Abstract
An efficient protocol for the establishment of transformed root culture of Verbascum xanthophoeniceum using sonication-assisted Agrobacterium rhizogenes-mediated transformation is reported. Only 10 days after the inoculation with A. rhizogenes ATCC 15834 and 45 s ultrasound exposure, hairy roots appeared on 75% of the Verbascum leaves. Ten hairy root lines were isolated, although only half of them were free of bacterial contamination and started growing when excised from mother explants. The transgenic nature of the most vigorously growing hairy root clones (VX1 and VX6) was confirmed by polymerase chain reaction. Under submerged cultivation both hairy root clones accumulated high biomass amounts (12.8 and 14.3 g L−1, respectively) and significant amounts of bioactive phenylethanoid glycoside verbascoside (over 6-times more than in mother plant leaves). LC-APCI-MS analyses confirmed verbascoside accumulation in hairy root clones along with three other phenylethanoid glycosides (forsythoside B, leucosceptoside B and martynoside) and an iridoid glycoside aucubin. This is the first report on the induction of hairy roots of Verbascum plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Verbascum L., common name Mulleins, is a genus of 360 species of flowering plants in the Scrophulariaceae family, predominantly distributed in Asia, Europe, and North America (Heywood 1993). Mulleins are biennial or perennial, rarely annual plants, with a deep tap root. At the first year plants form a dense rosette of leaves, which is followed by growth of a stout flowering stem 0.3–2 m tall (Turker and Gurel 2005). Several records exist for the medical use of mulleins in folk medicine as a remedy for respiratory problems such as bronchitis, dry coughs, whooping cough, tuberculosis, and asthma. The leaves, roots and the flowers possess also anodyne, antimicrobial, anti-inflammatory, and sedative properties (Turker and Gurel 2005). Although Verbascum plants have been used medicinally since ancient times, their popularity increased commercially in the past few years. Today, the dried leaves and flowers, swallow capsules, alcohol extracts and the flower oil of common mullein can be found in United States health stores (Turker and Gurel 2005). Verbascum xanthophoeniceum Griseb. is an endemic plant species for the Balkan region, as well as for Northwestern and South parts of Turkey. It produces several bioactive iridoid and phenylethanoid glycosides (Kostadinova et al., in preparation), therefore it might serve as attractive source of potent anti-inflammatory and antioxidant compounds. In a previous report we found that phenylethanoid glycosides (forsythoside B, verbascoside and leucosceptoside B) accumulating in V. xanthophoeniceum aerial parts possess strong antioxidant, and acetylcholine and butyrylcholine inhibitory activities (Georgiev et al. 2010).
Genetic transformations with the ‘natural genetic engineer’ Agrobacterium rhizogenes for hairy root culture induction have attracted considerable attention since their immense potential to produce similar (or even higher) amounts of desired compounds (compared to mother plants) was realized about 25 years ago (Georgiev et al. 2007). The genetic and biochemical stability (being theoretically initiated from a single cell) and the fast growth in media free of exogenous phytohormones offer substantial advantages of transformed roots over dedifferentiated cell cultures (Vasilev et al. 2006; Georgiev et al. 2007; Zhang et al. 2009). It was recently estimated that hairy roots of more than 450 different plant species have been induced, including a diverse host range of dicotyledonous plants (the natural hosts of Agrobacterium; Georgiev et al. 2008). A major drawback of Agrobacterium-mediated transformations is that some plant species (mainly monocotyledonous plant families) are still considered as difficult-to-transform or even non-susceptible to Agrobacterium infections. Trick and Finer (1997) developed a new and potentially more efficient method to deliver Agrobacterium to target plant tissues. This technique, called by them Sonication-assisted Agrobacterium-mediated transformation (SAAT), involves subjecting the explants to short periods of ultrasound in the presence of Agrobacterium (Trick and Finer 1997, 1998). Performed scanning electron and light microscopy observations revealed that ultrasound treatment produces small and uniform fissures and channels throughout the plant tissue, which allows Agrobacterium access to internal plant tissue (Trick and Finer 1997). Furthermore, the SAAT technology has been successfully applied for A. rhizogenes transformation of difficult-to-transform Papaver somniferum plants (Le Flem-Bonhomme et al. 2004).
