Abstract
To understand the genetic and molecular mechanisms underlying floral development in Populus tomentosa, we isolated PtLFY, a LEAFY homolog, from a P. tomentosa floral bud cDNA library. DNA gel blot analysis showed that PtLFY is present as a single copy in the genomes of both male and female individuals of P. tomentosa. The genomic copy is composed of three exons and two introns. Relative expression levels of PtLFY in tissues of P. tomentosa were estimated by RT-PCR; our results revealed that PtLFY mRNA is highly abundant in roots and both male and female floral buds. A low level of gene expression was detected in stems and vegetative buds, and no PtLFY-specific transcripts were detected in leaves. PtLFY expression patterns were analyzed during the development of both male and female floral buds in P. tomentosa via real-time quantitative RT-PCR. Continuous, stable and high-level expression of PtLFY-specific mRNA was detected in both male and female floral buds from September 13th to February 25th, but the level of PtLFY transcripts detected in male floral buds was considerably higher than in female floral buds. Our results also showed an inverted repeat PtLFY fragment (PtLFY-IR) effectively blocked flowering of transgenic tobacco plants, and that this effect appeared to be due to post-transcriptional silencing of the endogenous tobacco LFY homologs NFL1 and NFL2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chinese white poplar (Populus tomentosa Carr.) is a tree species native to a large area of northern China. It is an important species in forest production and forest reclamation projects along the Yellow River. In addition, Populus (poplar) species are an important model system for molecular genetic studies of woody plants.
Two commonly used model plant genera, Populus and Arabidopsis, are both eudicots (Soltis and Soltis 1999; Wikström et al. 2001) and are characterized by a monopodial shoot system (Bradley et al. 1997; Reinhardt and Kuhlemeiera 2002; Yuceer et al. 2003). However, the two genera differ in many ways (Boes and Strauss 1994). Arabidopsis is an annual herbaceous plant and completes its life cycle in 2 months, with only a short juvenile phase followed by the production of flowers and seeds in the reproductive phase (Somerville and Koornneef 2002). In addition, Arabidopsis plants do not undergo specific phases of seasonal vegetative growth and floral development in the reproductive phase (Hsu et al. 2006). Moreover, the Arabidopsis flower conforms to the general angiosperm pattern in consisting of four types of floral organ arranged in a series of concentric whorls. From the outermost whorl inwards, the flower consists of sepals in whorl 1, petals in whorl 2, stamens in whorl 3, and carpels in whorl 4 (Weigel and Meyerowitz 1994; Yanofsky 1995; Ng and Yanofsky 2001; Lohmann and Weigel 2002).
In contrast, poplars are perennial trees with a lifespan of about 100–200 years and a long juvenile phase (Braatne et al. 1996). In general, poplar seedlings begin flowering after at least 7–10 years and thereafter annual flowering occurs during the reproductive phase. After completion of flowering and fruiting, shoots initiate early vegetative buds (vegetative zone I), floral buds (floral zone), and late vegetative buds (vegetative zone II) in a sequential manner, indicating that the shoot goes through repeated phase-change cycles between vegetative and reproductive growth (Yuceer et al. 2003). Such recurrent developmental transitions between vegetative and reproductive growth are absent in Arabidopsis (Boss et al. 2004). Flower development in poplars also differs markedly from that of Arabidopsis in that the male and female flowers are borne on separate trees from axillary inflorescences. Instead of four concentric whorls of organs, poplar flowers have only two whorls, comprising a reduced perianth cup surrounding either the stamens or carpels (Boes and Strauss 1994; Sheppard 1997; Rottmann et al. 2000).
Both physiological changes and expression of floral genes in the shoot apex are involved in the floral transition. The switch from vegetative to reproductive growth requires activation of genes involved in flower differentiation (Böhlenius 2007). Several floral meristem identity genes have been isolated from Arabidopsis and other model plant species. One of these genes, LFY (LEAFY) plays a crucial role in the transition from vegetative to reproductive development in Arabidopsis (Weigel et al. 1992; Weigel and Nilsson 1995). Another floral meristem identity gene, AP1 (APETALA1), is required for sepal and petal development and is also involved in controlling the transition mentioned above (Mandel et al. 1992). Loss-of-function mutations in LFY lead to plants in which shoots replace most flowers (Weigel et al. 1992). LFY expression is first detectable in leaf primordia and reaches maximal levels in young floral meristems (Blazquez et al. 1997; Blázquez et al. 1998). Constitutive expression of either LFY or AP1 results in flowering of transgenic Arabidopsis plants in vitro in just 10 days (Mandel and Yanofsky 1995; Weigel and Nilsson 1995). When the LFY gene from Arabidopsis is expressed constitutively in hybrid aspen (P. tremula × P. tremuloides), plants flowered in vitro within 7 months (Weigel and Nilsson 1995). Overexpression of MdMADS5, an APETALA1-like gene of apple, causes early flowering in transgenic Arabidopsis (Kotoda et al. 2002). Expression of Arabidopsis LFY and AP1 genes in Citrus induces flowering within the first year. This shortening of the juvenile period is stable and occurs in both zygotic and nucellar-derived seedlings (Peña et al. 2001). However, the influence of flowering genes is more complex in poplar. Overexpression of PTLF, a LFY homolog from P. trichocarpa, in several poplar hybrids induced precocious flowering in a tiny proportion of transgenic lines, although overexpression of PTLF in Arabidopsis accelerated flowering (Rottmann et al. 2000).
In the present study, a LFY gene homolog, PtLFY, was isolated from a P. tomentosa floral bud cDNA library. The tissue-specific expression pattern of PtLFY was determined by RT-PCR, while the time course of PtLFY expression in developing floral buds of both male and female plants was quantified by qRT-PCR. Furthermore, the potential function of the PtLFY gene was investigated using an inverted repeat (IR) structure of PtLFY (PtLFY-IR) to induce PTGS in a heterologous plant species, tobacco.
