117B, 2011ZX08009-003), and the Chenguang Project of Wuhan Municipality (Grant No. 200950431185).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Lu.
H. Zhong and Q.-Q. Guo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhong, H., Guo, QQ., Chen, L. et al. Two Brassica napus genes encoding NAC transcription factors are involved in response to high-salinity stress. Plant Cell Rep 31, 1991–2003 (2012). https://doi.org/10.1007/s00299-012-1311-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1311-3