The aim of the present report was to develop an efficient protocol for A. rhizogenes-mediated transformation of Verbascum plants for induction of hairy roots and to show that these hairy roots are able to produce bioactive compounds. According to the best of our knowledge this is the first report for induction of hairy roots in Verbascum plants.
Materials and methods
Intact plant material
The V. xanthophoeniceum plants were identified and seeds were collected from Lesovo village (Yambol district, Bulgaria) by Dr. S. Bancheva (Institute of Botany, Bulgarian Academy of Sciences). A voucher specimen was deposited in the Herbarium of the Institute of Botany, Sofia, Bulgaria (SOM). Verbascum seeds were sowed in decontaminated soil and grown in the laboratory, at 26°C with an illumination period of 16 h light:8 h dark. After 2 months of growth V. xanthophoeniceum plants formed ~10 cm (in diameter) dense rosettes. Young haired leaves were cut and gently washed with tap water. Surface sterilization was performed with 70% ethanol for 10 s, followed by a second sterilization with 6% Ca(OCl)2 for 6 min. Few drops of Tween 80 were added to both sterilization agents for surface tension reduction. Sterile explants were cut to ~1 cm2 pieces and used for transformation experiments.
Agrobacterium strains and hairy roots induction protocol
Three different A. rhizogenes strains were used to infect V. xanthophoeniceum plants: A. rhizogenes ATCC 15834 (a gift from Prof. Dr. Thomas Bley, TU Dresden, Germany), LBA 9402 and TR 105 (a gift from Prof. Dr. Iliana Ionkova, Medical University, Sofia, Bulgaria). All Agrobacterium strains were maintained on YEB agar medium (8 g L−1 beef extract, 1 g L−1 yeast extract, 5 g L−1 sucrose, 0.5 g L−1 MgSO4 and 20 g L−1 agar, pH 7.0) at 26°C. Prior to transformation experiments three strains were grown overnight (16 h) in YEB medium (minus agar), on an orbital shaker (110 rpm), at 26°C. The bacterial suspensions were centrifuged at 5,000 rpm for 25 min and then the pellets were resuspended in MS medium (Murashige and Skoog 1962) to an OD600 nm between 0.6 and 0.8. Transformations of sterile explants were performed either through co-cultivation or through direct infection methods (Georgiev et al. 2007). For the SAAT experiments ten explants (~1 cm2) were transferred to sterile 50 mL Falcon tubes filled out with properly diluted in MS medium A. rhizogenes ATCC 15834 suspension. The plastic tubes were individually placed in a sonicator and subjected to ultrasound with frequency of 35 kHz (UCI-50Raypa® ultrasonic cleaner bath; R. Espinar S.L., Barcelona, Spain). The duration of treatments was 0, 30, 45 or 60 s. After ultrasound treatment, explants were blotted on filter paper to remove excess bacteria and transferred to solid MS media. All transformation procedures were performed in dark, at 26°C. After 3 days all explants from co-cultivation, direct infection and SAAT transformations were transferred to solid MS media, supplemented with 30 g L−1 sucrose, 300 mg L−1 cefotaxime sodium and 5.5 g L−1 Plant agar (all from Duchefa, Haarlem, The Netherlands).
Polymerase chain reaction analysis of transformation
Genomic DNA from two hairy root lines (VX1 and VX6) and untransformed roots of in vitro grown Verbascum plants was isolated following a cetyltrimethylammonium bromide (CTAB)-Protocol that was modified after Pirttilä et al. (2001). Plant material was pulverized with a mortar and pestle under liquid nitrogen and 100–300 mg were placed in Eppendorf tubes. Seven hundred microlitre CTAB buffer (without mercaptoethanol) was added to the samples, mixed and incubated under continuous stirring for 45 min at 65°C. After centrifugation for 10 min at 15,900g the supernatant was extracted with 1 volume of chloroform/isoamyl alcohol (24:1/v:v). The aqueous phase was mixed with 1/10 volume of 3 M sodium acetate (pH 5.2) and 1 volume of ice cold (–20°C) isopropyl alcohol. DNA was precipitated by incubation for 10–15 min at room temperature and centrifugation for 20 min at 4°C and 15,900g. The pellet was washed two times with ice cold ethanol (70%/v/v), air dried and dissolved in 50 μL distilled water.