Materials and methods
Plant materials
Samples of vegetative and reproductive tissues were collected during 2004 and 2005 from both female (5082) and male (LM50) trees of P. tomentosa growing in the Beijing Forestry University nursery. Young leaves were used for extraction of genomic DNA. Floral buds of P. tomentosa were collected at different developmental stages, from floral bud initiation to maturity, between September 13th 2004 and February 25th 2005 for the RNA extraction assay. After each collection, the samples were frozen rapidly in liquid nitrogen and stored at −80°C until use. Besides, root, stem and leaf samples were taken from one-month-old tissue-cultured plantlets of P. tomentosa, followed by immediate RNA extraction.
Sterile tobacco (Nicotiana tabacum) leaf discs were used for Agrobacterium-mediated transformations. Young leaves of transgenic tobacco plants were used for extraction of genomic DNA and RNA.
cDNA library construction and cloning of PtLFY
To construct a cDNA library, total RNA was extracted from a mixed sample of floral buds at different developmental stages using a CTAB-based method as described previously (Chang et al. 1993). The mRNA corresponding to the respective total RNA was then isolated and purified with the PolyATtract® mRNA Isolation System (Promega). Subsequently, the cDNA library was constructed with the mRNA as template using the SMART™ cDNA Library Construction Kit (Clontech).
Primers used for screening of PtLFY and DNA probe labeling were designed from the nucleotide sequence of PTLF (GenBank accession no. U93196), a LFY homolog from Populus balsamifera, and were synthesized by Sangon Co. (Table 1). PtLFY was obtained by PCR screening of the P. tomentosa female floral bud cDNA library. The 20-μl PCR reaction mixture was composed of 1× PCR buffer (10 mM Tris–HCl, pH 8.3, 50 mM KCl, 2.5 mM MgCl2), 1 μl SM buffer (0.01% gelatin, 50 mM Tris–HCl, pH 8.0, 0.1 M NaCl, 8 mM MgSO4) containing recombinant bacteriophages, 0.4 μl of each 10 μM forward primer (P1F) and reverse primer (P1R), and 1 unit Taq DNA polymerase. Thermal cycling was performed at 94°C for 5 min, then 94°C for 30 s, 60°C for 20 s, 72°C for 30 s min for 30 cycles, and 72°C for 10 min and finally kept at 4°C, using a GeneAmp® PCR System 9700 (ABI). The positive clones of the Lambda phage clones screened by PCR were sequenced directly with an ABI 377 DNA sequencer (Applied Biosystems).
Protein structure and phylogenetic relationship analysis
The amino acid sequences of LFY homologous genes were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi) and aligned using ClustalX 1.81. The secondary structure of the PtLFY protein was predicted using ANTHEPROT 2000 6.0, and the conserved protein domain sequences were analyzed with the Proteomics Server of the Expert Protein Analysis System (ExPASy) of the Swiss Institute of Bioinformatics (http://cn.expasy.org; Gasteiger et al. 2003). The tertiary structure of the PtLFY protein was predicted using 3D-JIGSAW 2.0 (http://bmm.cancerresearchuk.org/~3djigsaw/), and viewed with RasMol 2.7.2.1. Genetic distance matrices were obtained from the alignments and neighbor-joining trees constructed with bootstrap sampling of 1000 replications using MEGA 4.1 (Tamura et al. 2007).
DNA gel blot analysis
A 1076-bp fragment amplified by the P2F and P2R primers (Table 1) was used as the PCR probe for DNA gel blot analysis. Fifteen micrograms of genomic DNA from both male and female P. tomentosa was digested with the restriction enzymes BamHI, HindIII and EcoRI, and electrophoresed on a 0.8% agarose gel. After blotting onto a positively charged nylon membrane by capillary transfer using 20× SSC, the blots were hybridized with a DIG-labeled DNA probe at 58°C, and washed at high stringency (2× SSC, 0.1% SDS, at 15–25°C for 2 × 5 min, then 0.5× SSC, 0.1% SDS at 65–68°C for 2 × 15 min). Immunological detection was performed with the DIG DNA Labeling and Detection Kit (Roche) according to the manufacturer’s protocol.
RT-PCR and qRT-PCR analysis
Total RNA was extracted from root, stem, leaf, vegetative bud and floral bud respectively according to the method described previously (Chang et al., 1993). Five micrograms of total RNA was pretreated with RQ1 DNase I (Promega) to remove genomic DNA contaminants. The concentration of total RNA was measured using a SPEKOL 1300 spectrophotometer (Jena). First-strand cDNA was synthesized using 1.0 μg DNase-treated total RNA, Superscript III (Invitrogen), and oligo(dT)20 in a total volume of 20 μl. The first-strand cDNA was diluted 1:10 with ddH2O, and 2 μl of the diluted cDNA was used as a template for RT-PCR and qRT-PCR analysis. RT-PCR system were performed in a total volume of 20 μl, with 2 μl of 10× PCR buffer, 1.6 μl of 2.5 mM dNTPs and each 0.4 μl of 10 μM P1F and P1R (Table 1), and 1 unit Taq DNA polymerase in the reaction. PCR conditions were the same as used previously for the library screening. qRT-PCR reactions were performed in a total volume of 20 μl, with 0.4 μl of 10 μM P1F and P1R (Table 1), and 10 μl 2× SYBR® Green PCR Master Mix (Invitrogen), using a DNA Engine Opticon 2 system (MJ Research). The qRT-PCR program included a preliminary step of 2 min at 50, 94°C respectively, followed by 35 cycles of 94°C for 15 s, 56°C for 30 s, and 72°C for 30 s, with final extension at 72°C for 7 min. No-template controls for each primer pair were included in each run. According to a previous study, the poplar ACTIN gene (GenBank accession: AY261523.1) is a stably expressed internal control (Zhang et al. 2008; Zheng et al. 2009); therefore, it was employed as an internal reference gene to normalize small differences in template amounts with the forward primer 5′-CTCCATCATGAAATGCGATG-3′ and reverse primer 5′-TTGGGGCTAGTGCTGAGATT-3′. At least three different RNA isolations and cDNA syntheses were used as replicates for the qRT-PCR.
Construction and transformation of PtLFY-IR structure in tobacco
To examine the biological function of PtLFY, the PCR primers P2F, P2aR, and P2bR (Table 1), designed according to the sequence of the highly conserved region between the tobacco LFY homologs NFL1 (GenBank accession no. U16172), NFL2 (U15799) and PtLFY, were used to amplify PtLFY fragments from genomic DNA. A common NcoI restriction enzyme site was found in each PtLFY fragment that amplified using primers P2F and P2aR, and P2F, and P2bR. The resulting fragments were digested with XbaI and NcoI, and BamHI and NcoI, respectively. In addition, pBI121 plasmid DNA was digested with XbaI and BamHI. The corresponding fragments were purified using the QIAquick™ Gel Extraction Kit (QIAGEN) and ligated with T4 DNA ligase (Promega) at 16°C overnight, then transformed into competent cells of E. coli TG1. The fragment containing the IR sequence of the PtLFY fragment was verified by digestion with BamHI and XbaI, and by PCR amplification using primers P3F and P3R (Table 1). The PtLFY-IR structure was assembled into the binary vector pBI121, which gave plasmid pBI121-PtLFY-IR. Subsequently, pBI121-PtLFY-IR was introduced into the Agrobacterium tumefaciens strain GV3101 according to a direct and efficient liquid nitrogen freezing—thawing method reported previously (Tzfira et al. 1997). Agrobacterium tumefaciens GV3101 manipulation and N. tabacum leaf disc transformation were performed as described previously (Li et al. 1992).
Identification of transformants by PCR and DNA gel blots analysis
Genomic DNA was extracted from young leaves of transformants and used as the template for PCR identification. The PCR reaction conditions were similar to those used previously with the exception that primers P3F and P3R were used, and thermal cycling was performed at 94°C for 5 min, then at 94°C for 40 s, 58°C for 40 s, 72°C for 40 s for 30 cycles and 72°C for 10 min. The PCR product amplified with primers P3F and P3R was then used as DNA probe against transgenic tobacco DNA. DNA gel blotting and immunological detection was performed as described above.
Detection of NFL1 and NFL2 gene silencing in transgenic tobacco plants
Total RNA was extracted from the whole plantlet of transgenic tobacco using SV Total RNA Isolation System (Promega). First-strand cDNA was synthesized using Reverse Transcription System (Promega), and diluted 1:10 with ddH2O. Semi-quantitative RT-PCR was performed in 20 μl reactions containing 0.4 μl of 10 μM P4F and P4R primers which were designed according to the conserved sequence of NFL homologs (Table 1), 1 μl of cDNA template, 2 μl of 10× PCR buffer, 1.6 μl of 2.5 mM dNTPs, and 1 unit Taq DNA polymerase in reaction system. Thermal cycling was performed at 94°C for 3 min, then at 94°C for 20 s, 60°C for 20 s, 72°C for 20 s for 30 cycles and 72°C for 7 min. The tobacco ACTIN gene (GenBank accession: U60491) was selected as an endogenous reference gene to normalize small differences in template amounts because of its relatively expression levels. Sequences of primers P5F and P5R are given Table 1.
Phenotype analysis of transgenic tobacco plants
The verified transgenic tobacco plants were propagated and synchronized using vegetative stem cuttings containing an axillary bud from primary transformants. Transplanting of the rooted transgenic tobacco plantlets into an artificial soil mix (humus:perlite:vermiculite = 3:1:1) in individual pots, the plantlets were covered with clear plastic and the pots placed in a shaded greenhouse. After hardening for 2–3 weeks and once the plantlets had produced four to five leaves (June 15th), the plants were moved to the outside of the greenhouse and covered with clear plastic to maintain humidity for 2 weeks. Subsequently, the plastic was removed for a period each day to harden the plants. Plant height and number of leaves of each T0 transgenic line were recorded on August 11th, August 21st, September 1st, September 11th, September 21st, and October 2nd. In addition, the date was recorded when flower primordia were first visible.
Results
Cloning and DNA blot analysis of PtLFY from P. tomentosa
Three positive phage plaques were isolated by PCR screening. The longest of the PtLFY cDNA fragments was 1314 bp encoding 377 amino acids. Comparison of the cDNA sequence against our previously submitted PtLFY genomic sequence (GenBank accession: AY211519) from P. tomentosa showed that the gene is composed of three exons and two introns (Fig. 1a). Using Seqaid II and the SPL (search for potential splice sites) tool available at http://dot.imgen.bcm.tmc.edu:9331/gene-finder/gf.htm, the donor and acceptor sites of the first intron (596 bp) were found to be at 458 and 1054 bp. The donor and acceptor sites of the second intron (669 bp) were at 1388 and 2057 bp. The positions of splice sites were the same as those of LFY homologs of other plants (Frohlich and Parker 2000), but the length of the introns differed greatly. For example, the intron of Arabidopsis LFY is 910-bp-long in the position of the second intron. The splice junctions in PtLFY followed the “GT······AG” rule, the splice junctions and restriction endonuclease sites are shown in Fig. 1a.
To determine the number of PtLFY copies in the P. tomentosa genome, hybridization of genomic DNA from both male (LM50) and female (5082) clones were performed. The DNA blots revealed the same pattern of hybridization in both male and female clones. A single band was visualized in three different digestions in both male and female clones. This indicated that there was only one gene copy in the genomic DNA, and no additional bands were observed at a lower hybridization temperature and with reduced washing stringency (Fig. 1b).
Prediction of protein structure and phylogenetic relationships of PtLFY
A comparison of the amino acid sequences of PtLFY and other FLO/LFY homologs LFY (Weigel et al. 1992), FLO (Coen et al. 1990), VFL (Carmona et al. 2002), SdF (AY230817), and AFL1 (AB162028) showed the presence of two highly conserved regions, and the C-terminal conserved region of PtLFY protein is mainly composed of seven α-helix and two β-sheet structures (Fig. 2).