Ri plasmid DNA was isolated after Hayman and Farrand (1990). Polymerase chain reaction (PCR) was performed according to Grabkowska et al. (2010) with the following modifications: 10 pM of each primer and 100 ng DNA were in the PCR mixture, elongation time was 1 min per cycle, and all primer pairs were combined in one PCR (multiplex PCR). Primers were 5′-GCT CTT GCA GTG CTA GAT TT-3′ and 5′-GAA GGT GCA AGC TAC CTC TC-3′ for rolB (expected size of product 423 bp), 5′-CTC CTG ACA TCA AAC TCG TC-3′ and 5′-TGC TTC GAG TTA TGG GTA CA-3′ for rolC (expected size of product 626 bp) according to Grabkowska et al. (2010) and 5′-ACT GAA TAT CAG GCA ACG CC-3′ and 5′-GCG TCA AAG AAA TAG CCA GC-3′ for VirG (expected size of product 350 bp) according to Sidwa-Gorycka et al. (2009). The amplified fragments were separated in a 1.5% agarose gel and stained with ethidium bromide. The gels were documented with a Gel Doc™ XR + System (BioRad, München, Germany).
Hairy root submerged cultivation and growth analyses
V. xanthophoeniceum hairy root culture lines (VX1, VX2, VX5, VX6 and VX7) used in the experiments were grown in liquid MS medium in 250-mL Erlenmeyer flasks with 20% net volume shaken at 110 rpm, in the dark at 25°C. All flasks were inoculated with ~1.0 g fresh 14-day-old roots per flask. The growth of the roots was assessed by harvesting cultures 2 and 3 weeks after inoculation. On each occasion, the hairy roots were separated from the culture medium by filtration. The final fresh and dried biomasses of the harvested roots were then gravimetrically determined (the latter after freeze-drying to constant weight). The growth of V. xanthophoeniceum hairy roots was monitored through determination of accumulated dry biomass (ADB) and growth index (GI) as described before (Georgiev et al. 2006). ADB = final dry biomass − initial dry biomass (g L−1); GI = (final dry biomass − initial dry biomass)/initial dry biomass.
Metabolite extraction and high performance liquid chromatography with diode array detection analysis
Freeze-dried V. xanthophoeniceum leaves and hairy roots samples (~100 mg) were extracted with methanol (solid:liquid ratio 1:50) in an ultrasonic bath (3 × 20 min), pooled, filtered through 0.2-μm filters and directly injected into an high performance liquid chromatography with diode array detection (HPLC–DAD) system, to quantify the verbascoside content. The HPLC–DAD system consisted of an 1200 Series instrument equipped with a G1310A pump, a G1329A autosampler, a G1322A degasser, a G1316A column oven, a G1315D diode array detector controlled by ChemStation (all from Agilent Technologies, Inc., Santa Clara, CA, USA) and a Luna reverse phase (C18) column (150 × 4.6 mm i.d., 5 μm particle size; Phenomenex, Utrecht, The Netherlands). The metabolites were then separated using a mobile phase consisting of methanol/water 5:95 (A:B) for 3 min, followed by linear gradients to 25:75 (A:B) after 7 min then to 60:40 (A:B) after 13 min and the flow rate was 1 mL min−1 (Gyurkovska et al. 2011). Eluting verbascoside was detected, and quantified, by monitoring the eluate at a wavelength of 330 nm. A standard calibration curve was plotted using various concentration ranges (0–400 μg mL−1) of verbascoside (a gift from Dr. I. Koleva, University of Food Technology, Plovdiv, Bulgaria).
Liquid chromatography-atmospheric pressure chemical ionization mass spectrometry analyses
Extracts from V. xanthophoeniceum leaves and hairy roots (10 μL) were injected into an Liquid chromatography-atmospheric pressure chemical ionization mass spectrometry (LC-APCI-MS) system consisting of an 1100 Series instrument equipped with a G1312A binary pump, a G1367A autosampler, a G1379A degasser, a G1316A column oven, a G1315B diode array detector, a simple quadrupole SL mass spectrometer driven by ChemStation software, all supplied by Agilent Technologies. The same column and mobile phase as described above were used. The eluent was monitored by the UV detector at 330 nm, and by the MS from mass-to-charge ratio (m/z) 300–850 (negative ionization mode) with drying gas at 10 mL min−1, 350°C, 50 psig and capillary voltage at 3,000 V.