Sequence alignments of PtLFY with SdLFY (GenBank accession no. AY230817), VFL (AF450278), AFL1 (AB162028), FLO (M55525) and LFY (NM_125579). The line beneath indicates the conserved region. Asterisks and dots indicate totally conserved and conservatively replaced amino acids, respectively. The arrows indicate the predicted β-sheets, and rectangles indicate positions of the predicted the α-helices
The phylogenetic relationships of the FLO/LFY homologs are represented in Fig. 3. The earliest divergence occurred between the angiosperms and gymnosperms, and another important event was the divergence of monocotyledons and dicotyledons. The duplicated homologs are indicated to have arisen from relatively recent events (e.g. as in maize, tobacco, pear, loquat and apple), with the exception of a duplication that predated species diversification within Maloideae. No duplication of PtLFY has occurred in P. tomentosa.
Neighbor-joining tree representing relationships of PtLFY (framed) with gymnosperm and other angiosperm LFY/FLO homologs. Bootstrap support values (%) from 1,000 replications are indicated when over 50%. GenBank accession number for each sequence: AAL (AY229891), AcOrcLFY (AB088457), AFL1 (AB162028), AFL2 (AB056159), ALF (AF030171), BdLFY (AY734564), BjLFY (DQ471932), ClLFY (AY672542), CjNdly (AB074568), CmLFY (DQ989225), CoLFY-1 (AB162031), CoLFY-2 (AB162037), CrLFY (DQ995349), CsLFY (AY338976), CsLFY-1 (AB162032), CsLFY-2 (AB162038), CuLFY (DQ995347), DFL (AY559245) EjLFY-1 (AB162033), EjLFY-2 (AB162039), FcLFY (DQ497003), FLO (M55525), GinLFY (AF108228), GinNdly (AF105111), GmLFY (DQ448809), HLY (AY520841), LcLFY (AY770393), LEAFY2 (AF184589), LeLFY (AF197934), LFY (NM_125579), NFL1 (U16172), NFL2 (U15799), OrcLFY (AB088454), OSL (AF065992), PaLFY (AY701763), PcLFY1 (AY640316), PdFL (AY947465), PFL (DQ054794), PmNEEDLY (AY957473), PpLFY-1 (AB162029), PpLFY-2 (AB162035), PrFLL (AF109149), Psuni (AF010190), PTLF (U93196), SdLFY (AY230817), TaLFY (AB231889), ZmFL1 (AY179883), ZmFL2 (AY789046)
Expression pattern of PtLFY in P. tomentosa
The differential expression of PtLFY in various tissues of P. tomentosa was detected by RT-PCR, and the result indicated that PtLFY-specific mRNA was relatively abundant in seedling roots and both male and female floral buds. PtLFY-specific transcripts were only faintly detected in seedling shoots and vegetative buds and appeared to be absent from leaves (Fig. 4a). Furthermore, the relative difference of PtLFY expression in female and male floral buds indicated by qRT-PCR analysis is shown in Fig. 4b and c. The PtLFY expression patterns during both male and female floral bud development were similar. In both male and female floral buds PtLFY expression was continuous and stable from September 13th to February 25th. However, the expression of PtLFY in male floral buds was 25-fold higher than that in female buds.
The expression patterns of PtLFY in P. tomentosa. a Expression of PtLFY in different tissues of P. tomentosa. R, S and L (root, stem and leaf from one-month-old tissue-cultured plantlets, respectively), VB (vegetative bud), Fme (floral bud-male-early stage, on July 5th), Ffe (floral bud-female-early stage, on July 5th), Fml (floral bud-male-late stage, on March 10th in next year), Ffl (floral bud-female-late stage, on March 10th in next year), + detectable, − undetectable. The expression patterns of PtLFY during floral bud development in P. tomentosa derived from real time quantitative RT-PCR analysis in female floral buds (b) and male floral buds (c)
Potential function of PtLFY revealed by post-transcriptional gene silencing
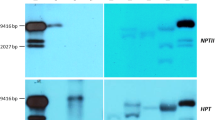
To investigate the function of PtLFY gene in planta, a plasmid containing a PtLFY IR sequence that released from PtLFY fragment (from 668 to 1076 bp) was constructed (Fig. 5a) and used for genetic transformation of tobacco plants. Both PCR amplification and DNA gel blot analysis showed that PtLFY-IR structure had been successfully integrated into the tobacco genome (Fig. 5b). Notably, it was observed that the resulting transgenic tobacco plants presented a significantly low NFL1 and NFL2 transcript level (slight/faint) when compared with the wild-type control, suggesting that PtLFY-IR in tissues was responsible for silencing the endogenous genes (Fig. 5c).
Molecular identification of transgenic tobacco plants carrying the PtLFY-IR silencing construct. a Schematic representation of pBI121-PtLFY-IR for gene silencing of NFL1 and NFL2 in transgenic tobacco. RB (T-DNA right border), LB (T-DNA left border), Nosp (Nos promoter), nptII (Neomycin phosphotransferase II), Nost (Nos terminator), 35S (CaMV 35S promoter), gus (β-glucuronidase gene), PtLFY-IR (inverted repeat PtLFY fragment, two black boxes respectively represent two 285-bp-long inverted sequences from the 3rd exon of PtLFY, the box intermediated represents a 248-bp-long sequence from the 2nd intron of PtLFY). b Transgenic tobacco plants identified by PCR amplification and DNA gel blot analysis. T24, T25, T26, T27, T34 and T39 are six transgenic lines, WT is the wild type, P is positive control (the recombinant plasmid pBI121-PtLFY-IR). c The suppression of NFL1 and NFL2 expression in transgenic tobacco plantlets identified by semi-quantitative RT-PCR (using tobacco ACTIN as reference control). T24, T25, T26, T27, T34 and T39 are six transgenic lines, WT indicates wild type
In order to observe the flowering characteristics of tobacco plants expressing the PtLFY-IR silencing construct, some transgenic tobacco lines and wild-type (WT) plants were transferred from the greenhouse to natural growing conditions for further analysis (Fig. 6). Only one transgenic line (T35) flowered out of a total of 15 lines, whereas all 10 WT plants flowered (Table 2). Compared with WT plants, the transgenic tobacco plants produced fewer leaves (Fig. 6c) and were slightly shorter (Fig. 6d), but almost no variation in leaf shape or size was observed on the transgenic plants (Fig. 6a, b).
Phenotypic characteristics and growth traits of transgenic tobacco plants carrying PtLFY-IR grown in natural conditions. a Transgenic plants compared with wild-type tobacco plants (60-day-old after synchronized culture of vegetative stem cuttings). b Comparison of height in transgenic and wild-type tobacco plants (120-day-old after synchronized culture of vegetative stem cuttings). T4 and T5 (corresponding to very the fourth and fifth plants from left to right in front row in (a) represent two transgenic lines. c Change in leaf number during the development of transgenic tobacco plants and wild-type tobacco plants. d Height of transgenic and wild-type tobacco plants from August 11th to October 2nd (the ruler unit: cm). No significant differences between controls (wild type) and transgenic tobacco plants were observed (P > 0.05) according to the ANOVA FISHER’s LSD test
Discussion
A number of LFY (LEAFY) homologs have been isolated from many plant species, including annual and herbaceous dicots such as Arabidopsis thaliana LFY (Weigel et al. 1992), Antirrhinum majus FLO (Coen et al. 1990), and Petunia hybrida ALF (Souer et al. 1998); monocots such as Oryza sativa RFL (Junko et al. 1998) and Zea mays ZmFL1 and ZmFL2 (Bomblies et al. 2003); the gymnosperm Pinus NEEDLY and PrFLL (Mellerowicz et al. 1998; Mouradov et al. 1998) as well as many other woody angiosperms including Populus trichocarpa PTLF (Rottmann et al. 2000). LFY is found in all land plants, which evolved during the past 400 million years (Maizel et al. 2005). These LFY homologs have two distinct characteristics: they are composed of three exons and two introns, and have two highly conserved regions (the N- and C-terminals) in diverse species (Maizel et al. 2005). The stability and conserved nature of the primary structure of the protein indicated that LFY homologs have a similar function even in different species. In Arabidopsis, LFY is a crucial integrator of endogenous and environmental signals, including hormonal cues, photoperiodic changes, and exposure to cold temperature. Its expression is upregulated in response to these signals (Chae et al. 2008) and, in turn, LFY acts to coordinate initial expression of floral homeotic genes (Weigel and Meyerowitz 1993; Nilsson et al. 1998; Blazquez and Weigel 2000). LFY is involved in regulating the transition from an inflorescence meristem to a floral meristem (Weigel et al. 1992), and it serves as a developmental switch for floral initiation in a diversity of plants (Weigel and Nilsson 1995). Our tertiary structure predictions based on the inferred protein sequence imply that LFY adopts a novel seven-helix fold that binds to DNA as a cooperative dimer, forming base-specific contacts in both the major and minor grooves. Cooperativity is mediated by two basic residues and this may explain the effectiveness with which LFY triggers sharp developmental transitions (Hamès et al. 2008). As in the Arabidopsis LFY protein, the PtLFY C terminus is composed of a seven-helix fold (Fig. 2), indicating that PtLFY might play a role in triggering the floral transition in P. tomentosa.
The DNA gel blot analysis of restriction endonuclease-digested P. tomentosa genomic DNA showed that both the male and female P. tomentosa genomes contain only one copy of a LFY homolog (Fig. 1b). Gymnosperm species have two homologs of LFY genes, whereas angiosperm species generally possess only one copy of the gene (Frohlich and Parker 2000). The Ginkgo biloba tree has two homologous genes, GinLFY (GenBank accession no. AF108228) and GinNdly (AF105111). The Pinus radiata genome contains the homologs NEEDLY and PRFLL (Mellerowicz et al. 1998; Mouradov et al. 1998). NEEDLY is expressed during vegetative and reproductive development, while PRFLL is expressed preferentially in male cones during reproductive development. However, two LFY copies have been reported to occur in some angiosperm species such as tobacco (Ahearn et al. 2001), apple (Wada et al. 2002), maize (Bomblies et al. 2003), and pear. In addition, the neighbor-joining tree presented in Fig. 3 showed that the duplication event that gave rise to the apple LFY/FLO homolog occurred before the divergence of the Maloideae. No PtLFY duplication event has occurred during the evolution of P. tomentosa, implying that PtLFY gene might be indispensable and irreplaceable for floral bud initiation and floral organ development.