Statistical analysis of the data
All transformation experiments were repeated three times. The data from shake-flask cultures are averages obtained from three independent experiments with two biological replicates. HPLC–DAD quantitative measurements and LC-APCI-MS analyses were done in triplicate. The results are presented as mean ± standard errors (SE).
Results and discussions
Establishment of hairy root cultures
Three different strains of A. rhizogenes—ATCC 15834, LBA 9402 and TR 105—were tested for their ability to induce the formation of hairy roots on V. xanthophoeniceum explants. We performed a huge experimental scheme with all Agrobacterium strains as both co-cultivation and direct infection methods were applied. Acetosyringone (in concentrations of 100 and 200 μM) was also added. Although over 600 explants were wounded and treated with A. rhizogenes (Table 1) no hairy root formation was observed. These data suggest that V. xanthophoeniceum plants are not susceptible to infection with A. rhizogenes possible due to the hairy leaf surface, which might be responsible for reduced access of Agrobacteria. Only one report exists in literature on the transformation of V. phoeniceum plants with A. tumefaciens (McCaskill and Turgeon 2007); however, there is no information available on the successful transformation of Verbascum plants with A. rhizogenes and hairy root induction (at least in SCOPUS database, accessed on 22.11.2010).
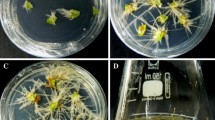
Thus we decided to apply the SAAT technology for V. xanthophoeniceum transformation. Based on the available literature data the exposure of wounded explants to ultrasound was chosen as 30, 45 and 60 s. The best performance was observed when explants were treated with ultrasound for 45 s, because in this case ~75% of explants treated with A. rhizogenes ATCC 15834 showed hairy roots formation (Table 1). In the other two conditions also hairy root growth was observed; however, the transformation frequency was significantly lower (15–28%) and even necrosis of mother plant tissue when exposed to 60 s ultrasound was observed (Table 1). Neoplastic hairy roots growth at the wound site of Verbascum leaves occurred already after 7 days of culture (10 days after transformation), which significantly reduces the time for hairy roots induction (Fig. 1). Furthermore, we successfully transformed via the above-described SAAT protocol V. densiflorum (using A. rhizogenes ATCC 15834) and V. phlomoides (with both A. rhizogenes strains ATCC 15834 and LBA 9402), which proves the high reliability of the SAAT technology for the transformation of Verbascum species. The infection frequency of ATCC 15834 strain was similar to that reported above (77 and 72% in the case of V. densiflorum and V. phlomoides, respectively), while when LBA 9402 was used for transformation of V. phlomoides the infection frequency was over 90% (data not shown). In all cases first neoplastic roots appeared 10–14 days after transformation. However, it should be noted that the time for hairy roots formation depends on species, age and type of plant tissue. For instance, when Papaver hypocotyls were infected with A. rhizogenes LBA 9402 via ultrasound, hairy roots occurred after 5 weeks of culture (Le Flem-Bonhomme et al. 2004).
After 3 weeks of cultivation 10 hairy root lines were isolated from different explants, denoted as VX1–10. When excited and transferred to new MS solid media three hairy root lines showed bacterial contamination (lines VX3, VX4 and VX10), which was not possible to be eliminated even after repeated antibiotic treatment. Two other lines (VX8 and VX9) did not show any growth when hairy roots were excised from mother explants and transferred to a new media. All remaining hairy roots lines (VX1, VX2, VX5, VX6 and VX7) showed stable growth and absence of bacterial contamination. V. xanthophoeniceum VX1 and VX6 lines showed typical morphology of thick roots with high degree of lateral branching, plagiotropic root growth and profusion of root hairs, while VX2, VX5 and VX7 lines differed in morphology, grew like fine thin branched roots.