P. tomentosa is a dioecious tree, although the sporadic occurrence of bisexual (perfect) flowers has been noted in some Populus species (Boes and Strauss 1994). Previous studies on the expression pattern of PTLF, a LFY homolog of Populus trichocarpa, showed the gene was expressed most strongly not only in developing inflorescences, but also in leaf primordia, very young leaves, apical vegetative buds, and seedlings (Rottmann et al. 2000). The pattern of expression does, in general, agree with that seen in Arabidopsis and Antirrhinum (Weigel et al. 1992; Coen et al. 1990; Blazquez et al. 1997). In our study, however, RT-PCR analysis showed a high level of PtLFY transcripts in roots and developing female and male floral buds, lower levels in stems and vegetative buds, and virtually no expression in leaves (Fig. 4a). Further qRT-PCR analysis showed that a high and stable quantity of PtLFY transcripts accumulated in both male and female developing floral buds, and that the relative expression level of PtLFY in developing male floral buds is much higher than that in developing female floral buds (Fig. 4b, c). The differential expression of PtLFY in floral buds might have something to do with the observation that the developmental progression of male floral buds occurs prior to that of female floral buds (An et al. unpublished data). Alternatively, this difference might indicate that a much higher level of PtLFY expression could be required for male floral bud development in P. tomotosa. In poplar, there are more androecium primordia present in male floral buds than there are gynoecium primordia in female floral buds. Thus, a much higher and constant level of PtLFY-specific mRNA may be necessary to ensure stamen morphogenesis. A previous in situ hybridization study also showed that a PTLF antisense probe hybridized strongly to the floral meristems and developing flowers in both male and female poplars (Rottmann et al. 2000). Although Hevea brasiliensis also has male, female and bisexual flowers, in that species all floral meristems were shown to express HbLFY transcripts equally (Dornelas and Rodriguez 2005). In contrast, the distinction between male and female inflorescences in maize apparently requires the differential expression of distinct maize FLO/LFY paralogs (Bomblies et al. 2003). In other woody species, the expression patterns of LFY homologs are not always related to reproductive development. LFY homologs are expressed in leaf primordia of Eucalyptus and grape (Southerton et al. 1998; Carmona et al. 2002; Dornelas et al. 2004). In transgenic poplar, the PTLF promoter was found to direct the highest level of GUS gene expression in shoots (Wei et al. 2006). In contrast, we found a relatively low level of PtLFY expression in stems, with expression the highest in both male and female floral buds and roots in P. tomentosa (Fig. 4a). In addition, seasonally dependent expression of LFY homologs has been observed in other woody species such as kiwifruit, grape, and apple (Walton et al. 2001; Carmona et al. 2002; Almada et al. 2009; Wada et al. 2002).
This study has contributed to a better understanding of the biological role of PtLFY during reproductive development and also explored a new method of controlling flower initiation in P. tomentosa. The high expression level in developing male and female floral buds implies that PtLFY might play an important role in development of floral buds. To reveal the potential function of PtLFY, a common nucleotide sequence among PtLFY, NFL1 and NFL2 that encodes the C-terminal region, which is comprised of several conserved secondary domains such as α2, α3, α4, α5, α6, and α7 helices, was targeted for RNAi analysis (Figs. S1, S2). The helix-turn-helix (HTH, helices α2 and α3) motif is involved in sequence-specific contact between LFY and both the minor and major grooves of the target DNA sequence (Hamès et al. 2008). The PtLFY-IR silencing construct was designed based on conserved sequence of the target region (Fig. 5a), and transgenic tobacco lines were obtained that carried the PtLFY-IR structure (Fig. 6a). Semi-quantitative RT-PCR analysis showed that the expression of endogenous NFL1 and NFL2 was reduced by the presence of PtLFY-IR in the transgenic tobacco lines (Fig. 5c). Fourteen out of 15 transgenic tobacco lines possessed a non-flowering phenotype (Table 2), indicating that PtLFY has a similar function to Arabidopsis LFY in planta. This result suggested that the PtLFY-IR silencing construct reduced expression of the endogenous NFL1 and NFL2, and thereby inhibited flowering in transgenic tobacco. Similarly, suppression of Sus activity caused by construct Sus-IR in the ovule epidermis led to a fiberless phenotype in transgenic cotton plants (Ruan et al. 2003). This approach to generate hpRNAs in plants is known as IR silencing triggers (Chuang and Meyerowitz 2000; Wesley et al. 2001). The most potent variation is a hairpin in which the terminal loop is initially formed by a short intron. Although there is some variation, the success rate for gene silencing has been reported to exceed 90% in some publications (Chuang and Meyerowitz 2000; Wesley et al. 2001; Kerschen et al. 2004). Therefore, hpRNAi has been widely adopted for effecting gene knock-down in many plant species (Ossowski et al. 2008). In another respect, a large number of non-coding RNAs (e.g. microRNA) have been shown to be conserved among diverse plant species, implying that an identical or similar RNAi sequence could function across different plant families (Sunkar and Jagadeeswaran 2008). The PtLFY-IR-mediated gene silencing of LFY orthologs in tobacco presented in our study suggests a down-regulation of transcription that could be due to an RNAi mechanism. In previous study, clustered shoots on crown, growth rate change, and not flowering, were seen in some transgenic poplar lines with strong PTLF expression and early flowering by overexpression of PTLF were still observed in others (Rottmann et al. 2000). Consequently, we infer that the PtLFY-IR construct may be of general use to control flowering in poplars.
P. tomentosa is a versatile species important in wood production, ecological rehabilitation, pulping for paper, and biomass production. It also provides shade and esthetic beauty in rural and urban areas. Given that the production of flowers might be energetically expensive and possibly have a negative impact on vegetative growth, along with the known allergenic properties of pollen, and other environmental issues such as catkins production, the regulation of flowering in P. tomentosa is an important issue that needs to be considered when planting this species. Our results provided evidence for the important role of PtLFY in flowering of P. tomentosa, but further investigation is needed to elucidate the molecular mechanisms underlying the function and regulation of PtLFY in P. tomentosa.