To confirm the complete and stable genetic transformation, PCR analyses of DNA from two V. xanthophoeniceum lines VX1 and VX6 with primer pairs for the amplification of rolB, rolC, and VirG (Fig. 2) were performed. Although the molecular mechanism of hairy roots induction is not completely understood, it is known that the agropine type of A. rhizogenes transfers two independent T-DNAs, denoted TL-DNA and TR-DNA, to the host plant genome (Nilsson and Olsson 1997). Both fragments are independently transferred and integrated to the host genome; however, the transfer of TL-DNA is essential to induce hairy roots. The sequence analyses of TL-DNA identified 18 open reading frames (ORF), four of which are essential for hairy root induction, namely ORF10 (rolA), ORF11 (rolB), ORF12 (rolC) and ORF15 (rolD; Nilsson and Olsson 1997). Amplification of rolB and rolC primers showed 423 and 626 bp bands for the transformed root, respectively (Fig. 2, lanes 5–8), which is a proof for the successful genetic transformation of lines VX-1 and VX-6. No band for the VirG genes in untransformed Verbascum roots (lane 4) and transformed root culture lines (lanes 5–8) was observed, which is an indication for the absence of bacterial contamination in the roots. When the Ri plasmid of A. rhizogenes was analyzed it gave three PCR fragments as expected (lane 3). As negative control, untransformed Verbascum roots were also analyzed to demonstrate the specificity of the primer pairs (Fig. 2, lane 2).
PCR analysis of DNA from transformed Verbascum xanthophoeniceum root lines VX1 and VX6: lane 1 contains GeneRuler™ (Fermentas); lanes 2–8 contain amplified PCR products with different templates: 2 DNA from untransformed Verbascum roots (negative control); 3 Ri plasmid which is rolC (626 bp), rolB (423 bp), and VirG (350 bp) positive; 4 water control; 5, 6 DNA from VX1 (first and second isolation); 7, 8 DNA from VX6 (first and second isolation)
Hairy roots submerged cultivation and chemical analyses
V. xanthophoeniceum hairy roots lines VX1, VX2, VX5, VX6 and VX7 were grown 4 months on solid MS medium with 21-day period of subculture. That period was enough to get hairy roots with stable growth and morphological characteristics and for multiplication of hairy roots mass. Furthermore, we performed submerged cultivation of all five hairy root lines and the root mass was harvested after 2 and 3 weeks of culture (Fig. 3a). The results indicate that the most significant growth with a large number of lateral branching was observed for hairy root lines VX6 and VX1, which accumulated 14.33 ± 0.47 and 12.82 ± 0.13 g L−1 of biomass, respectively. Lines VX2, VX5 and VX7, although showing visible multiplication of root mass, accumulated lower biomass amounts (<7 g L−1; Fig. 3a). Calculated GI values 3 weeks after inoculation were 6.9–7.8 for best growing VX6 and VX1 lines. These values are similar or slightly higher than those reported for hairy root cultures of Beta vulgaris and Harpagophytum procumbens (Pavlov et al. 2007; Homova et al. 2010).
HPLC–DAD analyses of V. xanthophoeniceum hairy roots lines revealed the presence of significant amounts of verbascoside (Fig. 3b). The highest content of verbascoside was detected in VX1, VX2 and VX6 lines (23.31 ± 0.42, 21.90 ± 0.66 and 22.00 ± 0.72 mg g−1, respectively). It is worth mentioning that these amounts were over six times higher compared to the verbascoside content in the original V. xanthophoeniceum leaves used for transformation (3.48 ± 0.02 mg g−1 dry weight). Verbascoside levels in transformed root cultures (2.19–2.33%) are also several times higher compared to those detected in the flowers of V. densiflorum (0.688–0.742%) and V. phlomoides (0.167–0.206%; Klimek et al. 2010). Verbascoside (also known as acteoside, kusaginin and orobanchin; Fig. 4) is a very active member of the phenylethanoid group, which has been isolated from many plant species (Dembitsky 2005). It exhibits a wide spectrum of biological activities, including anti-leukemic and cytotoxic activity against a murine cell line (Pettit et al. 1990) and anti-inflammatory activity through inhibition of cyclooxygensase-2 expression and inhibition of complement activity in human serum (Gyurkovska et al. 2011). Verbascoside was reported as major compound in hairy root cultures of Gmelina arborea and H. procumbens, however, in significantly lower amounts than in V. xanthophoeniceum (0.91 and 1.1 mg g−1 dry weight, respectively; Dhakulkar et al. 2005; Homova et al. 2010). Therefore, V. xanthophoeniceum VX1 and VX6 hairy root clones might be attractive producers of bioactive verbascoside.