Abbreviations
- E. coli :
-
Escherichia coli
- SDS:
-
Sodium dodecyl sulfate
- SSC:
-
Saline-sodium citrate
- WT:
-
Wild type
- qRT-PCR:
-
Quantitative reverse transcription polymerase chain reaction
- IR:
-
Inverted repeat
- PTGS:
-
Post-transcriptional gene silencing
References
Ahearn KP, Johnson HA, Weigel D, Wagner DR (2001) NFL1, a Nicotiana tabacum LEAFY-like gene, controls meristem initiation and floral structure. Plant Cell Physiol 42:1130–1139
Almada R, Cabrera N, Casaretto JA, Ruiz-Lara S, González Villanueva E (2009) VvCO and VvCOL1, two CONSTANS homologous genes, are regulated during flower induction and dormancy in grapevine buds. Plant Cell Rep 28(8):1193–1203
Blazquez MA, Weigel D (2000) Integration of floral inductive signals in Arabidopsis. Nature 404:889–892
Blazquez MA, Soowal LN, Lee I, Weigel D (1997) LEAFY expression and flower initiation in Arabidopsis. Development 124:3835–3844
Blázquez MA, Santos E, Flores CL, Martínez-Zapater JM, Salinas J, Gancedo C (1998) Isolation and molecular characterization of the Arabidopsis TPS1 gene, encoding trehalose-6-phosphate synthase. Plant J 13:685–689
Boes TK, Strauss SH (1994) Floral phenology and morphology of black cottonwood, Populus trichocarpa (Salicaceae). Am J Bot 81:562–567
Böhlenius H (2007) Control of flowering time and growth Cessation in Arabidopsis and Populus trees. Doctoral Thesis, Swedish University of Agricultural Sciences, Umeå, Sweden
Bomblies K, Wang R-L, Ambrose BA, Schmidt RJ, Meeley RB, Doebley J (2003) Duplicate FLORICAULA/LEAFY homologues zfl1 and zfl2 control inflorescence architecture and flower patterning in maize. Dev 130:2385–2395
Boss PK, Bastow RM, Mylne JS, Dean C (2004) Multiple pathways in the decision to flower enabling, promoting, and resetting. Plant Cell 16(Suppl):S18–S31
Braatne JH, Rood SB, Heilman PE (1996) Life history, ecology and conservation of riparian cottonwoods in North America. In: Stettler RF, Bradshaw HD, Heilman PE, Hinckley TM (eds) Populus and its implications for management and conservation, part I, chap 3. NRC Research Press, National Research Council of Canada, Ottawa, pp 57–85
Bradley D, Ratcliffe OJ, Vincent C, Carpenter R, Coen ES (1997) Inflorescence commitment and architecture in Arabidopsis. Science 275:80–83
Carmona MJ, Cubas P, Martinez-Zapater JM (2002) VFL, the Grapevine FLORICAULA/LEAFY ortholog, is expressed in meristematic regions independently of their fate. Plant Physiol 130:68–77
Chae E, Tan QK-G, Hill TA, Irish VF (2008) An Arabidopsis F-box protein acts as a transcriptional co-factor to regulate floral development. Development 135:1235–1245
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine tree. Plant Mol Biol Rep 11:113–116
Chuang CF, Meyerowitz EM (2000) Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc Natl Acad Sci USA 97:4985–4990
Coen ES, Romero JM, Doyle S, Elliott R, Murphy G, Carpenter R (1990) Floricaula: a homeotic gene required for flower development in Antirrhinum majus. Cell 63:1311–1322
Dornelas MC, Rodriguez APM (2005) The rubber tree (Hevea brasiliensis Muell. Arg.) homologue of the LEAFY/FLORICAULA gene is preferentially expressed in both male and female floral meristems. J Exp Bot 56:1965–1974
Dornelas MC, Amaral WAN, Rodriguez APM (2004) EgLFY, the Eucalyptus grandis homolog of the Arabidopsis gene LFY is expressed in reproductive and vegetative tissues. Braz J Plant Physiol 16:105–114
Frohlich MW, Parker DS (2000) The mostly male theory of flower evolution origins: from genes to fossils. Sys Bot 25:155–170
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788
Hamès C, Ptchelkine D, Grimm C, Thevenon E, Moyroud E, Gérard F, Martiel JL, Benlloch R, Parcy F, Müller CW (2008) Structural basis for LEAFY floral switch function and similarity with helix-turn-helix proteins. EMBO J 27:2628–2637
Hsu CY, Liu YX, Luthe DS, Yuceer C (2006) Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. Plant Cell 18:1846–1861
Junko K, Saeko K, Keisuke N, Takeshi I, Ko S (1998) Down-regulation of RFL, the FLO/LFY homolog of rice, accompanied with panicle branch initiation. PNAS 95:1979–1982
Kerschen A, Napoli CA, Jorgensen RA, Müller AE (2004) Effectiveness of RNA interference in transgenic plants. FEBS Lett 566:223–228
Kotoda N, Wada M, Kusaba S, Kano-Murakami Y, Masuda T, Soejima J (2002) Overexpression of dMADS5, an APETALA1-like gene of apple, causes early flowering in transgenic Arabidopsis. Plant Sci 162:679–687
Li Y, Hagen G, Guilfoyle TJ (1992) Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Dev Biol 153:386–395
Lohmann JU, Weigel D (2002) Building beauty: the genetic control of floral patterning. Dev Cell 2:135–142
Maizel A, Busch MA, Tanahashi T, Perkovic J, Kato M, Hasebe M, Weigel D (2005) The floral regulator LEAFY evolves by substitutions in the DNA binding domain. Science 308:260–263
Mandel MA, Yanofsky MF (1995) A gene triggering flower formation in Arabidopsis. Nature 377:522–524
Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360:273–277
Mellerowicz EJ, Horgan K, Walden A, Coker A, Walter C (1998) PRFLL: a Pinus radiata homologue of FLORICAULA and LEAFY is expressed in buds containing vegetative and undifferentiated male cone primordia. Planta 206:619–629
Mouradov A, Glassick T, Hamdorf B, Murphy L, Fowler B, Marla S, Teasdale RD (1998) NEEDLY, a Pinus radiata ortholog of FLORICAULA/LEAFY genes, expressed in both reproductive and vegetative meristems. PNAS 95:6537–6542
Ng M, Yanofsky MF (2001) Function and evolution of the plant MADS-box gene family. Nat Rev Genet 2:186–195
Nilsson O, Lee I, Blazquez MA, Weigel D (1998) Flowering time genes modulate the response to LEAFY activity. Genetics 150:403–410
Ossowski S, Schwab R, Weigel D (2008) Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J 53(4):674–690
Peña L, Martin-Trillo MM, Juarez J, Pina JA, Navarro L, Martinez-Zapater JM (2001) Constitutive expression of Arabidopsis LEAFY or APETALA1 genes in citrus reduces their generation time. Nat Biotechnol 19:263–267
Reinhardt D, Kuhlemeiera C (2002) Plant architecture. EMBO Rep 3:846–851
Rottmann WH, Meilan R, Sheppard LA, Brunner AM, Skinner JS, Ma C, Cheng S, Jouanin L, Pilate G, Strauss SH (2000) Diverse effects of overexpression of LEAFY and PTLF, a poplar (Populus) homolog of LEAFY/FLORICAULA, in transgenic poplar and Arabidopsis. Plant J 22:235–245
Ruan YL, Llewellyn DJ, Furbank RT (2003) Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. Plant Cell 15(4):952–964
Sheppard LA (1997) PTD: a Populus trichocarpa gene with homology to floral homeotic transcription factors. PhD Dissertation, Oregon State University, Corvallis, USA
Soltis DE, Soltis PS (1999) Polyploidy: recurrent formation and genome evolution. Trends Ecol Evol 14:348–352
Somerville C, Koornneef M (2002) A fortunate choice: the history of Arabidopsis as a model plant. Nat Rev Genet 3:883–889
Souer E, van der Krol A, Kloos D, Spelt C, Bliek M, Mol J, Koes R (1998) Genetic control of branching pattern and floral identity during Petunia inflorescence development. Development 125:733–742
Southerton SG, Strauss SH, Olive MR, Harcourt RL, Decroocq V, Zhu X, Llewellyn DJ, Peacock WJ, Dennis ES (1998) Eucalyptus has a functional equivalent of the Arabidopsis floral meristem identity gene LEAFY. Plant Mol Biol 37:897–910
Sunkar R, Jagadeeswaran G (2008) In silico identification of conserved microRNAs in large number of diverse plant species. BMC Plant Biol 8:37
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tzfira T, Jensen CS, Wang WG, Zuker A, Vinocur B, Altman A, Vainstein A (1997) Transgenic Populus tremula: a step-by-step protocol for its Agrobacterium-mediated transformation. Plant Mol Biol Rep 15:219–235
Wada M, Cao Q-F, Kotoda N, Soejima J-I, Masuda T (2002) Apple has two orthologues of FLORICAULA/LEAFY involved in flowering. Plant Mol Biol 49:567–577
Walton EF, Podivinsky E, Wu RM (2001) Bimodal pattern of floral gene expression over the two seasons that kiwifruit flowers develop. Physiol Plantarum 111:396–404
Wei H, Meilan R, Brunner AM, Skinner JS, Ma C, Strauss SH (2006) Transgenic sterility in Populus: expression properties of the poplar PTLF, Agrobacterium NOS and two minimal 35S promoters in vegetative tissues. Tree Physiol 26(4):401–410
Weigel D, Meyerowitz EM (1993) Activation of floral homeotic genes in Arabidopsis. Science 261:1723–1726
Weigel D, Meyerowitz EM (1994) The ABCs of floral homeotic genes. Cell 78:203–209
Weigel D, Nilsson O (1995) A developmental switch sufficient for flower initiation in diverse plants. Nature 377:495–500
Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 69:843–859
Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27:581–590
Wikström N, Savolainen V, Chase MW (2001) Evolution of the angiosperms: calibrating the family tree. Proc R Soc Lond B 268:2211–2220
Yanofsky MF (1995) Floral meristems to floral organs: Genes controlling early events in Arabidopsis flower development. Annu Rev Plant Physiol Plant Mol 46:167–188
Yuceer C, Land SB Jr, Kubiske ME, Harkess RL (2003) Shoot morphogenesis associated with flowering in Populus deltoides (Salicaceae). Am J Bot 90:196–206
Zhang Q, Zhang ZY, Lin SZ, Zheng HQ, Lin YZ, An XM, Li Y, Li HX (2008) Characterization of resistance gene analogs with a nucleotide binding site isolated from a triploid white poplar. Plant Biol (Stuttg) 10(3):310–322
Zheng H, Lin S, Zhang Q, Lei Y, Zhang Z (2009) Functional analysis of 5′ untranslated region of a TIR-NBS-enconding gene from triploid white poplar. Mol Genet Genomics 282(4):381–394
Acknowledgments
We gratefully acknowledge Prof. Phillip F. Elliott, East Hartford University, CT, USA, and Dr. Jean W. H. Yong, Nanyang Technological University, Singapore, for invaluable advice and assistance. We thank Huiquan Zheng, a Ph.D. student currently in our laboratory, for assistance with figure preparation. This work was supported by grants from the Natural Science Foundation of China (No. 30571511), National High-tech R&D Program of China (No. 2009AA10Z107) and Key Project of Education of Chinese Ministry of Education (No. 108017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Li.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
The predicted secondary and tertiary structures of the PtLFY protein. (A) The secondary structure of PtLFY. The blue, yellow, green and light blue colors indicate the helix, sheet, turn and coil structure, respectively. (B) Putative proportion of helix, sheet, turn and coil in PtLFY. (C) The tertiary structure of PtLFY. The highly conserved PtLFY C-terminal is composed of α1, α2, α3, α4, α5, α6 and α7 helices (TIFF 4662 kb)
Fig. S2
The alignments of C-terminal amino acid sequences (A) and nucleotide sequences (B) of PtLFY (GenBank accession no. AY211519) with NFL1 (U16172) and NFL2 (U15799). PtLFY shares 94.7% amino acid identity with NFL1 and NFL2 at C-terminal designed as the target of PtLFY-IR structure (A). Nucleic acid of PtLFY shows 82.1% similarity to that of NFL1 and NFL2 at C-terminal designed as the target of PtLFY-IR structure (B) (TIFF 1128 kb)
Rights and permissions
About this article
Cite this article
An, XM., Wang, DM., Wang, ZL. et al. Isolation of a LEAFY homolog from Populus tomentosa: expression of PtLFY in P. tomentosa floral buds and PtLFY-IR-mediated gene silencing in tobacco (Nicotiana tabacum). Plant Cell Rep 30, 89–100 (2011). https://doi.org/10.1007/s00299-010-0947-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0947-0