In addition, we partially analyzed the metabolite profiles of methanolic extracts of V. xanthophoeniceum hairy roots by LC-APCI-MS. Except verbascoside with m/z 623.2 [M]− (1) two other phenylethanoid glycosides, leucosceptoside B with m/z 783.2 [M]− (3) and martynoside with m/z 651.2 M]− (4) and one iridoid glycoside, aucubin with m/z 345.2 [M]− (5), were identified in both mother plant leaves and V. xanthophoeniceum hairy root lines VX1 and VX6. Typical HPLC–DAD chromatograms are presented in Fig. 5. Forsythoside B (m/z 755.2 [M]−; (2) was detected only in small amounts in transformed root cultures in opposite of intact Verbascum leaves, where it appeared as the most abundant phenylethanoid glycoside (Fig. 5B). On the contrary, the levels of verbascoside were higher in the hairy roots (Fig. 5C, D) than in intact leaves, indicating a possible shift in the biosynthetic pathways. It should be noted that aucubin could not be seen at these conditions (330 nm) as it shows absorbance maximum at 204 nm.
Conclusion
V. xanthophoeniceum plant leaves were not susceptible to transformation with three A. rhizogenes strains when co-cultivation and direct infection methods (with or without addition of acetosyringone) were applied. Therefore, we developed an efficient SAAT protocol for Verbascum hairy root culture induction. Most vigorous hairy root clones showed stable growth under submerged cultivation and accumulated high biomass amounts. Verbascoside was the most abundant secondary metabolite and its amounts in hairy root clones were several times higher than in mother plant tissue. Therefore, these hairy root cultures will be promising material for possible large scale production of this bioactive metabolite in the future.
Abbreviations
- ADB:
-
Accumulated dry biomass (g L−1)
- CTAB:
-
Cetyltrimethylammonium bromide
- GI:
-
Growth index
- HPLC–DAD:
-
High performance liquid chromatography with diode array detection
- LC-APCI-MS:
-
Liquid chromatography-atmospheric pressure chemical ionization mass spectrometry
- m/z :
-
Mass-to-charge ratio
- ORF:
-
Open reading frames
- PCR:
-
Polymerase chain reaction
- SAAT:
-
Sonication-assisted Agrobacterium-mediated transformation
References
Dembitsky VM (2005) Astonishing diversity of natural surfactants: 5. Biologically active glycosides of aromatic metabolites. Lipids 40:869–900
Dhakulkar S, Ganapathi TR, Bhargava S, Bapat VA (2005) Induction of hairy roots in Gmelina arborea Roxb. And production of verbascoside in hairy root. Plant Sci 169:812–818
Georgiev M, Heinrich M, Kerns G, Bley T (2006) Production of iridoids and phenolics by transformed Harpagophytum procumbens root cultures. Eng Life Sci 6:593–596
Georgiev M, Pavlov A, Bley TH (2007) Hairy root type plant in vitro systems as sources of bioactive substances. Appl Microbiol Biotechnol 74:1175–1185
Georgiev M, Georgiev V, Weber J, Bley TH, Ilieva M, Pavlov A (2008) Agrobacterium rhizogenes-mediated genetic transformations: a powerful tool for the production of metabolites. In: Wolf T, Koch J (eds) Genetically modified plants. Nova Science Publishers Inc., New York, pp 99–126
Georgiev M, Alipieva K, Orhan I, Abrashev R, Denev P, Angelova M (2010) Antioxidant and cholinesterases inhibitory activities of Verbascum xanthophoeniceum Griseb. and its active constituents. Food Chem (submitted)
Grabkowska R, Krolicka A, Mielicki W, Wielanek M, Wysokinska H (2010) Genetic transformation of Harpagophytum procumbens by Agrobacterium rhizogenes: iridoid and phenylethanoid glycoside accumulation in hairy root cultures. Acta Physiol Plant 32:665–673
Gyurkovska V, Alipieva K, Maciuk A, Dimitrova P, Ivanovska N, Haas C, Bley T, Georgiev M (2011) Anti-inflammatory activity of devil’s claw in vitro systems and their active constituents. Food Chem 125:171–178
Hayman GT, Farrand SK (1990) Agrobacterium plasmids encode structurally and functionally different loci for catabolism of agrocinopine-type opines. Mol Gen Genet 223:465–473
Heywood VH (1993) Flowering plants in the world. Oxford University Press, New York
Homova V, Weber J, Schulze J, Alipieva K, Bley Th, Georgiev M (2010) Devil’s claw hairy root culture in flasks and in a 3-L bioreactor: bioactive metabolite accumulation and flow cytometry. Z Naturforsch C 65:472–478
Klimek B, Olszewska MA, Tokar M (2010) Simultaneous determination of flavonoids and phenylethanoids in the flowers of Verbascum densiflorum and V. phlomoides by high-performance liquid chromatography. Phytochem Anal 21:150–156
Le Flem-Bonhomme V, Laurain-Mattar D, Fliniaux MA (2004) Hairy root induction of Papaver somniferum var. album, a difficult-to-transform plant by A. rhizogenes LBA 9402. Planta 218:890–893
McCaskill A, Turgeon R (2007) Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose-family oligosaccharides. Proc Natl Acad Sci USA 104:19619–19624
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nilsson O, Olsson O (1997) Getting to the root: the role of the Agrobacterium rhizogenes rol-genes in the formation of hairy roots. Physiol Plant 100:463–473
Pavlov A, Georgiev M, Bley T (2007) Batch and fed-batch production of betalains by Red beet (Beta vulgaris) hairy roots in a bubble column reactor. Z Naturforsch C 62:439–446
Pettit GR, Numata A, Takemura T, Ode RH, Narula AS, Schmidt JM, Cragg GM, Pase CP (1990) Antineoplastic agents, 107. Isolation of acteoside and isoacteoside from Castilleja linariaefolia. J Nat Prod 53:456–458
Pirttilä AM, Hirsikorpi M, Kämäräinen T, Jaakola L, Hohtola A (2001) DNA isolation methods for medicinal and aromatic plants. Plant Mol Biol Rep 19:273a–273f
Sidwa-Gorycka M, Krolicka A, Orlita A, Malinski E, Golebiowski M, Kumirska J, Chromik A, Biskup E, Stepnowski P, Lojkowska E (2009) Genetic transformation of Ruta graveolens L. by Agrobacterium rhizogenes: hairy root cultures a promising approach for production of coumarins and furanocoumarins. Plant Cell Tissue Organ Cult 97:59–69
Trick HN, Finer JJ (1997) SAAT: sonication-assisted Agrobacterium-mediated transformation. Transgenic Res 6:329–336
Trick HN, Finer JJ (1998) Sonication-assisted Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merrill] embryogenic suspension culture tissues. Plant Cell Rep 17:482–488
Turker AU, Gurel E (2005) Common mullein (Verbascum thapsus L.): recent advances in research. Phytother Res 19:733–739
Vasilev N, Elfahmi BosR, Kayser O, Momekov G, Konstantinov S, Ionkova I (2006) Production of justicidine B, a cytotoxic arylnaphthalene lignan from genetically transformed root cultures of Linum leonii. J Nat Prod 69:1014–1017
Zhang H-C, Liu J-M, Lu H-Y, Gao S-L (2009) Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over-expression of chalcone isomerase gene with the elicitation treatment. Plant Cell Rep 28:1205–1213
Acknowledgments
The authors are cordially grateful to Prof. Dr. Robert Verpoorte (Institute of Biology, Leiden, The Netherlands) for helpful discussions and Dr. Vasil Georgiev (Institute of Microbiology, Plovdiv, Bulgaria) for his help with hairy root clones maintenance. This work has been supported by a Marie Curie Fellowship of the European Community programme “Intra-European Fellowships” project SYSBIOPRO (contract number PIEF-GA-2009-252558) and by a grant from the National Science Fund of Bulgaria (contract number DO-02-261/2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Feher.
A contribution to the Special Issue: Plant Biotechnology in Support of the Millennium Development Goals.
Rights and permissions
About this article
Cite this article
Georgiev, M.I., Ludwig-Müller, J., Alipieva, K. et al. Sonication-assisted Agrobacterium rhizogenes-mediated transformation of Verbascum xanthophoeniceum Griseb. for bioactive metabolite accumulation. Plant Cell Rep 30, 859–866 (2011). https://doi.org/10.1007/s00299-010-0981-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0981-y